Synthesis of Solar Light Active Reduced Graphene Oxide-ZnS Nanomaterial for Photocatalytic Degradation and Antibacterial Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analytical Techniques
2.2. Irradiation Experiments
2.3. Synthesis of GO
2.4. Synthesis of rGO
2.5. Synthesis of rGO-ZnS
3. Results
3.1. X-ray Diffraction Pattern of Reduced Graphene Oxide-ZnS
3.2. Scanning Electron Microscope Analysis
3.3. Transmission Electron Microscope Analysis
3.4. Energy Dispersive X-ray Analysis
3.5. Fourier Transfer-Infra Red Spectra
3.6. X-ray Photoelectron Spectroscopy
3.7. Photocatalytic Activity Studies
3.7.1. Effect of Catalyst Weight
3.7.2. Effect of pH
3.7.3. Effect of Initial Dye Concentration
3.7.4. Radical Scavengers Test
3.7.5. Stability of the Catalyst
3.7.6. Kinetic Analysis and Literature Comparison
3.7.7. COD Measurements
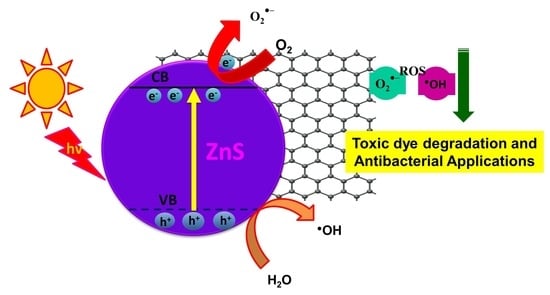
3.7.8. Mechanism of Degradation
4. Antibacterial Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Y.; Li, L.; Xiao, K.; Xi, J. Constructing Three-Dimensional Hierarchical Architectures by Integrating Carbon Nanofibers into Graphite Felts for Water Purification. ACS Sustain. Chem. Eng. 2016, 4, 2351–2358. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Lv, L.; Zhao, Y.; Qu, L. Vertically Aligned Graphene Sheets Membrane for Highly Efficient Solar Thermal Generation of Clean Water. ACS Nano. 2017, 11, 5087–5093. [Google Scholar] [CrossRef]
- Guo, K.; Gao, B.; Tian, B.X.; Yue, Q.; Zhang, P.; Shen, X.; Xu, X. Synthesis of polyaluminium chloride/papermaking sludge-based organic polymer composites for removal of disperse yellow and reactive blue by flocculation. Chemosphere 2019, 231, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Amanulla, B.; Sannasi, S.; Abubakker, A.K.M.; Ramaraj, S.K. A magnetically recoverable bimetallic Au-FeNPs decorated on g-C3N4 for efficient photocatalytic degradation of organic contaminants. J. Mol. Liq. 2018, 249, 754–763. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Jing, L.; Zhou, W.; Tian, G.; Fu, H. Surface tuning for oxide-based nanomaterials as efficient photocatalysts. Chem. Soc. Rev. 2013, 42, 9509–9549. [Google Scholar] [CrossRef] [PubMed]
- Kuzhalosai, V.; Subash, B.; Senthilraja, A.; Dhatshanamurthi, P.; Shanthi, M. Synthesis, characterization and photocatalytic properties of SnO2–ZnO composite under UV-A light. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Terashima, C. Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys. Chem. Chem. Phys. 2014, 16, 8751–8760. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Ren, Z.; Fan, H.; Yang, X. Elementary photocatalytic chemistry on TiO2 surfaces. Chem. Soc. Rev. 2016, 45, 3701–3730. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, J.; Sun, D.D. Graphene oxide enwrapped Ag3PO4 composite: Towards a highly efficient and stable visible-light-induced photocatalyst for water purification. Catal. Sci. Technol. 2012, 2, 2525–2532. [Google Scholar] [CrossRef]
- Xiang, X.; Xie, L.; Li, Z.; Li, F. Ternary MgO/ZnO/In2O3 heterostructured photocatalysts derived from a layered precursor and visible-light-induced photocatalytic activity. Chem. Eng. J. 2013, 221, 7975. [Google Scholar] [CrossRef]
- Dhatshanamurthi, P.; Subash, B.; Senthilraja, A.; Kuzhalosai, V.; Krishnakumar, B.; Shanthi, M. Synthesis and Characterization of ZnS–TiO2 Photocatalyst and Its Excellent Sun Light Driven Catalytic Activity. J. Nanosci. Nanotechnol. 2014, 14, 4930–4939. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Sun, Z.; Liu, X.; Jia, H.; Du, P. Cadmium sulfide/graphitic carbon nitride heterostructure nanowire loading with a nickel hydroxide cocatalyst for highly efficient photocatalytic hydrogen production in water under visible light. Nanoscale 2016, 8, 4748–4756. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Zong, R.; Li, Z.; Yao, W.; Zhu, Y. Photocatalytic hydrogen generation on bifunctional ternary heterostructured In2S3/MoS2/CdS composites with high activity and stability under visible light irradiation. J. Mater. Chem. A 2015, 3, 18406–18412. [Google Scholar] [CrossRef]
- Khanchandani, S.; Kundu, S.; Patra, A.; Ganguli, A.K. Shell Thickness Dependent Photocatalytic Properties of ZnO/CdS. J. Phys. Chem. C 2012, 116, 23653. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Zhou, J.; Jing, D.; Sun, Y. Localized nano-solid-solution induced by Cu doping in ZnS for efficient solar hydrogen generation. Dalt. Trans. 2014, 43, 11533–11541. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, C.; Ho, G.W. In situ dissolution-diffusion toward homogeneous multiphase Ag/Ag2S@ZnS core-shell heterostructures for enhanced photocatalytic performance. J. Phys. Chem. C 2015, 119, 1667–1675. [Google Scholar] [CrossRef]
- Han, L.; Wang, P.; Dong, S. Progress in graphene-based photoactive nanocomposites as a promising class of photocatalyst. Nanoscale 2012, 4, 5814–5825. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, R.; Yang, Y.; Yan, D.; Xiang, X. Highly Enhanced Photoelectrochemical Water Oxidation Efficiency Based on Triadic Quantum Dot/Layered Double Hydroxide/BiVO4 Photoanodes. ACS Appl. Mater. Interfaces 2016, 8, 19446–19455. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Xiang, X.; Yan, D.; Li, F. Engineering of ZnCo-layered double hydroxide nanowalls toward high-efficiency electrochemical water oxidation. J. Mater. Chem. A 2014, 2, 13250–13258. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Kesarla, M.K.; Nimmala, A.R.; Godavarthi, S.; Kukkambakam, C.M.; Gomez, L.M.; Sarada, N.C. ZnS nanoparticles capped with watermelon rind extract and their potential application in dye degradation. Res. Chem. Intermed. 2017, 43, 1329–1339. [Google Scholar] [CrossRef]
- Wang, R.; Pan, K.; Han, D.; Jiang, J.; Xiang, C.; Huang, Z.; Zhang, L.; Xiang, X. Solar-Driven H2O2 Generation From H2O and O2 Using Earth-Abundant Mixed-Metal Oxide@Carbon Nitride Photocatalysts. ChemSusChem 2016, 9, 2470–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Wang, R.; Zhang, L.; Zhu, J.; Xiang, X.; Li, F. Enhanced photoelectrochemical water oxidation on a BiVO4 photoanode modified with multi-functional layered double hydroxide nanowalls. J. Mater. Chem. A 2015, 3, 17977–17982. [Google Scholar] [CrossRef]
- Wei, X.X.; Chen, C.M.; Guo, S.Q.; Guo, F.; Li, X.M.; Wang, X.X.; Cui, H.T.; Zhao, L.F.; Li, W. Advanced visible-light-driven photocatalyst BiOBr-TiO2-graphene composite with graphene as a nano-filler. J. Mater. Chem. A 2014, 2, 4667–4675. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Yao, Y.; Qian, C.; Zhang, X.; Wei, X. Microwave-assisted synthesis and photocatalytic properties of carbon nanotube/zinc sulfide heterostructures. J. Phys. Chem. C 2008, 112, 16779–16783. [Google Scholar] [CrossRef]
- Ye, A.; Fan, W.; Zhang, Q.; Deng, W.; Wang, Y. CdS-graphene and CdS-CNT nanocomposites as visible-light photocatalysts for hydrogen evolution and organic dye degradation. Catal. Sci. Technol. 2012, 2, 969–978. [Google Scholar] [CrossRef]
- Akcöltekin, S.; El Kharrazi, M.; Köhler, B.; Lorke, A.; Schleberger, M. Graphene on insulating crystalline substrates. Nanotechnology 2009, 20, 155601. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Borovikov, V.; Zangwill, A. Step-edge instability during epitaxial growth of graphene from SiC(0001). Phys. Rev. B-Condens. Matter Mater. Phys. 2009, 80, 121406. [Google Scholar] [CrossRef] [Green Version]
- Shin, B.H.; Kim, K.K.; Benayad, A.; Yoon, S.; Park, K.; Jung, I.; Jin, M.H.; Jeong, H.; Kim, J.M.; Choi, J.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Khamboonrueang, D.; Srirattanapibula, S.; Tang, I.-M.; Thongmeea, S. TiO2∙rGO nanocomposite as a photo catalyst for the reduction of Cr6+. Mater. Res. Bull. 2018, 107, 236–241. [Google Scholar] [CrossRef]
- Yuan, J.; Lu, Y.; Huang, B.; Xu, H.; Tao, Y.; Xu, H.; Zhang, W.; He, G.; Chen, H. Al-doping driven electronic structure of α-NiS hollow spheres modified by rGO as high-rate electrode for quasi-solid-state capacitor. Ceram. Int. 2022, 48, 36021–36028. [Google Scholar] [CrossRef]
- Srirattanapibul, S.; Tang, I.-M.; Thongmee, S. Photo catalytic reduction of Cr6+ by ZnO decorated on reduced graphene oxide (rGO) Nanocomposites. Mater. Res. Bull. 2020, 122, 110705. [Google Scholar] [CrossRef]
- Imboon, T.; Khumphon, J.; Yotkuna, K.; Tang, I.-M.; Thongmee, S. Enhancement of photocatalytic by Mn3O4 spinel ferrite decorated graphene oxide nanocomposites. SN Appl. Sci. 2021, 3, 653. [Google Scholar] [CrossRef]
- Liu, W.; Cai, J.; Li, Z. Self-Assembly of Semiconductor Nanoparticles/Reduced Graphene Oxide (RGO) Composite Aerogels for Enhanced Photocatalytic Performance and Facile Recycling in Aqueous Photocatalysis. ACS Sustain. Chem. Eng. 2015, 3, 277–282. [Google Scholar] [CrossRef]
- Nawaz, M.; Islam, M.U.; Nazir, M.A.; Bano, I.; Gul, I.H.; Ajmal, M. Transport properties in spinel ferrite/graphene oxide nanocomposites for electromagnetic shielding. Ceram. Int. 2021, 47, 25505–25513. [Google Scholar] [CrossRef]
- Lee, J.S.; You, K.H.; Park, C.B. Highly Photoactive, Low Bandgap TiO2 Nanoparticles Wrapped by Graphene. Adv Mater. 2012, 24, 1084–1088. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C. Graphene-based photocatalytic composites. RSC Adv. 2011, 1, 1426–1434. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Yang, Z.; Hu, N. Review of recent progress on graphene-based composite gas sensors. Ceram. Int. 2021, 47, 16367–16384. [Google Scholar] [CrossRef]
- Das, T.K.; Ghosh, S.K.; Das, N.C. Green synthesis of a reduced graphene oxide/silver nanoparticles-based catalyst for degradation of a wide range of organic pollutants. Nano-Struct. Nano-Objects 2023, 34, 100960. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Subash, B.; Swaminathan, M. AgBr–ZnO—An efficient nano-photocatalyst for the mineralization of Acid Black 1 with UV light. Sep. Purif. Technol. 2012, 85, 35–44. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, Q.; Zhu, M.; Li, Y.; Wang, H. One-step synthesis of magnetically-functionalized reduced graphite sheets and their use in hydrogels. Carbon 2011, 49, 47–53. [Google Scholar] [CrossRef]
- Lee, M.; Balasingam, S.K.; Jeong, H.Y.; Hong, W.G.; Lee, H.; Kim, B.H.; Jun, Y. One-step hydrothermal synthesis of graphene decorated V2O5 nanobelts for enhanced electrochemical energy storage. Sci. Rep. 2015, 5, 8151. [Google Scholar] [CrossRef] [Green Version]
- Muthoosamy, K.; Geetha Bai, R.; Abubakar, I.B.; Sudheer, S.M.; Lim, H.N.; Loh, H.S.; Huang, N.M.; Chia, C.H.; Manickam, S. Exceedingly biocompatible and thin-layered reduced graphene oxide nanosheets using an eco-friendly mushroom extract strategy. Int. J. Nanomed. 2015, 10, 1505–1519. [Google Scholar]
- De Silva, K.S.B.; Gambhir, S.; Wang, X.L.; Xu, X.; Li, W.X.; Officer, D.L.; Wexler, D.; Wallace, G.G.; Dou, S.X. The effect of reduced graphene oxide addition on the superconductivity of MgB2. J. Mater. Chem. 2012, 22, 13941–13946. [Google Scholar] [CrossRef] [Green Version]
- Phuruangrat, A.; Karthik, K.; Kuntalue, K.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Refuxing synthesis and characterization of ZnS nanoparticles and their photocatalytic properties. Chalcogenide Lett. 2019, 16, 387–393. [Google Scholar]
- Ramkumar, R.; Minakshi Sundaram, M. A biopolymer gel-decorated cobalt molybdate nanowafer: Effective graft polymer cross-linked with an organic acid for better energy storage. R. Soc. Chem. 2016, 40, 2863. [Google Scholar] [CrossRef]
- Yu, D.; Fang, H.; Qiu, P.; Meng, F.; Liu, H.; Wang, S.; Lv, P.; Cong, X.; Niu, Q.; Li, T. Improving the Performance of ZnS Photocatalyst in Degrading Organic Pollutants by Constructing Composites with Ag2O. Nanomater 2021, 11, 1451. [Google Scholar] [CrossRef]
- Bai, Y.; Li, J.P.; Wang, J.; Liu, L. Facile preparation 3D ZnS nanospheresreduced graphene oxide composites for enhanced photodegradation of norfloxacin. J. Alloys Compd. 2017, 729, 809–815. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, M. Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 2008, 46, 1994–1998. [Google Scholar] [CrossRef]
- Labiah, H.; Lahbib, K.; Hindouri, S.; Touil, S.; Chaabane, T.B. Insight of ZnS nanoparticles contribution in different biological uses. Asian Pac. J. Trop. Med. 2016, 9, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Feng, N.; Zhang, G.; Du, G. One-pot hydrothermal synthesis of ZnS–reduced graphene oxide composites with enhanced photocatalytic properties. Cryst. Eng. Comm. 2014, 16, 214–222. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Chen, B.; Guo, S.; Li, J.; Li, C. One-step hydrothermal synthesis of reduced graphene oxide/zinc sulfide hybrids for enhanced tribological properties of epoxy coatings. Surf. Coat. Technol. 2017, 326, 87–95. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X. ZnS-Graphene nanocomposite: Synthesis, characterization and optical properties. J. Solid State Chem. 2012, 191, 51–56. [Google Scholar] [CrossRef]
- Medidi, S.; Markapurapu, S.; Kotupalli, M.R.; Chinnam, R.K.R.; Susarla, V.M.; Gandham, H.B.; Sanasi, P.D. Visible Light Photocatalytic Degradation of Methylene Blue and Malachite Green Dyes with CuWO4-GO Nano Composite. Mod. Res. Catal. 2018, 7, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Jayamani, G.; Shanthi, M. An efficient nanocomposite CdS-ZnWO4 for the degradation of Naphthol Green B dye under UV-A light illumination. Nano-Struct. Nano-Objects 2020, 22, 100452. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Shanthi, M. Photocatalytic degradation of an organic pollutant by zinc oxide–solar process. Arab. J. Chem. 2016, 9, S1858–S1868. [Google Scholar] [CrossRef] [Green Version]
- Baral, A.; Das, D.P.; Minakshi, M.G.; Ghosh, M.K.; Padhi, D.K. Probing Environmental Remediation of RhB Organic Dye Using a-MnO2 under Visible-Light Irradiation: Structural, Photocatalytic and Mineralization Studies. Chem. Sel. 2016, 1, 4277–4285. [Google Scholar]
- Karunakaran, C.; Dhanalakshmi, R. Photocatalytic performance of particulate semiconductors under natural sunshine—Oxidation of carboxylic acids. Sol. Energy Mater. Sol. Cells 2008, 92, 588–593. [Google Scholar] [CrossRef]
- Senthilraja, A.; Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. Novel Sr–Au–ZnO: Synthesis, characterization and photocatalytic activity. Superlattices Microstruct. 2014, 75, 701–715. [Google Scholar] [CrossRef]
- Sadollahkhani, A.; Nur, O.; Willander, M.; Kazeminezhad, I.; Khranovskyy, V.; Eriksson, M.O.; Yakimova, R.; Holtz, P.O. A detailed optical investigation of ZnO@ZnS core–shell nanoparticles and their photocatalytic activityat different pH values. Ceram. Int. 2015, 41, 7174–7184. [Google Scholar] [CrossRef]
- Palanisamy, G.; Bhuvaneswari, K.; Bharathi, G.; Pazhanivel, T.; Grace, A.N.; Pasha, S.K.K. Construction of magnetically recoverable ZnS@WO3@CoFe2O4 nanohybrid enriched photocatalyst for the degradation of MB dye under visible light irradiation. Chemosphere 2021, 273, 129687. [Google Scholar] [CrossRef] [PubMed]
- Janbandhu, S.Y.; Suhaila, C.T.; Munishwar, S.R.; Jayaramaiah, J.R.; Gedam, R.S. Borosilicate glasses containing CdS/ZnS QDs: A heterostructured composite with enhanced degradation of IC dye under visible-light. Chemosphere 2022, 286, 131672. [Google Scholar] [CrossRef]
- Kokilavani, S.; Al-Farraj, S.A.; Thomas, A.M.; El-Serehy, H.A.; Raju, L.L.; Sudheer Khan, S. Enhanced visible light driven photocatalytic and antibacterial activities of Ag2WO4 decorated ZnS nanocomposite. Ceram. Int. 2021, 47, 12997–13006. [Google Scholar] [CrossRef]
- Liu, D.; Gong, J.; Sun, C.; Xu, L.; Song, Y. Enhanced photocatalytic activity for degradation of ofloxacin and dye by hierarchical flower-like ZnS/MoS2/Bi2WO6 heterojunction: Synergetic effect of 2D/2D coupling interface and solid sulfide solutions. Catal. Commun. 2022, 172, 106546. [Google Scholar] [CrossRef]












| Different Radical Scavengers | NBB Dye Degradation Percentage (%) |
|---|---|
| No Scavengers | 88.7 |
| Isopropyl alcohol | 63.5 |
| Ethanol | 52.1 |
| Benzoquinone | 38.2 |
| Time (min) | COD Values (mg/L) | COD Removal (%) |
|---|---|---|
| 0 | 9049.6 | 0 |
| 90 | 5376.0 | 59.4 |
| 150 | 806.4 | 91.1 |
| Serial No. | Microbial Strains | Zone of Inhibition (mm) | |||
|---|---|---|---|---|---|
| Standard (30 µL) | rGO (30 µL) | ZnS (30 µL) | rGO-ZnS (30 µL) | ||
| Gram Positive (+) | |||||
| 1 | Staphylococcus aureus | 30 | 9 | 9 | 15 |
| 2 | Bacillus subtilis | 30 | 11 | 12 | 21 |
| Gram negative (−) | |||||
| 3 | Salmonella paratyphi A | 22 | 10 | 10 | 23 |
| 4 | Escherichia coli | 20 | 11 | 9 | 17 |
| 5 | Klebsiella pneumonia | 32 | 10 | 9 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathya priya, B.; Aruchamy, K.; Oh, T.H.; Avula, B.; Hasan, I.; Shanthi, M. Synthesis of Solar Light Active Reduced Graphene Oxide-ZnS Nanomaterial for Photocatalytic Degradation and Antibacterial Applications. Micromachines 2023, 14, 1324. https://doi.org/10.3390/mi14071324
Sathya priya B, Aruchamy K, Oh TH, Avula B, Hasan I, Shanthi M. Synthesis of Solar Light Active Reduced Graphene Oxide-ZnS Nanomaterial for Photocatalytic Degradation and Antibacterial Applications. Micromachines. 2023; 14(7):1324. https://doi.org/10.3390/mi14071324
Chicago/Turabian StyleSathya priya, B., Kanakaraj Aruchamy, Tae Hwan Oh, Balakrishna Avula, Imran Hasan, and M. Shanthi. 2023. "Synthesis of Solar Light Active Reduced Graphene Oxide-ZnS Nanomaterial for Photocatalytic Degradation and Antibacterial Applications" Micromachines 14, no. 7: 1324. https://doi.org/10.3390/mi14071324