Unexpected Negative Performance of PdRhNi Electrocatalysts toward Ethanol Oxidation Reaction
Abstract
:1. Introduction
2. Experimental Work
2.1. Chemicals
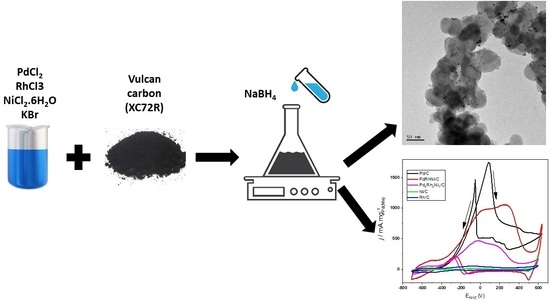
2.2. Catalyst Synthesis
2.3. Catalyst Evaluation
2.4. Catalyst Characterization
3. Results and Discussion
3.1. Electrochemical Characterization
3.2. Physiochemical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kleinikova, S.A.; Levchenko, M.G.; Yalmaev, A.B.; Talagaeva, N.V.; Dremova, N.N.; Gerasimova, E.V.; Zolotukhina, E.V. Some features of alcohols electrooxidation process on Pd, Rh and PdRh catalysts. Electrochim. Acta 2022, 409, 139998. [Google Scholar] [CrossRef]
- Ma, L.; Chu, D.; Chen, R. Comparison of ethanol electro-oxidation on Pt/C and Pd/C catalysts in alkaline media. Int. J. Hydrogen Energy 2012, 37, 11185–11194. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Yu, H.; Peng, F. Platinum-based ternary catalysts for the electrooxidation of ethanol. Particuology 2021, 58, 169–186. [Google Scholar] [CrossRef]
- Halim, E.M.; Chemchoub, S.; El Attar, A.; Salih, F.E.; Oularbi, L.; El Rhazi, M. Recent Advances in Anode Metallic Catalysts Supported on Conducting Polymer-Based Materials for Direct Alcohol Fuel Cells. Front. Energy Res. 2022, 10, 843736. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. A comprehensive and critical review of the recent progress in electrocatalysts for the ethanol oxidation reaction. RSC Adv. 2021, 11, 16768–16804. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Eid, K.; Lebechi, A.K.; Abdullah, A.M.; Ozoemena, K.I. Porous multi-metallic Pt-based nanostructures as efficient electrocatalysts for ethanol oxidation: A mini-review. Electrochem. Commun. 2022, 140, 107330. [Google Scholar] [CrossRef]
- Almeida, C.V.S.; Tremiliosi-Filho, G.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Improved ethanol electro-oxidation at Ni@Pd/C and Ni@PdRh/C core–shell catalysts. J. Catal. 2020, 391, 175–189. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Luo, L.M.; Zhang, R.H.; Chen, D.; Guo, Y.F.; Zhan, W.; Zhou, X.W. Hydrothermal synthesis of complex morphological bimetal PdRh nanocatalysts for methanol oxidation. J. Alloys Compd. 2020, 818, 152886. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef]
- Geraldes, A.N.; da Silva, D.F.; Pino, E.S.; da Silva, J.C.M.; de Souza, R.F.B.; Hammer, P.; Spinacé, E.V.; Neto, A.O.; Linardi, M.; dos Santos, M.C. Ethanol electro-oxidation in an alkaline medium using Pd/C, Au/C and PdAu/C electrocatalysts prepared by electron beam irradiation. Electrochim. Acta 2013, 111, 455–465. [Google Scholar] [CrossRef]
- Sheng, G.; Chen, J.; Ye, H.; Hu, Z.; Fu, X.Z.; Sun, R.; Huang, W.; Wong, C.P. Hollow PdCo alloy nanospheres with mesoporous shells as high-performance catalysts for methanol oxidation. J. Colloid Interface Sci. 2018, 522, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wen, D.; Oschatz, M.; Holzschuh, M.; Liu, W.; Herrmann, A.-K.K.; Simon, F.; Kaskel, S.; Eychmüller, A. Kinetically controlled synthesis of PdNi bimetallic porous nanostructures with enhanced electrocatalytic activity. Small 2015, 11, 1430–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Bin, D.; Yan, B.; Du, Y.; Majima, T.; Zhou, W. Porous bimetallic PdNi catalyst with high electrocatalytic activity for ethanol electrooxidation. J. Colloid Interface Sci. 2017, 493, 190–197. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, P.S.; Mandal, K.; Bhattacharjee, D.; Dasgupta, S.; Bhattacharya, S.K.; Kumar Bhattacharya, S. Improved catalysis of room temperature synthesized Pd-Cu alloy nanoparticles for anodic oxidation of ethanol in alkaline media. Electrochim. Acta 2015, 154, 447–455. [Google Scholar] [CrossRef]
- Carrion-Satorre, S.; Montiel, M.; Escudero-Cid, R.; Fierro, J.L.G.; Fatas, E.; Ocon, P. Performance of carbon-supported palladium and palladium-ruthenium catalysts for alkaline membrane direct ethanol fuel cells. Int. J. Hydrogen Energy 2016, 41, 8954–8962. [Google Scholar] [CrossRef]
- Bagchi, J.; Bhattacharya, S.K. Electrocatalytic activity of binary Palladium Ruthenium anode catalyst on Ni-support for ethanol alkaline fuel cells. Transit. Met. Chem. 2007, 32, 47–55. [Google Scholar] [CrossRef]
- Douk, A.S.; Saravani, H.; Noroozifar, M. Three-dimensional assembly of building blocks for the fabrication of Pd aerogel as a high performance electrocatalyst toward ethanol oxidation. Electrochim. Acta 2018, 275, 182–191. [Google Scholar] [CrossRef]
- Douk, A.S.; Saravani, H.; Zareie, M.; Abad, Y.; Noroozifar, M. Three-Dimensional Engineering of Nanoparticles to Fabricate the Pd-Au Aerogel as Advanced Support-Less Electrocatalyst in Low Temperatures Direct Ethanol Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 7527–7534. [Google Scholar] [CrossRef]
- Douk, A.S.; Saravani, H. Porous 3D Inorganic Superstructure of Pd−Ir Aerogel as Advanced Support-Less Anode Electrocatalyst toward Ethanol Oxidation. ACS Omega 2020, 5, 22031–22038. [Google Scholar] [CrossRef]
- Shafaei, A.; Saravani, H.; Noroozifar, M.; Kim, K. Microporous and Mesoporous Materials Tuning the morphology of Pd aerogels for advanced electrocatalysis of formic acid. Microporous Mesoporous Mater. 2022, 344, 112206. [Google Scholar] [CrossRef]
- Amin, R.S.; Hameed, R.M.A.; El-Khatib, K.M.; Youssef, M.E.; Abdel Hameed, R.M.; El-Khatib, K.M.; Elsayed Youssef, M.; Hameed, R.M.A.; El-Khatib, K.M.; Youssef, M.E. Electrocatalytic activity of nanostructured Ni and Pd-Ni on Vulcan XC-72R carbon black for methanol oxidation in alkaline medium. Int. J. Hydrogen Energy 2014, 39, 2026–2041. [Google Scholar] [CrossRef]
- Dutta, A.; Datta, J. Energy efficient role of Ni/NiO in PdNi nano catalyst used in alkaline DEFC. J. Mater. Chem. A 2014, 2, 3237. [Google Scholar] [CrossRef]
- Maksić, A.; Smiljanić, M.; Miljanić, Š.; Rakočević, Z.; Štrbac, S. Ethanol Oxidation on Rh/Pd(poly) in Alkaline Solution. Electrochim. Acta 2016, 209, 323–331. [Google Scholar] [CrossRef]
- Fontes, E.H.; Ramos, C.E.D.; Nandenha, J.; Piasentin, R.M.; Neto, A.O.; Landers, R. Structural analysis of PdRh/C and PdSn/C and its use as electrocatalysts for ethanol oxidation in alkaline medium. Int. J. Hydrogen Energy 2019, 44, 937–951. [Google Scholar] [CrossRef]
- Zhu, C.; Lan, B.; Wei, R.; Wang, C.; Yang, Y. Potential-Dependent Selectivity of Ethanol Complete Oxidation on Rh Electrode in Alkaline Media: A Synergistic Study of Electrochemical ATR-SEIRAS and IRAS. ACS Catal. 2019, 9, 4046–4053. [Google Scholar] [CrossRef]
- Lan, B.; Huang, M.; Wei, R.; Wang, C.; Wang, Q.; Yang, Y. Ethanol Electrooxidation on Rhodium—Lead Catalysts in Alkaline Media: High Mass Activity, Long-Term Durability, and Considerable CO2 Selectivity. Small 2020, 16, 2004380. [Google Scholar] [CrossRef]
- Lan, B.; Wang, Q.; Ma, Z.; Wu, Y.; Jiang, X.; Jia, W.; Zhou, C.; Yang, Y. Environmental Efficient electrochemical ethanol-to-CO2 conversion at rhodium and bismuth hydroxide interfaces. Appl. Catal. B Environ. 2022, 300, 120728. [Google Scholar] [CrossRef]
- Piwowar, J.; Lewera, A. On the lack of beneficial role of Rh towards C-C bond cleavage during low temperature ethanol electrooxidation on Pt-Rh nanoalloys. J. Electroanal. Chem. 2018, 875, 1–7. [Google Scholar] [CrossRef]
- Dutta, A.; Datta, J. Outstanding Catalyst Performance of PdAuNi Nanoparticles for the Anodic Reaction in an Alkaline Direct Ethanol (with Anion-Exchange Membrane) Fuel Cell. J. Phys. Chem. C 2012, 116, 25677–25688. [Google Scholar] [CrossRef]
- Su, P.P.-C.; Chen, H.-S.H.; Chen, T.T.-Y.; Liu, C.-W.C.; Lee, C.-H.; Lee, J.-F.; Chan, T.-S.; Wang, K.-W. Enhancement of electrochemical properties of Pd/C catalysts toward ethanol oxidation reaction in alkaline solution through Ni and Au alloying. Int. J. Hydrogen Energy 2013, 38, 4474–4482. [Google Scholar] [CrossRef]
- Shen, S.Y.; Guo, Y.G.; Wei, G.H.; Luo, L.X.; Li, F.; Zhang, J.L. A perspective on the promoting effect of Ir and Au on Pd toward the ethanol oxidation reaction in alkaline media. Front. Energy 2018, 12, 501–508. [Google Scholar] [CrossRef]
- Elsheikh, A.; McGregor, J. Synthesis and characterization of pdagni/c trimetallic nanoparticles for ethanol electrooxidation. Nanomaterials 2021, 11, 2244. [Google Scholar] [CrossRef] [PubMed]
- Hagos Gebre, S.; Getaye Sendeku, M. Trimetallic nanostructures and their applications in electrocatalytic energy conversions. J. Energy Chem. 2022, 65, 329–351. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, T.S.; Xu, J.; Li, Y. High performance of a carbon supported ternary PdIrNi catalyst for ethanol electro-oxidation in anion-exchange membrane direct ethanol fuel cells. Energy Environ. Sci. 2011, 4, 1428. [Google Scholar] [CrossRef]
- Henrique, R.S.; Ayoub, J.M.S.; Piasentin, R.M.; Linardi, M.; Santos, M.C. Preparation of Pt / C-In2O3. SnO2 Electrocatalysts by Borohydride Reduction Process for Ethanol Electro-Oxidation. Int. J. Electrochem. Sci. 2012, 7, 2036–2046. [Google Scholar] [CrossRef]
- Neto, A.O.; Tusi, M.M.; De Oliveira Polanco, N.S.; Da Silva, S.G.; Coelho Dos Santos, M.; Spinacé, E.V. PdBi/C electrocatalysts for ethanol electro-oxidation in alkaline medium. Int. J. Hydrogen Energy 2011, 36, 10522–10526. [Google Scholar] [CrossRef]
- Assumpção, M.H.M.T.; Da Silva, S.G.; De Souza, R.F.B.; Buzzo, G.S.; Spinacé, E.V.; Santos, M.C.; Neto, A.O.; Silva, J.C.M. Investigation of PdIr/C electrocatalysts as anode on the performance of direct ammonia fuel cell. J. Power Sources 2014, 268, 129–136. [Google Scholar] [CrossRef]
- Xiao, W.; Li, S.; Liu, J.; Fan, J.; Ma, L.; Cai, W. Lead as an effective facilitator for ethanol electrooxidation on Rh catalyst in alkaline media: RhPb / C vs RhRu / C. J. Electroanal. Chem. 2023, 936, 117386. [Google Scholar] [CrossRef]
- Delpeuch, A.B.; Maillard, F.; Chatenet, M.; Soudant, P.; Cremers, C.; Bach Delpeuch, A.; Maillard, F.; Chatenet, M.; Soudant, P.; Cremers, C. Ethanol oxidation reaction (EOR) investigation on Pt/C, Rh/C, and Pt-based bi- and tri-metallic electrocatalysts: A DEMS and in situ FTIR study. Appl. Catal. B Environ. 2016, 181, 672–680. [Google Scholar] [CrossRef]
- Obradović, M.D.; Stančić, Z.M.; Lačnjevac, U.Č.; Radmilović, V.V.V.R.; Gavrilović-Wohlmuther, A.; Radmilović, V.V.V.R.; Gojković, S.L. Electrochemical oxidation of ethanol on palladium-nickel nanocatalyst in alkaline media. Appl. Catal. B Environ. 2016, 189, 110–118. [Google Scholar] [CrossRef]
- Almeida, C.V.S.; Galiote, N.A.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Del Colle, V.; Tremiliosi-Filho, G. Evidence of surface restructuration on Pt–Rh/C and Pt–Rh–Ni/C nanoparticles applied to ethanol electrooxidation reaction. Electrochim. Acta 2020, 351, 136223. [Google Scholar] [CrossRef]
- Elsheikh, A.; Torrero, J.; Rojas, S.; McGregor, J. In-situ FTIR spectroscopy investigation of carbon-supported PdAuNi electrocatalysts for ethanol oxidation. J. Electroanal. Chem. 2023, 928, 116985. [Google Scholar] [CrossRef]





| C (mg) | PdCl2 (mg) | NiCl2 (mg) | RhCl3 (mg) | |
|---|---|---|---|---|
| Pd/C | 132 | 30 | - | - |
| Rh/C | 132 | - | - | 36 |
| Ni/C | 132 | - | 22 | - |
| PdRhNi/C | 132 | 11.91 | 8.67 | 11.51 |
| Pd4Rh2Ni1/C | 132 | 18.40 | 3.4 | 10.89 |
| Catalyst | Pd at.% | Rh at.% | Ni at.% | Notes (XPS) | Pd 3d5/2 (eV) | |||
|---|---|---|---|---|---|---|---|---|
| XPS | EDX | XPS | EDX | XPS | EDX | |||
| Pd/C | 2.1 | - | - | - | - | - | 0.45% Pd2+ | 335.43 |
| Pd4Rh2Ni1/C | 1.7 | 0.95 | 0.4 | 0.68 | 0.20 | 0.25 | 0.08% Pd2+ 0.14% Ni2+ | 335.54 |
| PdRhNi/C | 1.2 | 0.39 | 0.70 | 0.43 | 0.70 | 0.50 | 0.06% Ni0 0.09% Pd2+ 0.15% Rh2+ | 335.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElSheikh, A.; McGregor, J. Unexpected Negative Performance of PdRhNi Electrocatalysts toward Ethanol Oxidation Reaction. Micromachines 2023, 14, 957. https://doi.org/10.3390/mi14050957
ElSheikh A, McGregor J. Unexpected Negative Performance of PdRhNi Electrocatalysts toward Ethanol Oxidation Reaction. Micromachines. 2023; 14(5):957. https://doi.org/10.3390/mi14050957
Chicago/Turabian StyleElSheikh, Ahmed, and James McGregor. 2023. "Unexpected Negative Performance of PdRhNi Electrocatalysts toward Ethanol Oxidation Reaction" Micromachines 14, no. 5: 957. https://doi.org/10.3390/mi14050957