Free-Standing Self-Assemblies of Gallium Nitride Nanoparticles: A Review
Abstract
:1. Introduction
2. Syntheses of GaN Nanoparticles
2.1. Chemical Vapor Deposition
2.2. Nitridation
2.3. Solvothermal Techniques
2.4. Ball-Milling Techniques
2.5. Other Techniques
3. Physical Properties and Applications of GaN Nanoparticles
3.1. Physical Properties of Crystalline Nanoparticles
3.2. Defects of GaN Nanoparticles
3.3. Defect Effects on Physical Properties
3.4. Applications of Nanoparticles
4. Free-standing Self-assembly of GaN Nanoparticles
4.1. GaN Nanospheres
4.2. GaN Nanotubes
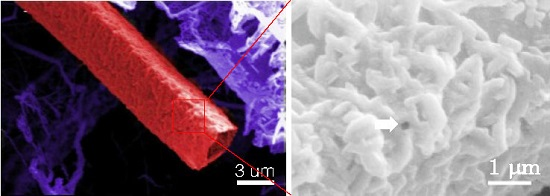
4.3. GaN Circular Microtubes
4.4. GaN Squared Microtubes
4.5. GaN Nanocomposite Bulks
5. Optical Properties of GaN Self-assemblies
5.1. Photoluminescence
5.2. Raman Scattering
6. Potential Applications of GaN Self-Assemblies
6.1. Photocatalytic Water Splitting
6.2. Piezoelectric Nanogenerators
6.3. Thermoelectric Devices
6.4. Other Potential Applications in Renewable Energy
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morkoç, H.; Strite, S.; Gao, G.B.; Lin, M.E.; Sverdlov, B.; Burns, M. Large band gap SiC, III-V nitride, and II-VI ZnSe-based semiconductor device technologies. J. Appl. Phys. 1994, 76, 1363–1398. [Google Scholar] [CrossRef]
- Mohammad, S.; Morkoç, H. Progress and prospects of group-III nitride semiconductors. Prog. Quant. Electron. 1996, 20, 361–525. [Google Scholar] [CrossRef]
- Akasaki, I.; Amano, H. Crystal Growth and Conductivity Control of Group III Nitride Semiconductors and Their Application to Short Wavelength Light Emitters. Jpn. J. Appl. Phys. 1997, 36, 5393. [Google Scholar] [CrossRef]
- Nakamura, S. The Roles of Structural Imperfections in InGaN-Based Blue Light-Emitting Diodes and Laser Diodes. Science 1998, 281, 956–961. [Google Scholar] [CrossRef]
- Chen, X.L.; Lan, Y.C.; Liang, J.K.; Cheng, X.R.; Xu, Y.P.; Xu, T.; Jiang, P.Z.; Lu, K.Q. Structure and Heat Capacity of Wurtzite GaN from 113 to 1073 K. Chin. Phys. Lett. 1999, 16, 107–108. [Google Scholar] [CrossRef]
- Monemar, B. Fundamental energy gap of GaN from photoluminescence excitation spectra. Phys. Rev. B 1974, 10, 676–681. [Google Scholar] [CrossRef]
- Korona, K.P.; Wysmołek, A.; Pakuła, K.; Stȩpniewski, R.; Baranowski, J.M.; Grzegory, I.; Łucznik, B.; Wróblewski, M.; Porowski, S. Exciton region reflectance of homoepitaxial GaN layers. Appl. Phys. Lett. 1996, 69, 788–790. [Google Scholar] [CrossRef]
- Nakamura, S.; Mukai, T.; Senoh, M. High-Power GaN P-N Junction Blue-Light-Emitting Diodes. Jpn. J. Appl. Phys. 1991, 30, L1998. [Google Scholar] [CrossRef]
- Nakamura, S.; Senoh, M.; Mukai, T. High-power InGaN/GaN double-heterostructure violet light emitting diodes. Appl. Phys. Lett. 1993, 62, 2390–2392. [Google Scholar] [CrossRef]
- Ponce, F.A.; Bour, D.P. Nitride-based semiconductors for blue and green light-emitting devices. Nature 1997, 386, 351–359. [Google Scholar] [CrossRef]
- Akasaki, I.; Amano, H.; Sota, S.; Sakai, H.; Tanaka, T.; Koike, M. Stimulated Emission by Current Injection from an AlGaN/GaN/GaInN Quantum Well Device. Jpn. J. Appl. Phys. 1995, 34, L1517. [Google Scholar] [CrossRef]
- Amano, H.; Asahi, T.; Akasaki, I. Stimulated Emission Near Ultraviolet at Room Temperature from a GaN Film Grown on Sapphire by MOVPE Using an AlN Buffer Layer. Jpn. J. Appl. Phys. 1990, 29, L205. [Google Scholar] [CrossRef]
- Asif Khan, M.; Kuznia, J.N.; Bhattarai, A.R.; Olson, D.T. Metal semiconductor field effect transistor based on single crystal GaN. Appl. Phys. Lett. 1993, 62, 1786–1787. [Google Scholar] [CrossRef]
- Brandt, M.S.; Herbst, P.; Angerer, H.; Ambacher, O.; Stutzmann, M. Thermopower investigation of n- and p-type GaN. Phys. Rev. B 1998, 58, 7786–7791. [Google Scholar] [CrossRef]
- Liu, W.; Balandin, A.A. Thermoelectric effects in wurtzite GaN and AlxGa1-xN alloys. J. Appl. Phys. 2005, 97, 123705. [Google Scholar] [CrossRef]
- Adachi, S. Lattice thermal conductivity of group-IV and III-V semiconductor alloys. J. Appl. Phys. 2007, 102, 063502. [Google Scholar] [CrossRef]
- Hurwitz, E.; Asghar, M.; Melton, A.; Kucukgok, B.; Su, L.; Orocz, M.; Jamil, M.; Lu, N.; Ferguson, I. Thermopower Study of GaN-Based Materials for Next-Generation Thermoelectric Devices and Applications. J. Electron. Mater. 2011, 40, 513–517. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, G.; Fu, K.; Su, D.; Hu, J. Gallium Nitride Schottky betavoltaic nuclear batteries. Energ. Convers. Manag. 2011, 52, 1955–1958. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, X.; San, H.; Feng, Z.; Liu, B. A high open-circuit voltage gallium nitride betavoltaic microbattery. J. Micromech. Microeng. 2012, 22, 074011. [Google Scholar] [CrossRef]
- San, H.; Yao, S.; Wang, X.; Cheng, Z.; Chen, X. Design and simulation of GaN based Schottky betavoltaic nuclear micro-battery. Appl. Radiat. Isot. 2013, 80, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Lin, S.; Zhong, S.; Wei, Y.; Meng, X.; Shen, X. Theoretical study on InxGa1-xN/Si hetero-junction solar cells. Proc. SPIE 2009, 7409, 740910. [Google Scholar]
- Lan, Y.; Ren, Z. Thermoelectric Nanocomposites for Thermal Energy Conversion. In Nanomaterials for Sustainable Energy; Springer-Verlag: New York, NY, USA, 2016; pp. 371–443. [Google Scholar]
- Lan, Y.C.; Chen, X.L.; Xu, Y.P.; Cao, Y.G.; Huang, F. Syntheses and structure of nanocrystalline gallium nitride obtained from ammonothermal method using lithium metal as mineralizator. Mater. Res. Bull. 2000, 35, 2325–2330. [Google Scholar] [CrossRef]
- Han, W.; Fan, S.; Li, Q.; Hu, Y. Synthesis of Gallium Nitride Nanorods through a Carbon Nanotube-Confined Reaction. Science 1997, 277, 1287–1289. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, D.; Park, Y.; Kim, D.; Kang, T.; Chung, K. Growth of GaN Nanorods by a Hydride Vapor Phase Epitaxy Method. Adv. Mater. 2002, 14, 991–993. [Google Scholar] [CrossRef]
- Deb, P.; Kim, H.; Rawat, V.; Oliver, M.; Kim, S.; Marshall, M.; Stach, E.; Sands, T. Faceted and Vertically Aligned GaN Nanorod Arrays Fabricated without Catalysts or Lithography. Nano Lett. 2005, 5, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Zhu, H. One-dimensional gallium nitride micro/nanostructures synthesized by a space-confined growth technique. Appl. Phys. A Mater. Sci. Process. 2007, 87, 651–659. [Google Scholar] [CrossRef]
- Li, J.Y.; Chen, X.L.; Qiao, Z.Y.; Cao, Y.G.; Lan, Y.C. Formation of GaN nanorods by a sublimation method. J. Cryst. Growth 2000, 213, 408–410. [Google Scholar] [CrossRef]
- Han, W.; Redlich, P.; Ernst, F.; Rühle, M. Synthesis of GaN-carbon composite nanotubes and GaN nanorods by arc discharge in nitrogen atmosphere. Appl. Phys. Lett. 2000, 76, 652–654. [Google Scholar] [CrossRef]
- Han, W.Q.; Zettl, A. Pyrolysis approach to the synthesis of gallium nitride nanorods. Appl. Phys. Lett. 2002, 80, 303–305. [Google Scholar] [CrossRef]
- Li, S.; Waag, A. GaN based nanorods for solid state lighting. J. Appl. Phys. 2012, 111, 071101. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Cao, Y.; Lan, Y.; Li, H.; He, M.; Wang, C.; Zhang, Z.; Qiao, Z. Straight and Smooth GaN Nanowires. Adv. Mater. 2000, 12, 1432–1434. [Google Scholar] [CrossRef]
- Chen, C.C.; Yeh, C.C.; Chen, C.H.; Yu, M.Y.; Liu, H.L.; Wu, J.J.; Chen, K.H.; Chen, L.C.; Peng, J.Y.; Chen, Y.F. Catalytic Growth and Characterization of Gallium Nitride Nanowires. J. Am. Chem. Soc. 2001, 123, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Lieber, C.M. Laser-Assisted Catalytic Growth of Single Crystal GaN Nanowires. J. Am. Chem. Soc. 2000, 122, 188–189. [Google Scholar] [CrossRef]
- Kuykendall, T.; Pauzauskie, P.; Lee, S.; Zhang, Y.; Goldberger, J.; Yang, P. Metalorganic Chemical Vapor Deposition Route to GaN Nanowires with Triangular Cross Sections. Nano Lett. 2003, 3, 1063–1066. [Google Scholar] [CrossRef]
- Bae, S.Y.; Seo, H.W.; Park, J.; Yang, H.; Kim, B. Porous GaN nanowires synthesized using thermal chemical vapor deposition. Chem. Phys. Lett. 2003, 376, 445–451. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Wang, R.; Yu, D. High-Quality Ultra-Fine GaN Nanowires Synthesized via Chemical Vapor Deposition. Adv. Mater. 2003, 15, 419–421. [Google Scholar] [CrossRef]
- Li, J.; Lu, C.; Maynor, B.; Huang, S.; Liu, J. Controlled Growth of Long GaN Nanowires from Catalyst Patterns Fabricated by Dip-Pen Nanolithographic Techniques. Chem. Mater. 2004, 16, 1633–1636. [Google Scholar] [CrossRef]
- Hersee, S.D.; Sun, X.; Wang, X. The Controlled Growth of GaN Nanowires. Nano Lett. 2006, 6, 1808–1811. [Google Scholar] [CrossRef] [PubMed]
- Calarco, R.; Meijers, R.J.; Debnath, R.K.; Stoica, T.; Sutter, E.; Lüth, H. Nucleation and Growth of GaN Nanowires on Si(111) Performed by Molecular Beam Epitaxy. Nano Lett. 2007, 7, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Stach, E.A.; Pauzauskie, P.J.; Kuykendall, T.; Goldberger, J.; He, R.; Yang, P. Watching GaN Nanowires Grow. Nano Lett. 2003, 3, 867–869. [Google Scholar] [CrossRef]
- He, M.; Minus, I.; Zhou, P.; Mohammed, S.N.; Halpern, J.B.; Jacobs, R.; Sarney, W.L.; Salamanca-Riba, L.; Vispute, R.D. Growth of large-scale GaN nanowires and tubes by direct reaction of Ga with NH3. Appl. Phys. Lett. 2000, 77, 3731–3733. [Google Scholar] [CrossRef]
- Goldberger, J.; He, R.; Zhang, Y.; Lee, S.; Yan, H.; Choi, H.J.; Yang, P. Single-crystal gallium nitride nanotubes. Nature 2003, 422, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Q.; Bando, Y.; Zhan, J.H.; Xu, F.F.; Sekiguchi, T.; Golberg, D. Growth of Single-Crystalline Cubic GaN Nanotubes with Rectangular Cross-Sections. Adv. Mater. 2004, 16, 1465–1468. [Google Scholar] [CrossRef]
- Yin, L.W.; Bando, Y.; Golberg, D.; Li, M.S. Growth of Single-Crystal Indium Nitride Nanotubes and Nanowires by a Controlled-Carbonitridation Reaction Route. Adv. Mater. 2004, 16, 1833–1838. [Google Scholar] [CrossRef]
- Yin, L.W.; Bando, Y.; Zhu, Y.C.; Golberg, D.; Yin, L.W.; Li, M.S. Indium-assisted synthesis on GaN nanotubes. Appl. Phys. Lett. 2004, 84, 3912–3914. [Google Scholar] [CrossRef]
- Liu, B.; Bando, Y.; Tang, C.; Shen, G.; Golberg, D.; Xu, F. Wurtzite-type faceted single-crystalline GaN nanotubes. Appl. Phys. Lett. 2006, 88, 093120. [Google Scholar] [CrossRef]
- Dwilinski, R.; Baranowski, J.A.M.; Kaminska, M.; Doradzinski, R.; Garczynski, J.; Siersputowski, L. On GaN Crystallization by Ammonothermal Method. Acta Phys. Pol. A 1996, 90, 763. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, X.; Cao, Y.; Xu, Y.; Xu, T.; Li, J.; Tao, Z.; Liang, J. Morphology of GaN in ammonia. J. Mater. Sci. Lett. 2000, 19, 2215–2217. [Google Scholar] [CrossRef]
- Song, Y.; Wang, W.; Yuan, W.; Wu, X.; Chen, X. Bulk GaN single crystals: Growth conditions by flux method. J. Cryst. Growth 2003, 247, 275–278. [Google Scholar] [CrossRef]
- Lan, Y.C.; Chen, X.L.; Crimp, M.A.; Cao, Y.G.; Xu, Y.P.; Xu, T.; Lu, K.Q. Single crystal growth of gallium nitride in supercritical ammonia. Phys. Status Solidi C 2005, 2, 2066–2069. [Google Scholar] [CrossRef]
- Hashimoto, T.; Wu, F.; Speck, J.S.; Nakamura, S. A GaN bulk crystal with improved structural quality grown by the ammonothermal method. Nat. Mater. 2007, 6, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A. Tuning Optical Properties of GaN-Based Nanostructures by Charge Screening. Phys. Status Solidi A 2001, 183, 81–85. [Google Scholar] [CrossRef]
- Arakawa, Y. Progress in GaN-based quantum dots for optoelectronics applications. IEEE J. Sel. Top. Quant. Electron. 2002, 8, 823–832. [Google Scholar] [CrossRef]
- Qian, F.; Gradečak, S.; Li, Y.; Wen, C.Y.; Lieber, C.M. Core/Multishell Nanowire Heterostructures as Multicolor, High-Efficiency Light-Emitting Diodes. Nano Lett. 2005, 5, 2287–2291. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.C.; Choi, H.J.; Knutsen, K.P.; Schaller, R.D.; Yang, P.; Saykally, R.J. Single gallium nitride nanowire lasers. Nat. Mater. 2002, 1, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Oh, H.; So, H.M.; Kim, J.J.; Kim, J.; Lee, C.J.; Lyu, S.C. Schottky diodes based on a single GaN nanowire. Nanotechnology 2002, 13, 701. [Google Scholar]
- Gradečak, S.; Qian, F.; Li, Y.; Park, H.G.; Lieber, C.M. GaN nanowire lasers with low lasing thresholds. Appl. Phys. Lett. 2005, 87, 173111. [Google Scholar]
- Huang, Y.; Duan, X.; Cui, Y.; Lieber, C.M. Gallium Nitride Nanowire Nanodevices. Nano Lett. 2002, 2, 101–104. [Google Scholar]
- Motayed, A.; Davydov, A.V.; He, M.; Mohammad, S.N.; Melngailis, J. 365 nm operation of n-nanowire/ p-gallium nitride homojunction light emitting diodes. Appl. Phys. Lett. 2007, 90, 183120. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, Y.H.; Lee, H.; Kim, S.I.; Ryu, S.R.; Kim, D.Y.; Kang, T.W.; Chung, K.S. High-Brightness Light Emitting Diodes Using Dislocation-Free Indium Gallium Nitride/Gallium Nitride Multiquantum-Well Nanorod Arrays. Nano Lett. 2004, 4, 1059–1062. [Google Scholar] [CrossRef]
- Zhang, X.M.; Lu, M.Y.; Zhang, Y.; Chen, L.J.; Wang, Z.L. Fabrication of a High-Brightness Blue-Light-Emitting Diode Using a ZnO-Nanowire Array Grown on p-GaN Thin Film. Adv. Mater. 2009, 21, 2767–2770. [Google Scholar]
- Hahn, C.; Zhang, Z.; Fu, A.; Wu, C.H.; Hwang, Y.J.; Gargas, D.J.; Yang, P. Epitaxial Growth of InGaN Nanowire Arrays for Light Emitting Diodes. ACS Nano 2011, 5, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Waag, A.; Wang, X.; Fündling, S.; Ledig, J.; Erenburg, M.; Neumann, R.; Al Suleiman, M.; Merzsch, S.; Wei, J.; Li, S.; et al. The nanorod approach: GaN NanoLEDs for solid state lighting. Phys. Status Solidi C 2011, 8, 2296–2301. [Google Scholar] [CrossRef]
- Huang, C.T.; Song, J.; Lee, W.F.; Ding, Y.; Gao, Z.; Hao, Y.; Chen, L.J.; Wang, Z.L. GaN Nanowire Arrays for High-Output Nanogenerators. J. Am. Chem. Soc. 2010, 132, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lai, C.H.; Hu, Y.; Zhang, Y.; Wang, X.; Xu, C.; Snyder, R.L.; Chen, L.J.; Wang, Z.L. High output nanogenerator based on assembly of GaN nanowires. Nanotechnology 2011, 22, 475401. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.G.; Chen, X.L.; Li, J.Y.; Lan, Y.C.; Liang, J.K. Observation of a quantum-confinement effect with GaN nanoparticles synthesized through a new gas reaction route. Appl. Phys. A Mater. Sci. Process. 2000, 71, 229–231. [Google Scholar]
- Balkaş, C.M.; Davis, R.F. Synthesis Routes and Characterization of High-Purity, Single-Phase Gallium Nitride Powders. J. Am. Ceram. Soc. 1996, 79, 2309–2312. [Google Scholar] [CrossRef]
- Drygaś, M.; Jeleń, P.; Bućko, M.M.; Olejniczak, Z.; Janik, J.F. Ammonolytical conversion of microcrystalline gallium antimonide GaSb to nanocrystalline gallium nitride GaN: Thermodynamics vs. topochemistry. RSC Adv. 2015, 5, 82576–82586. [Google Scholar] [CrossRef]
- Jia, L.; Xie, E.Q.; Pan, X.J.; Zhang, Z.X.; Zhang, Y.Z. Preparation and optical properties of GaN nanocrystalline powders. Mater. Sci. Technol. 2009, 25, 1498–1500. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Purushothaman, V.; Ramakrishnan, V.; Bhalerao, G.M.; Jeganathan, K. The effect of nitridation temperature on the structural, optical and electrical properties of GaN nanoparticles. CrystEngComm 2014, 16, 3584–3591. [Google Scholar] [CrossRef]
- Chen, X.L.; Cao, Y.G.; Lan, Y.C.; Xu, X.P.; Li, J.Q.; Lu, K.Q.; Jiang, P.Z.; Xu, T.; Bai, Z.G.; Yu, Y.D.; et al. Synthesis and structure of nanocrystal-assembled bulk GaN. J. Cryst. Growth 2000, 209, 208–212. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, Y.; Wang, W.; Zhang, S.; Zhang, Y. A benzene-thermal synthetic route to nanocrystalline GaN. Science 1996, 272, 1926–1927. [Google Scholar] [CrossRef] [PubMed]
- Millet, P.; Calka, A.; Williams, J.S.; Vantenaar, G.J.H. Formation of gallium nitride by a novel hot mechanical alloying process. Appl. Phys. Lett. 1993, 63, 2505–2507. [Google Scholar] [CrossRef]
- Johnson, W.C.; Parson, J.B.; Crew, M.C. Nitrogen Compounds of Gallium. III. J. Phys. Chem. 1931, 36, 2651–2654. [Google Scholar] [CrossRef]
- Frank, A.C.; Stowasser, F.; Sussek, H.; Pritzkow, H.; Miskys, C.R.; Ambacher, O.; Giersig, M.; Fischer, R.A. Detonations of Gallium Azides: A Simple Route to Hexagonal GaN Nanocrystals. J. Am. Chem. Soc. 1998, 120, 3512–3513. [Google Scholar] [CrossRef]
- Hwang, J.W.; Campbell, J.P.; Kozubowski, J.; Hanson, S.A.; Evans, J.F.; Gladfelter, W.L. Topochemical Control in the Solid-State Conversion of Cyclotrigallazane into Nanocrystalline Gallium Nitride. Chem. Mater. 1995, 7, 517–525. [Google Scholar] [CrossRef]
- Coffer, J.L.; Johnson, M.A.; Zhang, L.; Wells, R.L.; Janik, J.F. Influence of Precursor Route on the Photoluminescence of Bulk Nanocrystalline Gallium Nitride. Chem. Mater. 1997, 9, 2671–2673. [Google Scholar] [CrossRef]
- Shimada, M.; Wang, W.N.; Okuyama, K. Synthesis of Gallium Nitride Nanoparticles by Microwave Plasma-Enhanced CVD. Chem. Vapor Depos. 2010, 16, 151–156. [Google Scholar] [CrossRef]
- Mićić, O.; Ahrenkiel, S.P.; Bertram, D.; Nozik, A. Synthesis, structure, and optical properties of colloidal GaN quantum dots. Appl. Phys. Lett. 1999, 75, 478–480. [Google Scholar] [CrossRef]
- Lorenz, M.R.; Binkowski, B.B. Preparation, Stability, and Luminescence of Gallium Nitride. J. Electrochem. Soc. 1962, 109, 24–26. [Google Scholar] [CrossRef]
- Ogi, T.; Itoh, Y.; Abdullah, M.; Iskandar, F.; Azuma, Y.; Okuyama, K. Fabrication and photoluminescence of highly crystalline GaN and GaN:Mg nanoparticles. J. Cryst. Growth 2005, 281, 234–241. [Google Scholar] [CrossRef]
- Drygas, M.; Sitarz, M.; Janik, J.F. Ammonolysis of gallium phosphide GaP to the nanocrystalline wide bandgap semiconductor gallium nitride GaN. RSC Adv. 2015, 5, 106128–106140. [Google Scholar] [CrossRef]
- Drygaś, M.; Jeleń, P.; Radecka, M.; Janik, J.F. Ammonolysis of polycrystalline and amorphized gallium arsenide GaAs to polytype-specific nanopowders of gallium nitride GaN. RSC Adv. 2016, 6, 41074–41086. [Google Scholar] [CrossRef]
- Yu, L.; Lv, Y.; Zhang, X.; Zhao, Y.; Zhang, Y.; Huang, H.; Feng, Y. A soluble salt-assisted facile synthetic route to semiconducting GaN nanoparticles. CrystEngComm 2010, 12, 2037–2039. [Google Scholar] [CrossRef]
- Lv, Y.; Yu, L.; Ai, W.; Li, C. Scalable preparation and characterization of GaN nanopowders with high crystallinity by soluble salts-assisted route. J. Nanopart. Res. 2014, 16, 1–7. [Google Scholar] [CrossRef]
- Ren, Z.F.; Lan, Y.C.; Wang, Y. Aligned Carbon Nanotubes: Physics, Concepts, Fabrication and Devices; Springer-Verlag: Heidelberg/Berlin, Germany, 2013. [Google Scholar]
- Cao, Y.G.; Chen, X.L.; Lan, Y.C.; Xu, Y.P.; Xu, T.; Liang, J.K. Synthesis, Raman scattering, and infrared spectra of a new condensed form of GaN nanophase material. J. Mater. Res. 2000, 15, 267–269. [Google Scholar] [CrossRef]
- Purdy, A.P. Ammonothermal Synthesis of Cubic Gallium Nitride. Chem. Mater. 1999, 11, 1648–1651. [Google Scholar] [CrossRef]
- Jacobs, H.; Nöcker, B. Neubestimmung von Struktur und Eigenschaften isotyper Natriumtetraamidometallate des Aluminiums und Galliums. Z. Anorg. Allg. Chem. 1993, 619, 381–386. (In German) [Google Scholar] [CrossRef]
- Guarino, R.; Rouxel, J. L’amidogallate de potassium KGa(NH2)4 et l’imidogallate KGa(NH)2. L’obtention de l’amidure de gallium Ga(NH2)3. Bull. Soc. Chim. Fr. 1969, 7, 2284–2287. (In French) [Google Scholar]
- Zhang, S.; Hintze, F.; Schnick, W.; Niewa, R. Intermediates in Ammonothermal GaN Crystal Growth under Ammonoacidic Conditions. Eur. J. Inorg. Chem. 2013, 2013, 5387–5399. [Google Scholar] [CrossRef]
- Richter, T.M.M.; Niewa, R. Chemistry of Ammonothermal Synthesis. Inorganics 2014, 2, 29. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, Y.; Zhang, S.; Wang, W.; Liu, X.; Zhang, Y. Coexistence of wurtzite GaN with zinc blende and rocksalt studied by X-ray power diffraction and high-resolution transmission electron microscopy. Appl. Phys. Lett. 1996, 69, 334–336. [Google Scholar] [CrossRef]
- Pan, G.; Kordesch, M.E.; Patten, P.G.V. Room-Temperature Synthesis of GaN Nanopowder. Chem. Mater. 2006, 18, 5392–5394. [Google Scholar] [CrossRef]
- Grocholl, L.; Wang, J.; Gillan, E.G. Solvothermal Azide Decomposition Route to GaN Nanoparticles, Nanorods, and Faceted Crystallites. Chem. Mater. 2001, 13, 4290–4296. [Google Scholar] [CrossRef]
- Giroire, B.; Marre, S.; Garcia, A.; Cardinal, T.; Aymonier, C. Continuous supercritical route for quantum-confined GaN nanoparticles. React. Chem. Eng. 2016, 1, 151–155. [Google Scholar] [CrossRef]
- Guo, X.; Thomas, T.; Li, K.K.; Qi, J.; Wang, Y.; Chen, X.; Zhang, J.; Spencer, M.G.; Zhao, H.; Zou, Y.K.; et al. Size Reduction and Rare Earth Doping of GaN Powders through Ball Milling. MRS Proc. 2009, 1202. [Google Scholar] [CrossRef]
- Kano, J.; Kobayashi, E.; Tongamp, W.; Saito, F. Preparation of GaN powder by mechanochemical reaction between Ga2O3 and Li3N. J. Alloys Compd. 2008, 464, 337–339. [Google Scholar] [CrossRef]
- Yoshida, T.; Kakumoto, S.; Sugimura, A.; Umezu, I. Synthesis of GaN nanocrystallites by pulsed laser ablation in pure nitrogen background gases. Appl. Phys. A Mater. Sci. Process. 2011, 104, 907–911. [Google Scholar] [CrossRef]
- Li, E.; Ma, D.M.; Wang, X.W.; Qi, W.; Zhu, H. Synthesis and IR Vibrational Spectrum on GaN Nanocrystalline Prepared by Sol-gel Method. Chin. J. Electron. 2009, 18, 615–618. [Google Scholar]
- Manz, A.; Birkner, A.; Kolbe, M.; Fischer, R.A. Solution Synthesis of Colloidal Gallium Nitride at Unprecedented Low Temperatures. Adv. Mater. 2000, 12, 569–573. [Google Scholar] [CrossRef]
- Ramvall, P.; Tanaka, S.; Nomura, S.; Riblet, P.; Aoyagi, Y. Observation of confinement-dependent exciton binding energy of GaN quantum dots. Appl. Phys. Lett. 1998, 73, 1104–1106. [Google Scholar] [CrossRef]
- Ramvall, P.; Riblet, P.; Nomura, S.; Aoyagi, Y.; Tanaka, S. Optical properties of GaN quantum dots. J. Appl. Phys. 2000, 87, 3883–3890. [Google Scholar] [CrossRef]
- Cao, Y.G.; Chen, X.L.; Lan, Y.C.; Li, J.Y.; Zhang, Y.; Xu, Y.P.; Xu, T.; Liang, J.K. Red emission from GaN nanocrystalline solids. Mod. Phys. Lett. B 2000, 14, 583–588. [Google Scholar] [CrossRef]
- Guisbiers, G.; Liu, D.; Jiang, Q.; Buchaillot, L. Theoretical predictions of wurtzite III-nitride nano-materials properties. Phys. Chem. Chem. Phys. 2010, 12, 7203–7210. [Google Scholar] [CrossRef] [PubMed]
- Antoniammal, P.; Arivuoli, D. Size and Shape Dependence on Melting Temperature of Gallium Nitride Nanoparticles. J. Nanomater. 2012, 2012, 11. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, W.; Zhang, D.; Chen, X. Dielectric properties of GaN nanoparticles. J. Mater. Sci. 2001, 36, 4401–4403. [Google Scholar] [CrossRef]
- Kako, S.; Miyamura, M.; Tachibana, K.; Hoshino, K.; Arakawa, Y. Size-dependent radiative decay time of excitons in GaN/AlN self-assembled quantum dots. Appl. Phys. Lett. 2003, 83, 984–986. [Google Scholar] [CrossRef]
- Fonoberov, V.A.; Balandin, A.A. Excitonic properties of strained wurtzite and zinc-blende GaN/AlxGa1-xN quantum dots. J. Appl. Phys. 2003, 94, 7178–7186. [Google Scholar] [CrossRef]
- Reshchikov, M.A.; Morkoç, H.; Park, S.S.; Lee, K.Y. Yellow and green luminescence in a freestanding GaN template. Appl. Phys. Lett. 2001, 78, 3041–3043. [Google Scholar] [CrossRef]
- Ren, H.; Jian, J.; Chen, C.; Pan, D.; Ablat, A.; Sun, Y.; Li, J.; Wu, R. Ga-vacancy-induced room-temperature ferromagnetic and adjusted-band-gap behaviors in GaN nanoparticles. Appl. Phys. A Mater. Sci. Process. 2014, 116, 185–191. [Google Scholar] [CrossRef]
- Li, P.G.; Lei, M.; Du, Y.X.; Guo, X.; Tang, W.H. Synthesis of N-deficient GaN nanoparticles and its enhanced dielectric response. Appl. Surf. Sci. 2009, 255, 3843–3847. [Google Scholar] [CrossRef]
- Slimane, A.B.; Najar, A.; Elafandy, R.; San-Román-Alerigi, D.P.; Anjum, D.; Ng, T.K.; Ooi, B.S. On the phenomenon of large photoluminescence red shift in GaN nanoparticles. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Madhu, C.; Sundaresan, A.; Rao, C.N.R. Room-temperature ferromagnetism in undoped GaN and CdS semiconductor nanoparticles. Phys. Rev. B 2008, 77, 201306. [Google Scholar] [CrossRef]
- Reshchikov, M.A.; Morkoç, H. Luminescence properties of defects in GaN. J. Appl. Phys. 2005, 97, 061301. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Chang, Y.; Lin, C.; Han, H.; Tsai, M.; Kuo, H.C.; Yu, P.; Lin, S. Conversion Efficiency Enhancement of GaN/In0.11Ga0.89N Solar Cells With Nano Patterned Sapphire and Biomimetic Surface Antireflection Process. IEEE Photon. Technol. Lett. 2011, 23, 1304–1306. [Google Scholar] [CrossRef]
- Qian, F.; Peng, S.; Yu-Kun, L.; Kai, D.; Qiang, W.; Qing, F.; Yue, H. Hybrid solar cell based on polythiophene and GaN nanoparticles composite. Chin. Phys. B 2014, 23, 028802. [Google Scholar]
- Maeda, K.; Teramura, K.; Saito, N.; Inoue, Y.; Domen, K. Photocatalytic Overall Water Splitting on Gallium Nitride Powder. Bull. Chem. Soc. Jpn. 2007, 80, 1004–1010. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, J.; Qi, X.; He, M.; Li, J. Large-area synthesis of diameter-controllable porous single crystal gallium nitride micro/nanotube arrays. CrystEngComm 2013, 15, 9837–9840. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.D.; Wang, X.F.; Liang, C.H.; Peng, X.S.; Wang, Y.W. Fabrication and photoluminescence of ordered GaN nanowire arrays. J. Chem. Phys. 2001, 115, 5714–5717. [Google Scholar] [CrossRef]
- Yin, L.W.; Bando, Y.; Li, M.S.; Golberg, D. Growth of Semiconducting GaN Hollow Spheres and Nanotubes with Very Thin Shells via a Controllable Liquid Gallium-Gas Interface Chemical Reaction. Small 2005, 1, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Lin, F.; Li, Y.; Dias, Y.; Wang, H.; Liu, Y.; Yang, Z.; Zhou, H.; Lu, Y.; Bao, J.; et al. Gallium nitride porous microtubules self-assembled from wurtzite nanorods. J. Cryst. Growth 2015, 415, 139–145. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, H.; Lin, F.; Lu, Y.; Li, Y.; Liu, Y.; Bao, J.; Ren, Z.; Crimp, M. Nanoporous gallium nitride square microtubes. J. Mater. Sci. 2013, 48, 7703–7707. [Google Scholar] [CrossRef]
- Lagzi, I.; Kowalczyk, B.; Grzybowski, B.A. Liesegang Rings Engineered from Charged Nanoparticles. J. Am. Chem. Soc. 2010, 132, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Govor, L.V.; Reiter, G.; Bauer, G.H.; Parisi, J. Nanoparticle ring formation in evaporating micron-size droplets. Appl. Phys. Lett. 2004, 84, 4774–4776. [Google Scholar] [CrossRef]
- Nicholls, D. Inorganic Chemistry in Liquid Ammonia; Elsevier Scientificc Publishing Company: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Azuhata, T.; Sota, T.; Suzuki, K.; Nakamura, S. Polarized Raman spectra in GaN. J. Phys. Condens. Matter 1995, 7, L129. [Google Scholar] [CrossRef]
- Wang, D.; Pierre, A.; Kibria, M.G.; Cui, K.; Han, X.; Bevan, K.H.; Guo, H.; Paradis, S.; Hakima, A.R.; Mi, Z. Wafer-Level Photocatalytic Water Splitting on GaN Nanowire Arrays Grown by Molecular Beam Epitaxy. Nano Lett. 2011, 11, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Hong, Y.J.; Li, Y.; Cho, J.; Kim, Y.J.; Yi, G.C. Photocatalysis Using GaN Nanowires. ACS Nano 2008, 2, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.B.; Chen, Z.H.; Song, H.S.; Lee, C.S.; Cong, H.T.; Cheng, H.M.; Zhang, W.J.; Bello, I.; Lee, S.T. Vertically Aligned p-Type Single-Crystalline GaN Nanorod Arrays on n-Type Si for Heterojunction Photovoltaic Cells. Nano Lett. 2008, 8, 4191–4195. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lee, S.; You, J.; Kim, T.; Lee, K.; Lee, J.; Kwon, Y.; Kang, T. UV photovoltaic cells fabricated utilizing GaN nanorod/Si heterostructures. J. Cryst. Growth 2010, 312, 2320–2323. [Google Scholar] [CrossRef]
- Fujii, K.; Karasawa, T.; Ohkawa, K. Hydrogen Gas Generation by Splitting Aqueous Water Using n-Type GaN Photoelectrode with Anodic Oxidation. Jpn. J. Appl. Phys. 2005, 44, L543. [Google Scholar] [CrossRef]
- Fujii, K.; Ohkawa, K. Photoelectrochemical Properties of p-Type GaN in Comparison with n-Type GaN. Jpn. J. Appl. Phys. 2005, 44, L909. [Google Scholar] [CrossRef]
- Ono, M.; Fujii, K.; Ito, T.; Iwaki, Y.; Hirako, A.; Yao, T.; Ohkawa, K. Photoelectrochemical reaction and H2 generation at zero bias optimized by carrier concentration of n-type GaN. J. Chem. Phys. 2007, 126. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Nakamura, S.; Yokojima, S.; Goto, T.; Yao, T.; Sugiyama, M.; Nakano, Y. Photoelectrochemical Properties of InxGa1–xN/GaN Multiquantum Well Structures in Depletion Layers. J. Phys. Chem. C 2011, 115, 25165–25169. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Wu, C.H.; Hahn, C.; Jeong, H.E.; Yang, P. Si/InGaN Core/Shell Hierarchical Nanowire Arrays and their Photoelectrochemical Properties. Nano Lett. 2012, 12, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- AlOtaibi, B.; Harati, M.; Fan, S.; Zhao, S.; Nguyen, H.P.T.; Kibria, M.G.; Mi, Z. High efficiency photoelectrochemical water splitting and hydrogen generation using GaN nanowire photoelectrode. Nanotechnology 2013, 24, 175401. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.W.; Zhang, Y.; Leung, B.; Yerino, C.; Han, J. Improved photoelectrochemical water splitting efficiency of nanoporous GaN photoanode. Semicond. Sci. Technol. 2012, 27, 015014. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Chowdhury, R.; Adhikari, S. Size- and temperature-dependent piezoelectric properties of gallium nitride nanowires. Scripta Mater. 2013, 68, 627–630. [Google Scholar] [CrossRef]
- SuŁkowski, C.; ChuchmaŁa, A.; Zaleski, A.J.; Matusiak, M.; Mucha, J.; GŁuchowski, P.; Strȩk, W. Transport properties, specific heat and thermal conductivity of GaN nanocrystalline ceramic. J. Solid State Chem. 2010, 183, 2501–2505. [Google Scholar] [CrossRef]
- Lu, W.; Fang, J.; Stokes, K.L.; Lin, J. Shape Evolution and Self Assembly of Monodisperse PbTe Nanocrystals. J. Am. Chem. Soc. 2004, 126, 11798–11799. [Google Scholar] [CrossRef] [PubMed]













| Method | Precursors | Catalysts | Reaction Temperature () | Average Diameter () | Reference |
|---|---|---|---|---|---|
| CVD | Ga | - | 900–1150 | 5–8 | [67] |
| nitridation | GaO | - | 800–1100 | 40–500 | [68] |
| GaSb | - | 900 | 14–23 | [69] | |
| Ga(NO) | - | 850 | 5 | [70] | |
| GaO(OH) | - | 900–1000 | 12–15 | [71] | |
| solvothermal | Ga | Li | 350–500 | 30–70 | [23] |
| Ga | NHI | 350–500 | 12 | [72] | |
| GaCl | LiN | 280 | 30 | [73] | |
| ball milling | Ga and NH | - | 100 | 10–30 | [74] |
| Mode | E (low) | A(TO) | E(TO) | E(high) | SO(A1) | SO(E1) | A(LO) | E(LO) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| (cm) | (cm) | (cm) | (cm) | (cm) | (cm) | (cm) | (cm) | ||
| crystals | 144 | 533 | 561 | 569 | - | - | 735 | 743 | [128] |
| nanoparticles † | - | 528 | 552 | 564 | 656 | - | 730 | - | [88] |
| microtubes ‡ | - | 519 | 544 | 564 | 656 | 704 | 719 | - | [123] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Li, J.; Wong-Ng, W.; Derbeshi, R.M.; Li, J.; Lisfi, A. Free-Standing Self-Assemblies of Gallium Nitride Nanoparticles: A Review. Micromachines 2016, 7, 121. https://doi.org/10.3390/mi7090121
Lan Y, Li J, Wong-Ng W, Derbeshi RM, Li J, Lisfi A. Free-Standing Self-Assemblies of Gallium Nitride Nanoparticles: A Review. Micromachines. 2016; 7(9):121. https://doi.org/10.3390/mi7090121
Chicago/Turabian StyleLan, Yucheng, Jianye Li, Winnie Wong-Ng, Rola M. Derbeshi, Jiang Li, and Abdellah Lisfi. 2016. "Free-Standing Self-Assemblies of Gallium Nitride Nanoparticles: A Review" Micromachines 7, no. 9: 121. https://doi.org/10.3390/mi7090121