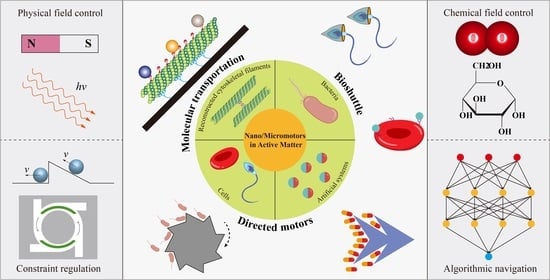
Nano/Micromotors in Active Matter
Abstract
:1. Introduction
2. NMMs Consisted of RCFs
2.1. Classification of the RCF’s Behavior
2.2. Mimicking Cellular Structures via RCF-Based NMMs
2.3. RCF-Based NMMs for Self-Organizing Molecular Transport
2.4. RCF-Based NMMs as Bioshuttle
3. NMMs Consisted of Bacterial Suspensions
3.1. Bacterium-Driven Directed NMMs
3.2. Bacterium-Based NMMs Applying to Particles Transport and Separation
4. NMMs Consisted of Cells
4.1. NMMs Powered by Sperm Cells
4.2. NMMs Consisted of Somatic Cells
5. Regulation and Control of NMMs
5.1. Physical Field
5.1.1. Light
5.1.2. Magnetic Field

5.2. Topological Constraint
5.3. Chemical Field
5.4. Algorithmic Navigation and Control
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Rozen, I.; Wang, J. Rocket Science at the Nanoscale. ACS Nano 2016, 10, 5619–5634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, S.; Soler, L.; Katuri, J. Chemically Powered Micro- and Nanomotors. Angew. Chem. Int. Ed. 2015, 54, 1414–1444. [Google Scholar] [CrossRef] [PubMed]
- Woehlke, G.; Schliwa, M. Walking on two heads: The many talents of kinesin. Nat. Rev. Mol. Cell Biol. 2000, 1, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Abuhaimed, G.N.; Liu, Q.; Smalyukh, I.I. Self-assembled nematic colloidal motors powered by light. Nat. Commun. 2018, 9, 5040. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, M.C.; Joanny, J.F.; Ramaswamy, S.; Liverpool, T.B.; Prost, J.; Rao, M.; Simha, R.A. Hydrodynamics of soft active matter. Rev. Mod. Phys. 2013, 85, 1143–1189. [Google Scholar] [CrossRef] [Green Version]
- Bechinger, C.; Di Leonardo, R.; Löwen, H.; Reichhardt, C.; Volpe, G.; Volpe, G. Active particles in complex and crowded environments. Rev. Mod. Phys. 2016, 88, 045006. [Google Scholar] [CrossRef]
- Ramaswamy, S. The Mechanics and Statistics of Active Matter. Annu. Rev. Condens. Matter Phys. 2010, 1, 323–345. [Google Scholar] [CrossRef] [Green Version]
- Chaté, H. Dry Aligning Dilute Active Matter. Annu. Rev. Condens. Matter Phys. 2020, 11, 189–212. [Google Scholar] [CrossRef] [Green Version]
- Doostmohammadi, A.; Ignes-Mullol, J.; Yeomans, J.M.; Sagues, F. Active nematics. Nat. Commun. 2018, 9, 3246. [Google Scholar] [CrossRef]
- Prost, J.; Julicher, F.; Joanny, J.-F. Active gel physics. Nat. Phys. 2015, 11, 111–117. [Google Scholar] [CrossRef]
- Angelani, L.; Di Leonardo, R. Geometrically biased random walks in bacteria-driven micro-shuttles. New J. Phys. 2010, 12, 113017. [Google Scholar] [CrossRef]
- Koumakis, N.; Lepore, A.; Maggi, C.; Di Leonardo, R. Targeted delivery of colloids by swimming bacteria. Nat. Commun. 2013, 4, 2588. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Medina-Sánchez, M.; Maitz, M.F.; Werner, C.; Schmidt, O.G. Sperm micromotors for cargo delivery through flowing blood. ACS Nano 2020, 14, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, T.; Chen, D.T.N.; DeCamp, S.J.; Heymann, M.; Dogic, Z. Spontaneous motion in hierarchically assembled active matter. Nature 2012, 491, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Senoussi, A.; Kashida, S.; Voituriez, R.; Galas, J.-C.; Maitra, A.; Estevez-Torres, A. Tunable corrugated patterns in an active nematic sheet. Proc. Natl. Acad. Sci. USA 2019, 116, 22464–22470. [Google Scholar] [CrossRef]
- Huber, L.; Suzuki, R.; Krüger, T.; Frey, E.; Bausch, A.R. Emergence of coexisting ordered states in active matter systems. Science 2018, 361, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Amos, L.; Klug, A. Arrangement of subunits in flagellar microtubules. J. Cell Sci. 1974, 14, 523–549. [Google Scholar] [CrossRef]
- Zhang, R.; Mozaffari, A.; de Pablo, J.J. Autonomous materials systems from active liquid crystals. Nat. Rev. Mater. 2021, 6, 437–453. [Google Scholar] [CrossRef]
- Giomi, L.; Bowick, M.J.; Ma, X.; Marchetti, M.C. Defect annihilation and proliferation in active nematics. Phys. Rev. Lett. 2013, 110, 228101. [Google Scholar] [CrossRef]
- Zhang, R.; Kumar, N.; Ross, J.L.; Gardel, M.L.; de Pablo, J.J. Interplay of structure, elasticity, and dynamics in actin-based nematic materials. Proc. Natl. Acad. Sci. USA 2018, 115, E124–E133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.-Y.; Zhang, D.-Q.; Lin, S.-Z.; Li, B. Pattern formation and defect ordering in active chiral nematics. Phys. Rev. Lett. 2020, 125, 098002. [Google Scholar] [CrossRef] [PubMed]
- Ventejou, B.; Chaté, H.; Montagne, R.; Shi, X.-Q. Susceptibility of orientationally ordered active matter to chirality disorder. Phys. Rev. Lett. 2021, 127, 238001. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhu, H.; Liu, P.; Liu, R.; Shi, Q.; Chen, K.; Zheng, N.; Ye, F.; Yang, M. Topologically protected transport of cargo in a chiral active fluid aided by odd-viscosity-enhanced depletion interactions. Phys. Rev. Lett. 2021, 126, 198001. [Google Scholar] [CrossRef]
- Strübing, T.; Khosravanizadeh, A.; Vilfan, A.; Bodenschatz, E.; Golestanian, R.; Guido, I. Wrinkling instability in 3D active nematics. Nano Lett. 2020, 20, 6281–6288. [Google Scholar] [CrossRef]
- Wollman, A.J.M.; Sanchez-Cano, C.; Carstairs, H.M.J.; Cross, R.A.; Turberfield, A.J. Transport and self-organization across different length scales powered by motor proteins and programmed by DNA. Nat. Nanotechnol. 2014, 9, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, T.; Welch, D.; Nicastro, D.; Dogic, Z. Cilia-like beating of active microtubule bundles. Science 2011, 333, 456–459. [Google Scholar] [CrossRef] [Green Version]
- Loiseau, E.; Schneider, J.A.M.; Keber, F.C.; Pelzl, C.; Massiera, G.; Salbreux, G.; Bausch, A.R. Shape remodeling and blebbing of active cytoskeletal vesicles. Sci. Adv. 2016, 2, e1500465. [Google Scholar] [CrossRef] [Green Version]
- Dürre, K.; Keber, F.C.; Bleicher, P.; Brauns, F.; Cyron, C.J.; Faix, J.; Bausch, A.R. Capping protein-controlled actin polymerization shapes lipid membranes. Nat. Commun. 2018, 9, 1630. [Google Scholar] [CrossRef] [Green Version]
- Hess, H.; Ross, J.L. Non-equilibrium assembly of microtubules: From molecules to autonomous chemical robots. Chem. Soc. Rev. 2017, 46, 5570–5587. [Google Scholar] [CrossRef]
- Aoyama, S.; Shimoike, M.; Hiratsuka, Y. Self-organized optical device driven by motor proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 16408–16413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juniper, M.P.N.; Weiss, M.; Platzman, I.; Spatz, J.P.; Surrey, T. Spherical network contraction forms microtubule asters in confinement. Soft Matter 2018, 14, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Hess, H.; Clemmens, J.; Brunner, C.; Doot, R.; Luna, S.; Ernst, K.H.; Vogel, V. Molecular self-assembly of “nanowires” and “nanospools” using active transport. Nano Lett. 2005, 5, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Hess, H.; Matzke, C.M.; Doot, R.K.; Clemmens, J.; Bachand, G.D.; Bunker, B.C.; Vogel, V. Molecular shuttles operating undercover: A new photolithographic approach for the fabrication of structured surfaces supporting directed motility. Nano Lett. 2003, 3, 1651–1655. [Google Scholar] [CrossRef]
- Nitta, T.; Hess, H. Dispersion in active transport by kinesin-powered molecular shuttles. Nano Lett. 2005, 5, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Tanahashi, A.; Hirano, M.; Hess, H. Simulating molecular shuttle movements: Towards computer-aided design of nanoscale transport systems. Lab Chip 2006, 6, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Bachand, G.D.; Rivera, S.B.; Carroll-Portillo, A.; Hess, H.; Bachand, M. Active capture and transport of virus particles using a biomolecular motor-driven, nanoscale antibody sandwich assay. Small 2006, 2, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Ernst, K.-H.; Bachand, G.D.; Vogel, V.; Hess, H. Selective loading of kinesin-powered molecular shuttles with protein cargo and its application to biosensing. Small 2006, 2, 330–334. [Google Scholar] [CrossRef]
- Nicolau, D.V., Jr.; Lard, M.; Korten, T.; van Delftf, F.C.M.J.M.; Persson, M.; Bengtsson, E.; Månsson, A.; Diez, S.; Linke, H.; Nicolau, D.V. Parallel computation with molecular-motor-propelled agents in nanofabricated networks. Proc. Natl. Acad. Sci. USA 2016, 113, 2591–2596. [Google Scholar] [CrossRef] [Green Version]
- Fischer, T.; Agarwal, A.; Hess, H. A smart dust biosensor powered by kinesin motors. Nat. Nanotechnol. 2009, 4, 162–166. [Google Scholar] [CrossRef]
- Berg, H.C. Bacterial flagellar motor. Curr. Biol. 2008, 18, R689–R691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbons, I.R. Cilia and flagella of eukaryotes. J. Cell Biol. 1981, 91, 107s–124s. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, B.; Feng, X.-Q. Buckling of growing bacterial chains. J. Mech. Phys. Solids 2020, 145, 104146. [Google Scholar] [CrossRef]
- Feynman, R.P.; Leighton, R.B.; Sands, M. The Feynman Lectures on Physics. Mainly Mechanics, Radiaion and Heat; Addison-Wesley: Reading, MA, USA; London, UK, 1963; Volume 1. [Google Scholar]
- Astumian, R.D. Thermodynamics and kinetics of a brownian motor. Science 1997, 276, 917–922. [Google Scholar] [CrossRef] [Green Version]
- Reimann, P. Brownian motors: Noisy transport far from equilibrium. Phys. Rep. 2002, 361, 57–265. [Google Scholar] [CrossRef] [Green Version]
- Angelani, L.; Costanzo, A.; Di Leonardo, R. Active ratchets. Eur. Phys. Lett. 2011, 96, 68002. [Google Scholar] [CrossRef]
- Kaiser, A.; Peshkov, A.; Sokolov, A.; Hagen, B.T.; Löwen, H.; Aranson, I.S. Transport powered by bacterial turbulence. Phys. Rev. Lett. 2014, 112, 158101. [Google Scholar] [CrossRef] [Green Version]
- Angelani, L.; Di Leonardo, R.; Ruocco, G. Self-starting micromotors in a bacterial bath. Phys. Rev. Lett. 2009, 102, 048104. [Google Scholar] [CrossRef] [Green Version]
- Di Leonardo, R.; Angelani, L.; Dell’Arciprete, D.; Ruocco, G.; Iebba, V.; Schippa, S.; Conte, M.P.; Mecarini, F.; De Angelis, F.; Di Fabrizio, E. Bacterial ratchet motors. Proc. Natl. Acad. Sci. USA 2010, 107, 9541–9545. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, A.; Apodaca, M.M.; Grzybowski, B.A.; Aranson, I.S. Swimming bacteria power microscopic gears. Proc. Natl. Acad. Sci. USA 2010, 107, 969–974. [Google Scholar] [CrossRef] [Green Version]
- Vizsnyiczai, G.; Frangipane, G.; Maggi, C.; Saglimbeni, F.; Bianchi, S.; Di Leonardo, R. Light controlled 3D micromotors powered by bacteria. Nat. Commun. 2017, 8, 15974. [Google Scholar] [CrossRef] [PubMed]
- Angelani, L.; Maggi, C.; Bernardini, M.L.; Rizzo, A.; Di Leonardo, R. Effective interactions between colloidal particles suspended in a bath of swimming cells. Phys. Rev. Lett. 2011, 107, 138302. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, A.; Di Leonardo, R.; Ruocco, G.; Angelani, L. Transport of self-propelling bacteria in micro-channel flow. J. Phys. Condens. Matter 2012, 24, 065101. [Google Scholar] [CrossRef]
- Paoluzzi, M.; Di Leonardo, R.; Angelani, L. Self-sustained density oscillations of swimming bacteria confined in microchambers. Phys. Rev. Lett. 2015, 115, 188303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turiv, T.; Koizumi, R.; Thijssen, K.; Genkin, M.M.; Yu, H.; Peng, C.; Wei, Q.-H.; Yeomans, J.M.; Aranson, I.S.; Doostmohammadi, A.; et al. Polar jets of swimming bacteria condensed by a patterned liquid crystal. Nat. Phys. 2020, 16, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Du Nguyen, V.; Han, J.-W.; Choi, Y.J.; Cho, S.; Zheng, S.; Ko, S.Y.; Park, J.-O.; Park, S. Active tumor-therapeutic liposomal bacteriobot combining a drug (paclitaxel)-encapsulated liposome with targeting bacteria (Salmonella typhimurium). Sens. Actuators B Chem. 2016, 224, 217–224. [Google Scholar] [CrossRef]
- Suh, S.; Jo, A.; Traore, M.A.; Zhan, Y.; Coutermarsh-Ott, S.L.; Ringel-Scaia, V.M.; Allen, I.C.; Davis, R.M.; Behkam, B. Nanoscale bacteria-enabled autonomous drug delivery system (NanoBEADS) enhances intratumoral transport of nanomedicine. Adv. Sci. 2019, 6, 1801309. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, Y.; Chen, Z.; Sun, L.; Zhao, Y. Anisotropic structural color particles from colloidal phase separation. Sci. Adv. 2020, 6, eaay1438. [Google Scholar] [CrossRef] [Green Version]
- Reichhardt, C.; Reichhardt, C.J.O. Active matter ratchets with an external drift. Phys. Rev. E 2013, 88, 062310. [Google Scholar] [CrossRef] [Green Version]
- Koumakis, N.; Maggi, C.; Di Leonardo, R. Directed transport of active particles over asymmetric energy barriers. Soft Matter 2014, 10, 5695–5701. [Google Scholar] [CrossRef] [Green Version]
- Kantsler, V.; Dunkel, J.; Polin, M.; Goldstein, R.E. Ciliary contact interactions dominate surface scattering of swimming eukaryotes. Proc. Natl. Acad. Sci. USA 2013, 110, 1187–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galajda, P.; Keymer, J.; Chaikin, P.; Austin, R. A wall of funnels concentrates swimming bacteria. J. Bacteriol. 2007, 189, 8704–8707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, G.; Buttinoni, I.; Vogt, D.; Kümmerer, H.-J.; Bechinger, C. Microswimmers in patterned environments. Soft Matter 2011, 7, 8810–8815. [Google Scholar] [CrossRef] [Green Version]
- Mijalkov, M.; Volpe, G. Sorting of chiral microswimmers. Soft Matter 2013, 9, 6376–6381. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.-Q.; Chen, P.-C.; Guan, L.-Y.; Góźdź, W.T.; Feng, X.-Q.; Li, B. Collective migrations in an epithelial–cancerous cell monolayer. Acta Mech. Sin.-PRC 2021, 37, 773–784. [Google Scholar] [CrossRef]
- He, S.; Green, Y.; Saeidi, N.; Li, X.; Fredberg, J.J.; Ji, B.; Pismen, L.M. A theoretical model of collective cell polarization and alignment. J. Mech. Phys. Solids 2020, 137, 103860. [Google Scholar] [CrossRef]
- Lin, S.-Z.; Zhang, W.-Y.; Bi, D.; Li, B.; Feng, X.-Q. Energetics of mesoscale cell turbulence in two-dimensional monolayers. Commun. Phys. 2021, 4, 21. [Google Scholar] [CrossRef]
- Smith, D.J.; Gaffney, E.A.; Blake, J.R.; Kirkman-Brown, J.C. Human sperm accumulation near surfaces: A simulation study. J. Fluid Mech. 2009, 621, 289–320. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.V.; Ansari, M.H.D.; Mahajan, M.; Srivastava, S.; Kashyap, S.; Dwivedi, P.; Pandit, V.; Katha, U. Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines 2020, 11, 448. [Google Scholar] [CrossRef]
- Xu, H.; Medina-Sánchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-hybrid micromotor for targeted drug delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Magdanz, V.; Sanchez, S.; Schmidt, O.G. Development of a sperm-flagella driven micro-bio-robot. Adv. Mater. 2013, 25, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.S.M.; Klingner, A.; Magdanz, V.; Striggow, F.; Medina-Sánchez, M.; Schmidt, O.G.; Misra, S. Modeling of spermbots in a viscous colloidal suspension. Adv. Theory Simul. 2019, 2, 1900072. [Google Scholar] [CrossRef]
- Ridzewski, C.; Li, M.; Dong, B.; Magdanz, V. Gelatin microcartridges for onboard activation and antioxidant protection of sperm. ACS Appl. Bio Mater. 2020, 3, 1616–1627. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Klingner, A.; Hamed, Y.; Magdanz, V.; Toubar, M.; Misra, S. Characterization of flagellar propulsion of soft microrobotic sperm in a viscous heterogeneous medium. Front. Robot. AI 2019, 6, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, P.; Murata-Hori, M.; Lodish, H.F. Formation of mammalian erythrocytes: Chromatin condensation and enucleation. Trends Cell Biol. 2011, 21, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Alapan, Y.; Yasa, O.; Schauer, O.; Giltinan, J.; Tabak, A.F.; Sourjik, V.; Sitti, M. Soft erythrocyte-based bacterial microswimmers for cargo delivery. Sci. Robot. 2018, 3, eaar4423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Lin, Z.; Wang, D.; Wu, Z.; Xie, H.; He, Q. Red blood cell-mimicking micromotor for active photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 23392–23400. [Google Scholar] [CrossRef]
- Dreyfus, R.; Baudry, J.; Roper, M.; Fermigier, M.; Stone, H.A.; Bibette, J. Microscopic artificial swimmers. Nature 2005, 437, 862–865. [Google Scholar] [CrossRef]
- Wu, Z.; de Ávila, B.E.-F.; Martín, A.; Christianson, C.; Gao, W.; Thamphiwatana, S.K.; Escarpa, A.; He, Q.; Zhang, L.; Wang, J. RBC micromotors carrying multiple cargos towards potential theranostic applications. Nanoscale 2015, 7, 13680–13686. [Google Scholar] [CrossRef]
- Guo, J.; Agola, J.O.; Serda, R.; Franco, S.; Lei, Q.; Wang, L.; Minster, J.; Croissant, J.G.; Butler, K.S.; Zhu, W.; et al. Biomimetic rebuilding of multifunctional red blood cells: Modular design using functional components. ACS Nano 2020, 14, 7847–7859. [Google Scholar] [CrossRef]
- Williams, B.J.; Anand, S.V.; Rajagopalan, J.; Saif, M.T.A. A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun. 2014, 5, 3081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-J.; Gazzola, M.; Park, K.S.; Park, S.; Di Santo, V.; Blevins, E.L.; Lind, J.U.; Campbell, P.H.; Dauth, S.; Capulli, A.K.; et al. Phototactic guidance of a tissue-engineered soft-robotic ray. Science 2016, 353, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Shang, L.; Chen, Z.; Yu, Y.; Zhao, Y. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3, 8580. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Fu, F.; Yu, Y.; Wang, H.; Shang, Y.; Zhao, Y. Cardiomyocytes-actuated Morpho butterfly wings. Adv. Mater. 2019, 31, 1805431. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Chen, Z.; Fu, F.; Sun, L.; Shao, C.; Jin, W.; Liu, H.; Zhao, Y. Cardiomyocyte-driven structural color actuation in anisotropic inverse opals. ACS Nano 2019, 13, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, Z.; Bian, F.; Zhao, Y. Bioinspired soft robotic caterpillar with cardiomyocyte drivers. Adv. Funct. Mater. 2019, 30, 1907820. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, L.; Zhong, W.; Yan, Q.; Gao, Y.; Hong, W.; She, Y.; Yang, G. Recent advances in motion control of micro/nanomotors. Adv. Intell. Syst. 2020, 2, 2000049. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Shao, J.; Abdelghani, M.; Shen, G.; Cao, S.; Williams, D.S.; van Hest, J.C.M. Erythrocyte membrane modified Janus polymeric motors for thrombus therapy. ACS Nano 2018, 12, 4877–4885. [Google Scholar] [CrossRef]
- Hess, H.; Clemmens, J.; Qin, D.; Howard, J.; Vogel, V. Light-controlled molecular shuttles made from motor proteins carrying cargo on engineered surfaces. Nano Lett. 2001, 1, 235–239. [Google Scholar] [CrossRef]
- Ross, T.D.; Lee, H.J.; Qu, Z.J.; Banks, R.A.; Phillips, R.; Thomson, M. Controlling organization and forces in active matter through optically defined boundaries. Nature 2019, 572, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Redford, S.A.; Ruijgrok, P.V.; Kumar, N.; Mozaffari, A.; Zemsky, S.; Dinner, A.R.; Vitelli, V.; Bryant, Z.; Gardel, M.L.; et al. Spatiotemporal control of liquid crystal structure and dynamics through activity patterning. Nat. Mater. 2021, 20, 875–882. [Google Scholar] [CrossRef]
- Qu, Z.J.; Schildknecht, D.; Shadkhoo, S.; Amaya, E.; Jiang, J.; Lee, H.J.; Larios, D.; Yang, F.; Phillips, R.; Thomson, M. Persistent fluid flows defined by active matter boundaries. Commun. Phys. 2021, 4, 198. [Google Scholar] [CrossRef]
- Ahmad, R.; Kleineberg, C.; Nasirimarekani, V.; Su, Y.-J.; Pozveh, S.G.; Bae, A.; Sundmacher, K.; Bodenschatz, E.; Guido, I.; Vidaković-Koch, T.; et al. Light-powered reactivation of flagella and contraction of microtubule networks: Toward building an artificial cell. ACS Synth. Biol. 2021, 10, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.M.; Greenfield, D.; Bustamante, C.; Liphardt, J. Light-powering Escherichia coli with proteorhodopsin. Proc. Natl. Acad. Sci. USA 2007, 104, 2408–2412. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.Y.; Liu, Y.; Zhang, D.M.; Zhang, H.; Jiang, J.W.; Duan, R.M.; Xiao, J.; Xing, J.J.; Zhang, D.F.; Dong, B. Calligraphy/painting based on a bioinspired light-diven mcromotor with concentration-dependent motion direction reversal and dynamic swarming behavior. ACS Appl. Mater. Interfaces 2019, 11, 40533–40542. [Google Scholar] [CrossRef] [PubMed]
- Frangipane, G.; Dell’Arciprete, D.; Petracchini, S.; Maggi, C.; Saglimbeni, F.; Bianchi, S.; Vizsnyiczai, G.; Bernardini, M.L.; Di Leonardo, R. Dynamic density shaping of photokinetic E. coli. eLife 2018, 7, e36608. [Google Scholar] [CrossRef]
- Guillamat, P.; Ignés-Mullol, J.; Sagués, F. Control of active liquid crystals with a magnetic field. Proc. Natl. Acad. Sci. USA 2016, 113, 5498–5502. [Google Scholar] [CrossRef] [Green Version]
- Guillamat, P.; Ignés-Mullol, J.; Sagues, F. Taming active turbulence with patterned soft interfaces. Nat. Commun. 2017, 8, 564. [Google Scholar] [CrossRef]
- Zhang, B.; Snezhko, A.; Sokolov, A. Guiding self-assembly of active colloids by temporal modulation of activity. Phys. Rev. Lett. 2022, 128, 018004. [Google Scholar] [CrossRef]
- Sipos, O.; Nagy, K.; Di Leonardo, R.; Galajda, P. Hydrodynamic trapping of swimming bacteria by convex walls. Phys. Rev. Lett. 2015, 114, 258104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, S.; Saglimbeni, F.; Di Leonardo, R. Holographic imaging reveals the mechanism of wall entrapment in swimming bacteria. Phys. Rev. X 2017, 7, 011010. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, S.; Saglimbeni, F.; Frangipane, G.; Dell’Arciprete, D.; Di Leonardo, R. 3D dynamics of bacteria wall entrapment at a water–air interface. Soft Matter 2019, 15, 3397–3406. [Google Scholar] [CrossRef] [PubMed]
- Opathalage, A.; Norton, M.M.; Juniper, M.P.N.; Langeslay, B.; Aghvami, S.A.; Fraden, S.; Dogic, Z. Self-organized dynamics and the transition to turbulence of confined active nematics. Proc. Natl. Acad. Sci. USA 2019, 116, 4788–4797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardoüin, J.; Hughes, R.; Doostmohammadi, A.; Laurent, J.; Lopez-Leon, T.; Yeomans, J.M.; Ignés-Mullol, J.; Sagués, F. Reconfigurable flows and defect landscape of confined active nematics. Commun. Phys. 2019, 2, 121. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-Y.; Zhang, D.-Q.; Li, B. Formation and propagation of solitonlike defect clusters in confined active nematics with chiral anchoring. Phys. Rev. Res. 2021, 3, 023253. [Google Scholar] [CrossRef]
- Mahmud, G.; Campbell, C.J.; Bishop, K.J.M.; Komarova, Y.A.; Chaga, O.Y.; Soh, S.; Huda, S.; Kandere-Grzybowska, K.; Grzybowski, B.A. Directing cell motions on micropatterned ratchets. Nat. Phys. 2009, 5, 606–612. [Google Scholar] [CrossRef]
- Hess, H.; Clemmens, J.; Matzke, C.M.; Bachand, G.D.; Bunker, B.C.; Vogel, V. Ratchet patterns sort molecular shuttles. Appl. Phys. A 2002, 75, 309–313. [Google Scholar] [CrossRef]
- Doot, R.K.; Hess, H.; Vogel, V. Engineered networks of oriented microtubule filaments for directed cargo transport. Soft Matter 2007, 3, 349–356. [Google Scholar] [CrossRef]
- Clemmens, J.; Hess, H.; Doot, R.; Matzke, C.M.; Bachand, G.D.; Vogel, V. Motor-protein “roundabouts”: Microtubules moving on kinesin-coated tracks through engineered networks. Lab Chip 2004, 4, 83–86. [Google Scholar] [CrossRef]
- Inoue, D.; Gutmann, G.; Nitta, T.; Kabir, A.M.R.; Konagaya, A.; Tokuraku, K.; Sada, K.; Hess, H.; Kakugo, A. Adaptation of patterns of motile filaments under dynamic boundary conditions. ACS Nano 2019, 13, 12452–12460. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, K.; Khaladj, D.A.; Aghvami, S.A.; Gharbi, M.A.; Fraden, S.; Yeomans, J.M.; Hirst, L.S.; Shendruk, T.N. Submersed micropatterned structures control active nematic flow, topology, and concentration. Proc. Natl. Acad. Sci. USA 2021, 118, e2106038118. [Google Scholar] [CrossRef]
- Clemmens, J.; Hess, H.; Howard, A.J.; Vogel, V. Analysis of microtubule guidance in open microfabricated channels coated with the motor protein kinesin. Langmuir 2003, 19, 1738–1744. [Google Scholar] [CrossRef]
- Clemmens, J.; Hess, H.; Lipscomb, R.; Hanein, Y.; Böhringer, K.F.; Matzke, C.M.; Bachand, G.D.; Bunker, B.C.; Vogel, V. Mechanisms of microtubule guiding on microfabricated kinesin-coated surfaces: Chemical and topographic surface patterns. Langmuir 2003, 19, 10967–10974. [Google Scholar] [CrossRef]
- Saper, G.; Tsitkov, S.; Katira, P.; Hess, H. Robotic end-to-end fusion of microtubules powered by kinesin. Sci. Robot. 2021, 6, eabj7200. [Google Scholar] [CrossRef]
- Hess, H.; Clemmens, J.; Howard, J.; Vogel, V. Surface imaging by self-propelled nanoscale probes. Nano Lett. 2002, 2, 113–116. [Google Scholar] [CrossRef]
- Hess, H.; Howard, J.; Vogel, V. A piconewton forcemeter assembled from microtubules and kinesins. Nano Lett. 2002, 2, 1113–1115. [Google Scholar] [CrossRef]
- Wang, H.; Pumera, M. Fabrication of micro/nanoscale motors. Chem. Rev. 2015, 115, 8704–8735. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Park, S.-H.; Cho, S.; Kim, D.-M.; Lee, Y.; Ko, S.Y.; Hong, Y.; Choy, H.E.; Min, J.-J.; Park, J.-O.; et al. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci. Rep. 2013, 3, 3394. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.; Sitti, M. Chemotaxis of bio-hybrid multiple bacteria-driven microswimmers. Sci. Rep. 2016, 6, 32135. [Google Scholar] [CrossRef]
- Park, B.-W.; Zhuang, J.; Yasa, O.; Sitti, M. Multifunctional bacteria-driven microswimmers for targeted active drug delivery. ACS Nano 2017, 11, 8910–8923. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Hosseinidoust, Z.; Park, B.-W.; Yasa, O.; Sitti, M. Microemulsion-based soft bacteria-driven microswimmers for active cargo delivery. ACS Nano 2017, 11, 9759–9769. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Yuk, H.; Lin, S.T.; Parada, G.A.; Tang, T.C.; Tham, E.; de la Fuente-Nunez, C.; Lu, T.K.; Zhao, X.H. 3D printing of living responsive materials and devices. Adv. Mater. 2018, 30, 1704821. [Google Scholar] [CrossRef] [PubMed]
- Keya, J.J.; Kabir, A.M.R.; Inoue, D.; Sada, K.; Hess, H.; Kuzuya, A.; Kakugo, A. Control of swarming of molecular robots. Sci. Rep. 2018, 8, 11756. [Google Scholar] [CrossRef] [PubMed]
- Keya, J.J.; Suzuki, R.; Kabir, A.M.R.; Inoue, D.; Asanuma, H.; Sada, K.; Hess, H.; Kuzuya, A.; Kakugo, A. DNA-assisted swarm control in a biomolecular motor system. Nat. Commun. 2018, 9, 453. [Google Scholar] [CrossRef] [Green Version]
- Curatolo, A.I.; Zhou, N.; Zhao, Y.; Liu, C.; Daerr, A.; Tailleur, J.; Huang, J. Cooperative pattern formation in multi-component bacterial systems through reciprocal motility regulation. Nat. Phys. 2020, 16, 1152–1157. [Google Scholar] [CrossRef]
- Haeufle, D.F.B.; Bäuerle, T.; Steiner, J.; Bremicker, L.; Schmitt, S.; Bechinger, C. External control strategies for self-propelled particles: Optimizing navigational efficiency in the presence of limited resources. Phys. Rev. E 2016, 94, 012617. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.; Montiel, D.; Bregulla, A.; Cichos, F.; Yang, H. Harnessing thermal fluctuations for purposeful activities: The manipulation of single micro-swimmers by adaptive photon nudging. Chem. Sci. 2013, 4, 1420–1429. [Google Scholar] [CrossRef]
- Yang, Y.; Bevan, M.A. Optimal navigation of self-propelled colloids. ACS Nano 2018, 12, 10712–10724. [Google Scholar] [CrossRef]
- Yang, Y.; Bevan, M.A.; Li, B. Micro/nano motor navigation and localization via deep reinforcement learning. Adv. Theory Simul. 2020, 3, 2000034. [Google Scholar] [CrossRef]
- Yang, Y.; Bevan, M.A.; Li, B. Efficient navigation of colloidal robots in an unknown environment via deep reinforcement learning. Adv. Intell. Syst. 2019, 2, 1900106. [Google Scholar] [CrossRef]
- Yang, Y.; Bevan, M.A. Cargo capture and transport by colloidal swarms. Sci. Adv. 2020, 6, eaay7679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Yang, Y.; Li, B. Brownian cargo capture in mazes via intelligent colloidal microrobot swarms. Adv. Intell. Syst. 2021, 3, 2100115. [Google Scholar] [CrossRef]
- David, S.; Fleischner, R. Fantastic Voyage; Twentieth-Century Fox: Los Angeles, CA, USA, 1966. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Yang, Y.; Li, B. Nano/Micromotors in Active Matter. Micromachines 2022, 13, 307. https://doi.org/10.3390/mi13020307
Lv C, Yang Y, Li B. Nano/Micromotors in Active Matter. Micromachines. 2022; 13(2):307. https://doi.org/10.3390/mi13020307
Chicago/Turabian StyleLv, Chenglin, Yuguang Yang, and Bo Li. 2022. "Nano/Micromotors in Active Matter" Micromachines 13, no. 2: 307. https://doi.org/10.3390/mi13020307