Gravity-Based Precise Cell Manipulation System Enhanced by In-Phase Mechanism
Abstract
:1. Introduction
2. Related Works
3. Precise Pressure Control System with In-Phase Noise Cancelation
3.1. The Gravity-Based Pressure Control
3.2. The In-Phase Noise Cancelation
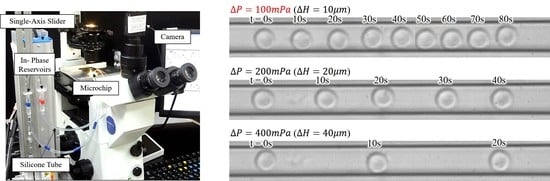
4. Experiments
4.1. Ambient Noises and the Effectiveness of Noise Cancelation
4.2. The Proposed System for Cell Manipulation
4.3. Preliminary Test on Cell Deformability under Precise Pressure Increments
5. Discussion
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mokken, F.C.; Kedaria, M.; Henny, C.P.; Hardeman, M.R.; Gelb, A.W. The clinical importance of erythrocyte deformability, a hemorrheological parameter. Ann. Hematol. 1992, 64, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.N.; Kim, S. Microfluidic platforms for single-cell analysis. Annu. Rev. Biomed. Eng. 2010, 12, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Kuroda, K.; Kaneko, M.; Arai, F. Realization of 240 Nanometer Resolution of Cell Positioning by a Virtual Flow Reduction Mechanism. In Proceedings of the 27th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 1031–1034.
- Monzawa, T.; Kaneko, M.; Tsai, C.; Sakuma, S.; Arai, F. On-chip actuation transmitter for enhancing the dynamic response of cell manipulation using a macro-scale pump. Biomicrofluidics 2015, 9, 014114. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Kuroda, K.; Tsai, C.; Fukui, W.; Arai, F.; Kaneko, M. Red blood cell fatigue evaluation based on the close-encountering point between extensibility and recoverability. Lab Chip 2014, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Sun, D.; Wang, J.; Huang, W. Mechanical characterization of human red blood cells under different osmotic conditions by robotic manipulation with optical tweezers. IEEE Trans. Biomed. Eng. 2010, 57, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Avci, E.; Ohara, K.; Nguyen, C.; Theeravithayangkura, C.; Kojima, M.; Tanikawa, T.; Mae, Y.; Arai, T. High-speed automated manipulation of microobjects using a two-fingered microhand. IEEE Trans. Ind. Electron. 2015, 62, 1070–1079. [Google Scholar] [CrossRef]
- Tanyeri, M.; Ranka, M.; Sittipolkul, N.; Schroeder, C.M. Microfluidic wheatstone bridge for rapid sample analysis. Lab Chip 2011, 11, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liang, Q.; Ma, S.; He, T.; Ai, X.; Hu, P.; Wang, Y.; Luo, G. A gravity-actuated technique for flexible and portable microfluidic droplet manipulation. Microfluid. Nanofluid. 2010, 9, 995–1001. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, Q.; Ai, X.; Hu, P.; Wang, Y.; Luo, G. Comprehensive two-dimensional manipulations of picoliter microfluidic droplets sampled from nanoliter samples. Anal. Chem. 2011, 83, 8029–8034. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Heo, Y.J.; Kim, D.S.; Chung, W.K. A Gravity-Actuated Micro-Manipulation Platform for Control of a Micro-Object with Minimal Hardware Setup. In Proceedings of the 19th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS), Gyeongju, Korea, 25–29 October 2015; pp. 1280–1282.
- Yamada, H.; Yoshida, Y.; Terada, N. Blood cell counter in gravity-driven microchannel. Jpn. J. Appl. Phys. 2005, 44, 8739. [Google Scholar] [CrossRef]
- Yao, B.; Luo, G.; Feng, X.; Wang, W.; Chen, L.; Wang, Y. A microfluidic device based on gravity and electric force driving for flow cytometry and fluorescence activated cell sorting. Lab Chip 2004, 4, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Morier, P.; Vollet, C.; Michel, P.E.; Reymond, F.; Rossier, J.S. Gravity-induced convective flow in microfluidic systems: electrochemical characterization and application to enzyme-linked immunosorbent assay tests. Electrophoresis 2004, 25, 3761–3768. [Google Scholar] [CrossRef] [PubMed]










© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizoue, K.; Phan, M.H.; Tsai, C.-H.D.; Kaneko, M.; Kang, J.; Chung, W.K. Gravity-Based Precise Cell Manipulation System Enhanced by In-Phase Mechanism. Micromachines 2016, 7, 116. https://doi.org/10.3390/mi7070116
Mizoue K, Phan MH, Tsai C-HD, Kaneko M, Kang J, Chung WK. Gravity-Based Precise Cell Manipulation System Enhanced by In-Phase Mechanism. Micromachines. 2016; 7(7):116. https://doi.org/10.3390/mi7070116
Chicago/Turabian StyleMizoue, Koji, Manh Hao Phan, Chia-Hung Dylan Tsai, Makoto Kaneko, Junsu Kang, and Wan Kyun Chung. 2016. "Gravity-Based Precise Cell Manipulation System Enhanced by In-Phase Mechanism" Micromachines 7, no. 7: 116. https://doi.org/10.3390/mi7070116