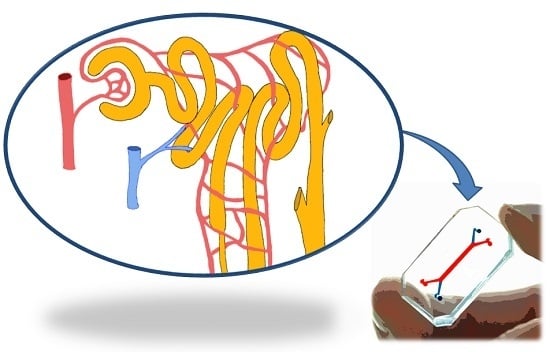
Mimicking the Kidney: A Key Role in Organ-on-Chip Development
Abstract
:1. Introduction
2. Microfluidic Organs-on-Chip
3. The Kidney
3.1. The Role of Kidneys in Drug R&D
3.2. The Kidney Functional Unit: The Nephron
4. Kidney Disease Modeling
4.1. Major Kidney Diseases
4.2. Current Models of Kidney Disease
4.3. Limitations of Current Models
5. Microfluidics Kidney-on-Chip
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| AKI | Acute Kidney Injury |
| (AD)PKD | (Autosomal Dominant) Polycystic Kidney Disease |
| ANCA | Anti-neutrophil cytoplasmic antibodies |
| CKD | Chronic Kidney Disease |
| ECM | Extra-cellular matrix |
| EMA | European Medicines Agency |
| ESRD | End-Stage Renal Disease |
| FDA | Food and Drug Administration |
| GN | Glomerulonephritis |
| HPTEC | Human Proximal Tubular Epithelial Cells |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| ID | Inner diameter |
| IMCD | Inner Medullary Collecting Duct |
| MCE | Mixed Cellulose Ester |
| MDCK | Madin Darby Canine Kidney |
| OD | Outer Diameter |
| OOC | Organ-on-chip |
| PES | Polyethersulfone |
| PK | Pharmacokinetics |
| PDMS | Poly-dimethylsiloxane |
| R&D | Drug research and development |
| RC | Regenerated cellulose |
References
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [PubMed]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [PubMed]
- Munos, B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov. 2009, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O.W.; Rønnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, K.; Ghosh, R. The Use of In Vitro Methods to Predict In Vivo Pharmacokinetics and Drug Interactions. Curr. Drug Metab. 2001, 2, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.-Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Samitier, J.; Sánchez, S. Bioprinting of 3D hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of human organs-on-chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef] [PubMed]
- Viravaidya, K.; Sin, A.; Shuler, M.L. Development of a Microscale Cell Culture Analog to Probe Naphthalene Toxicity. Biotechnol. Prog. 2004, 20, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Yoshimura, E.; Sato, K. Micro Total Bioassay System for Oral Drugs: Evaluation of Gastrointestinal Degradation, Intestinal Absorption, Hepatic Metabolism, and Bioactivity. Anal. Sci. 2012, 28, 197. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.; Materne, E.-M.; Brincker, S.; Süssbier, U.; Frädrich, C.; Busek, M.; Sonntag, F.; Sakharov, D.A.; Trushkin, E.V.; Tonevitsky, A.G.; et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013, 13, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Vozzi, F.; Mazzei, D.; Vinci, B.; Vozzi, G.; Sbrana, T.; Ricotti, L.; Forgione, N.; Ahluwalia, A. A flexible bioreactor system for constructing in vitro tissue and organ models. Biotechnol. Bioeng. 2011, 108, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Iori, E.; Vinci, B.; Murphy, E.; Marescotti, M.C.; Avogaro, A.; Ahluwalia, A.; Avogaro, A.; Crepaldi, C.; Miola, M.; Maran, A.; et al. Glucose and Fatty Acid Metabolism in a 3 Tissue in-vitro Model Challenged with Normo- and Hyperglycaemia. PLoS ONE 2012, 7, e34704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.S.; Bakht, M.; Aleman, J.; Shin, S.-R.; Yue, K.; Sica, M.; Ribas, J.; Duchamp, M.; Ju, J.; et al. Elastomeric Free-form Blood Vessels for Interconnecting Organs on Chip Systems. Lab Chip 2016, 16, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.; Labuz, J.M.; Leung, B.M.; Inoue, M.; Chun, T.-H.; Takayama, S. On being the right size: Scaling effects in designing a human-on-a-chip. Integr. Biol. 2013, 5, 1149. [Google Scholar] [CrossRef] [PubMed]
- Wikswo, J.P.; Curtis, E.L.; Eagleton, Z.E.; Evans, B.C.; Kole, A.; Hofmeister, L.H.; Matloff, W.J. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip 2013, 13, 3496. [Google Scholar] [CrossRef] [PubMed]
- Wikswo, J.P.; Block, F.E.; Cliffel, D.E.; Goodwin, C.R.; Marasco, C.C.; Markov, D.A.; McLean, D.L.; McLean, J.A.; McKenzie, J.R.; Reiserer, R.S.; et al. Engineering Challenges for Instrumenting and Controlling Integrated Organ-on-Chip Systems. IEEE Trans. Biomed. Eng. 2013, 60, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.-J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2016, 47, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, B.M.; Stanton, B.A. Renal Physiology: Mosby Physiology Monograph Series, 5th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Morrissey, K.M.; Stocker, S.L.; Wittwer, M.B.; Xu, L.; Giacomini, K.M. Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, J.; Miller, P. Trial watch: Phase II and phase III attrition rates 2011–2012. Nat. Rev. Drug Discov. 2013, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Pascual, M.T.; Soroko, S.; Savage, B.R.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004, 66, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Naughton, C.A. Drug-induced nephrotoxicity. Am. Fam. Physician 2008, 78, 743–750. [Google Scholar] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2013; United Nations Publication: New York, NY, USA, 2013. [Google Scholar]
- Food and Drug Administration (FDA). Guidance for Industry Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing ans Labeling. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM204959.pdf (accessed on 13 July 2016).
- EMA—Committee for Medicinal Products for Human Use (CHMP). Guideline on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Decreased Renal Function. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/02/WC500200841.pdf (accessed on 13 July 2016).
- Verbeeck, R.K.; Musuamba, F.T. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur. J. Clin. Pharmacol. 2009, 65, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Alpern, R.J.; Moe, O.W.; Caplan, M. Seldin and Giebisch’s The Kidney: Physiology & Pathophysiology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Haraldsson, B.; Nyström, J.; Deen, W.M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 2008, 88, 451–487. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Harris, P.C.; Torres, V.E. Polycystic Kidney Disease. Annu. Rev. Med. 2009, 60, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Bisceglia, M.; Galliani, C.A.; Senger, C.; Stallone, C.; Sessa, A. Renal cystic diseases: A review. Adv. Anat. Pathol. 2006, 13, 26–56. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.; Hein, K.Z.; Nin, V.; Edwards, M.; Chini, C.C.S.; Hopp, K.; Harris, P.C.; Torres, V.E.; Chini, E.N. Food Restriction Ameliorates the Development of Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Wynn, T.; Hakim, R.; Lazarus, J.; Hewitson, T.; Hewitson, T.; Darby, I.; Bisucci, T.; Jones, C.; Becker, G.; et al. Fibrosis in the kidney: Is a problem shared a problem halved? Fibrogenes. Tissue Repair 2007, 5, 524–529. [Google Scholar]
- Wynn, T.A.; Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A.; Friedman, S.L.; Li, M.O.; Wan, Y.Y.; Sanjabi, S.; et al. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, Y. Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 2016, 12, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Scales, C.D.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Prevalence of Kidney Stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, A.; Coppi, F.; Montanari, E.; Del Nero, A.; Zanetti, G.; Pisani, E. Increase in the Prevalence of Symptomatic Upper Urinary Tract Stones during the Last Ten Years. Eur. Urol. 2000, 37, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C.; Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular Mechanism of Renal Stone Formation and the Critical Role Played by Modulators. Biomed Res. Int. 2013, 2013, 1–21. [Google Scholar] [CrossRef] [PubMed]
- DesRochers, T.M.; Palma, E.; Kaplan, D.L. Tissue-engineered kidney disease models. Adv. Drug Deliv. Rev. 2014, 69–70, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Rossard, L.; Favreau, F.; Demars, J.; Robert, R.; Nadeau, C.; Cau, J.; Thuillier, R.; Hauet, T. Evaluation of early regenerative processes in a preclinical pig model of acute kidney injury. Curr. Mol. Med. 2012, 12, 502–505. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ye, J.; Li, Q.; Feng, Y.; Bai, X.; Chen, X.; Wu, C.; Yu, Z.; Zhao, Y.; Hu, X.; et al. Construction of a transgenic pig model overexpressing polycystic kidney disease 2 (PKD2) gene. Transgenic Res. 2013, 22, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Coffman, T.M. The kidney and hypertension: Novel insights from transgenic models. Curr. Opin. Nephrol. Hypertens. 2012, 21, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Jansson, K.; Nguyen, A.-N.T.; Magenheimer, B.S.; Reif, G.A.; Aramadhaka, L.R.; Bello-Reuss, E.; Wallace, D.P.; Calvet, J.P.; Blanco, G. Endogenous concentrations of ouabain act as a cofactor to stimulate fluid secretion and cyst growth of in vitro ADPKD models via cAMP and EGFR-Src-MEK pathways. AJP Ren. Physiol. 2012, 303, F982–F990. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.A.; Abrahamson, D.R.; St. John, P.; Clapp, W.L.; Williams, M.J.; Terada, N.; Hamazaki, T.; Ellison, G.W.; Batich, C.D. Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis 2012, 8, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.A.; Williams, M.J.; Hamazaki, T.; Terada, N.; Clapp, W.L.; Adin, C.; Ellison, G.W.; Jorgensen, M.; Batich, C.D. Embryonic Stem Cells Proliferate and Differentiate when Seeded into Kidney Scaffolds. J. Am. Soc. Nephrol. 2009, 20, 2338–2347. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Guyette, J.P.; Gilpin, S.E.; Gonzalez, G.; Vacanti, J.P.; Ott, H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 2013, 19, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Farney, A.C.; Iskandar, S.S.; Mirmalek-Sani, S.-H.; Sullivan, D.C.; Moran, E.; AbouShwareb, T.; Paolo, D.C.; Wood, K.J.; Stratta, R.J.; et al. Production and Implantation of Renal Extracellular Matrix Scaffolds From Porcine Kidneys as a Platform for Renal Bioengineering Investigations. Ann. Surg. 2012, 256, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.C.; Mirmalek-Sani, S.-H.; Deegan, D.B.; Baptista, P.M.; Aboushwareb, T.; Atala, A.; Yoo, J.J. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 2012, 33, 7756–7764. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.H.; Batchelder, C.A.; Lee, C.I.; Tarantal, A.F. Renal Tissue Engineering with Decellularized Rhesus Monkey Kidneys: Age-Related Differences. Tissue Eng. Part A 2011, 17, 2891–2901. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.H.; Lee, C.C.I.; Batchelder, C.A.; Tarantal, A.F.; Elliott, M.; DeCoppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.; Jungebluth, P.; et al. Tissue Specificity of Decellularized Rhesus Monkey Kidney and Lung Scaffolds. PLoS ONE 2013, 8, e64134. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.H.; Batchelder, C.A.; Lee, C.I.; Tarantal, A.F. Decellularized Rhesus Monkey Kidney as a Three-Dimensional Scaffold for Renal Tissue Engineering. Tissue Eng. Part A 2010, 16, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar] [PubMed]
- L’Azou, B.; Dubus, I.; Ohayon-Courtès, C.; Cambar, J. Human glomerular mesangial IP15 cell line as a suitable model for in vitro cadmium cytotoxicity studies. Cell Biol. Toxicol. 2007, 23, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Satchell, S.C.; Tasman, C.H.; Singh, A.; Ni, L.; Geelen, J.; von Ruhland, C.J.; O’Hare, M.J.; Saleem, M.A.; van den Heuvel, L.P.; Mathieson, P.W. Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int. 2006, 69, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Slater, S.C.; Beachley, V.; Hayes, T.; Zhang, D.; Welsh, G.I.; Saleem, M.A.; Mathieson, P.W.; Wen, X.; Su, B.; Satchell, S.C. An In Vitro Model of the Glomerular Capillary Wall Using Electrospun Collagen Nanofibres in a Bioartificial Composite Basement Membrane. PLoS ONE 2011, 6, e20802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astashkina, A.I.; Mann, B.K.; Prestwich, G.D.; Grainger, D.W. A 3-D organoid kidney culture model engineered for high-throughput nephrotoxicity assays. Biomaterials 2012, 33, 4700–4711. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Nivet, E.; Sancho-Martinez, I.; Gallegos, T.; Suzuki, K.; Okamura, D.; Wu, M.-Z.; Dubova, I.; Esteban, C.R.; Montserrat, N.; et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat. Cell Biol. 2013, 15, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Takasato, M.; Er, P.X.; Becroft, M.; Vanslambrouck, J.M.; Stanley, E.G.; Elefanty, A.G.; Little, M.H. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 2013, 16, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Sancho-Martinez, I.; Nivet, E.; Rodriguez Esteban, C.; Campistol, J.M.; Izpisua Belmonte, J.C. The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor-like cells. Nat. Protoc. 2014, 9, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Morizane, R.; Lam, A.Q.; Freedman, B.S.; Kishi, S.; Valerius, M.T.; Bonventre, J.V. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015, 33, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Xinaris, C.; Brizi, V.; Remuzzi, G. Organoid Models and Applications in Biomedical Research. Nephron 2015, 130, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Essig, M.; Terzi, F.; Burtin, M.; Friedlander, G. Mechanical strains induced by tubular flow affect the phenotype of proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2001, 281, F751–F762. [Google Scholar]
- Baudoin, R.; Griscom, L.; Monge, M.; Legallais, C.; Leclerc, E. Development of a renal microchip for in vitro distal tubule models. Biotechnol. Prog. 2007, 23, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.; Kaazempur-Mofrad, M.; Borenstein, J. Concept and computational design for a bioartificial nephron-on-a-chip. Int. J. Artif. Organs 2008, 31, 508–514. [Google Scholar] [PubMed]
- Jang, K.-J.; Suh, K.-Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 2010, 10, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Goldman, K.; Marchant, R.; Zydney, A.; Brown, D.; Fleischman, A.; Conlisk, A.; Desai, T.; Duffy, S.; Humes, H.; et al. Implanted renal replacement for end-stage renal disease. Panminerva Med. 2011, 53, 155–166. [Google Scholar] [PubMed]
- Fissell, W.H.; Dubnisheva, A.; Eldridge, A.N.; Fleischman, A.J.; Zydney, A.L.; Roy, S. High-Performance Silicon Nanopore Hemofiltration Membranes. J. Membr. Sci. 2009, 326, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, C.; Latancia, M.; Otterbein, L.E.; Netti, P.A. Update on Renal Replacement Therapy: Implantable Artificial Devices and Bioengineered Organs. Tissue Eng. Part B Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kensinger, C.; Karp, S.; Kant, R.; Chui, B.W.; Goldman, K.; Yeager, T.; Gould, E.R.; Buck, A.; Laneve, D.C.; Groszek, J.J.; et al. First Implantation of Silicon Nanopore Membrane Hemofilters. ASAIO J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Moll, S.; Ebeling, M.; Weibel, F.; Farina, A.; Araujo Del Rosario, A.; Hoflack, J.C.; Pomposiello, S.; Prunotto, M. Epithelial cells as active player in fibrosis: Findings from an in vitro model. PLoS ONE 2013, 8, e56575. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Amponsah, P.K.; Al-Shatti, M.; Nie, Z.; Bandyopadhyay, B.C. Engineering of polarized tubular structures in a microfluidic device to study calcium phosphate stone formation. Lab Chip 2012, 12, 4037–4040. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.P.; Zhuang, Y.; Lin, A.W.H.; Teo, J.C.M. A Fibrin-Based Tissue-Engineered Renal Proximal Tubule for Bioartificial Kidney Devices: Development, Characterization and In Vitro Transport Study. Int. J. Tissue Eng. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Zhu, W.; Li, J.; Liu, J. The cell engineering construction and function evaluation of multi-layer biochip dialyzer. Biomed. Microdevices 2013, 15, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Shankland, S.J.; Pippin, J.W.; Reiser, J.; Mundel, P. Podocytes in culture: Past, present, and future. Kidney Int. 2007, 72, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.; Endlich, N.; Kriz, W.; Endlich, K. Podocytes are sensitive to fluid shear stress in vitro. Am. J. Physiol. Ren. Physiol. 2006, 291, F856–F865. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meer, A.D.; Van Den Berg, A. Organs-on-chips: Breaking the in vitro impasse. Integr. Biol. 2012, 4, 461. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.L.; Di Giovanni, N.T.-T. On Exactitude in Science. In A universal History of Infamy; Penguin: London, UK, 1972. [Google Scholar]







© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paoli, R.; Samitier, J. Mimicking the Kidney: A Key Role in Organ-on-Chip Development. Micromachines 2016, 7, 126. https://doi.org/10.3390/mi7070126
Paoli R, Samitier J. Mimicking the Kidney: A Key Role in Organ-on-Chip Development. Micromachines. 2016; 7(7):126. https://doi.org/10.3390/mi7070126
Chicago/Turabian StylePaoli, Roberto, and Josep Samitier. 2016. "Mimicking the Kidney: A Key Role in Organ-on-Chip Development" Micromachines 7, no. 7: 126. https://doi.org/10.3390/mi7070126