1. Introduction
The field of microfluidics has seen rapid growth in the last two decades [
1], with some of the earliest successful examples being electrophoresis and gas chromatography developing into more current applications such as lab-on-a-chip devices [
1,
2]. Microfluidic devices contain feature sizes in the range of 1 to 500 µm and are constructed using microfabrication techniques in materials such as silicon, glass, plastic, and poly(dimethylsiloxane) (PDMS) [
1].
PDMS is of particular interest, as it has become the most commonly used material for research-based microfluidic devices due to its chemical inertness, high gas permeability, low polarity, low electrical conductivity, elasticity, optical clarity, and transparency in the ultraviolet and visible regions [
3]. PDMS can exhibit a broad range of mechanical properties depending on the crosslinking density of the network structure, resulting in both hard and soft types of PDMS [
4,
5].
In nearly all cases, soft lithography, a method in which a mold, usually constructed from photoresist on a silicon wafer, is used to form the desired features and thus microfluidic devices in PDMS. Soft lithography is simple, enables precise control over micron-scale features, and allows the creation of virtually any two-dimensional design. However, the resulting device is limited to the size of the mold, the creation of multilayer devices is difficult, and soft lithography is primarily a manual process [
6]. For these reasons, scaling up soft lithography to larger device volumes and larger device sizes remains a challenge [
7]. Further, truly three-dimensional (3D) devices cannot easily be created using soft lithography.
Recently, microfabrication via additive manufacturing (AM) has been realized, with advancements in 3D printing resolutions and capabilities [
6,
8,
9,
10,
11]. Stereolithography (SLA), multijet printing (MJP), and fused deposition modeling (FDM) are the most explored methods of 3D printing microfluidics, with SLA exhibiting higher resolution, tighter tolerances, and compatibility with thermoset polymers compared to MJP and FDM.
A fourth approach, two-photon photolithography (2PP), is relevant to microfluidics for its highly resolved printing capabilities in the nanometer regime. However, this technique is still confined to small-scale, small build volume, and low-throughput applications due to its high cost and slow printing speeds, relative to other commercial 3D printers [
12,
13]. Further, the photocurable resins being developed for 2PP are not directly relevant to the work presented in this paper, as these materials utilize two-photon absorption from tightly focused laser pulses, confining the polymerization region to the focal volume of the lasers resulting in high-resolution prints. This is unlike SLA in which the resolution of the part is dictated by layer-by-layer fabrication and Beer’s absorption law of the resin to resolve small features [
14,
15].
While state-of-the-art SLA 3D printers harnessing digital micromirror technology have resolutions down to 2 µm in the XY plane and 1 µm in the Z plane [
16,
17], there is still a gap in material development, limiting the successful fabrication of truly microfluidic features. This is especially true for PDMS, as there are currently no high-resolution (<100 um features) PDMS–SLA resins that are commercially available or presented in the literature. To the best of our knowledge, the smallest membranes and channels successfully printed with PDMS resins via SLA are >330 µm and >1000 µm, respectively [
18,
19].
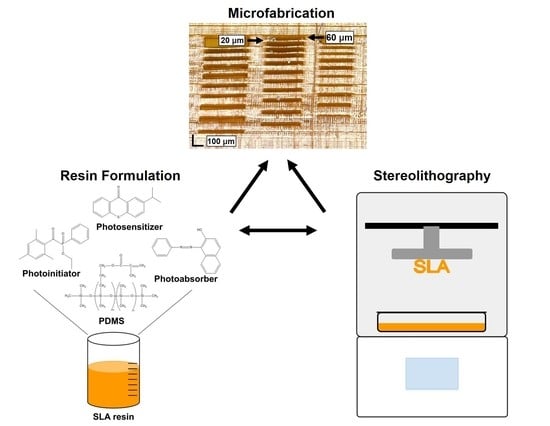
In this paper, we present the formulation of a high-resolution, gas-permeable, photopolymerizable PDMS resin and its successful implementation with a commercial SLA 3D printer to fabricate truly micron-scale parts with channels as small as 60 µm tall and membranes as thin as 20 µm.
2. Materials and Methods
2.1. Resin Formulation
Resin components were weighed out separately on a Quintix 125D-1S Semi-Micro Balance (Sartorius Lab Instruments GmbH & Co. KG, Goettingen, Germany) according to the desired w/w% of material. Components were combined and mixed by hand, heated for 2 h at 70 °C on a VMS-C7 S1 hot plate (VWR International, Radnor, PA, USA), and then sonicated with a Q700 sonicator (Qsonica LLC, Newtown, CT, USA) to ensure uniform mixing and particle size reduction. Sudan I was purchased from Sigma-Aldrich (St. Louis, MO, USA). 2-Isopropylthioxanthone (ITX) ≥ 98.0% was purchased from VWR International (Radnor, PA, USA). [7–9% (Methacryloxypropyl)methylsiloxane]-dimethylsiloxane copolymer (RMS-083) was purchased from Gelest, Inc. (Morrisville, PA, USA). 2, 4, 6-Trimethyl benzoyl diphenylphosphine oxide (TPO-L) was purchased from PL Industries of Esstech, Inc. (Essington, PA, USA).
The high-resolution formula for the resin described in this manuscript consists of a photoreactive methacrylate–PDMS copolymer of 98.6
w/
w% RMS-083 with 0.8
w/
w% TPO-L added as photoinitiator, 0.4
w/
w% ITX added as a photosensitizer, and 0.2
w/
w% Sudan I added as a photoabsorber. The Sudan I and ITX concentrations were chosen to maximize resolution while remaining below the solubility limit of the polymer. Various
w/
w% combinations of Sudan I and ITX were tested with the RMS-083 to determine the maximum amount of material that could be added while maintaining a uniform, stable resin. The
w/
w% of TPO-L was chosen based on previous work carried out by Bhattacharjee et al. looking at the solubility and curing parameters of the photoinitiator in RMS-083 [
18].
2.2. Resin Characterization
Resin characterization was performed by placing uncured resin on a glass slide and cured by exposing a small circle of light from the printer (MAX X27 UV, Asiga, Alexandria, Australia) at 15 mW/m2 at various time points. The excess, uncured resin was rinsed from the glass slide with ACS Grade ≥99.5% isopropyl alcohol (IPA) (LabChem, Zelienople, PA, USA) purchased from Fisher Scientific Company (Hampton, New Hampshire, USA). The thickness of the cured resin was measured by taking side view images of the cured spot with the AM413T Dino-Lite Digital Microscope using the DinoCapture 2.0 software (Dunwell Tech, Inc., Torrance, CA, USA) (camera resolution is ±3 µm). Resins were cured and measured three separate times with triplicate measurements taken for each thickness (n ≥ 9). Regressions were run in both GraphPad and Excel to cross-check the regression results and to determine the slope and x-intercepts of these curves (and corresponding errors). A working curve describing the relationship between cure energy and cure thickness was input to the material file for printing with the Asiga MAX X27 UV printer (Asiga, Alexandria, NSW 2015).
2.3. Printing
All resin characterization and builds were printed using the commercially available Asiga MAX X27 UV printer (Asiga, Alexandria, NSW 2015). This printer uses digital light processing (DLP) technology with a 385 nm light source (wavelength range of 370–400 nm), an X and Y pixel resolution of 27 µm, and a Z (vertical) resolution of 1 µm. Asiga Composer Software version 1.2.11 (Asiga, Alexandria, NSW 2015) was used as the interface for handling STL files and controlling print parameters. All 3D models were generated in SOLIDWORKS (Dassault Systems, Waltham, MA, USA) and exported to the STL file format. The printed test channel structure was designed with an array of channels with varying heights and membranes with varying thicknesses; see
Figure S1 for dimensions. The microfluidic channel junction was designed with channels that are 240 µm tall, 40 pixels (1080 µm) wide, and 2.7 mm long.
Glass slides were silanized with 3-(trimethoxysilyl)propyl methacrylate (Sigma Aldrich, St. Louis, MO, USA) following the procedure as described by A. Urrios et al. [
20], then attached to the build platform using a UV epoxy (Proto Glass, Proto Products, Ashland City, TN, USA) at the start of each print to ensure adhesion of the build to the build platform. Contact angles were taken to validate the proper coating of the slides with the silane via a custom goniometer described previously [
21]. The build platform was calibrated with the glass slide attached, and then printing proceeded as normal.
2.4. Print Post-Processing
Successful builds were removed from the build platform and soaked in IPA (Fisher Scientific Co., Waltham, MA, USA) to remove most of the uncured resin. For the test channels described below, a vacuum was applied to the open end of the channels to suction out the residual uncured, liquid resin. For the microfluidic channel junction, Silastic 2415569 Laboratory Tubing, 0.062” ID x 0.125” OD, 50’ (Cole Parmer, Vernon Hills, IL, USA) was attached to the inlet and outlet ports using 3140 RTV Silicone Conformal Coating (purchased from Ellsworth Adhesives, Germantown, WI, USA), then IPA was flowed through the channel junction at a rate of 0.2 mL/min using a Masterflex 7523-80 Peristaltic Pump (Cole Parmer, Vernon Hills, IL, USA) to ensure channels were completely void before testing. Flow channel and membrane dimensions were measured on the Dino-Lite Digital Microscope.
2.5. Mathematical Model of Dose Curves
A mathematical model previously described by Gong et al. [
22] was used to create exposure dose curves for the resin. Exposure dose curves can predict the total exposure dose throughout the printed part as well as channel and membrane dimensions.
2.6. Absorbance
Absorbance measurements of 3D printing resins were taken from 300 to 600 nm using a Varian Cary 50 Bio UV-Vis spectrophotometer (Aligent Technologies, Santa Clara, CA, USA). Triplicates of each sample were run in Hellma® absorption cuvettes (Hellma GmbH & Co., Müllheim, Germany) made of Herasil quartz with a spectral range of 260–2500 nm, pathlength of 10 mm, and chamber volume 3500 μL purchased from Sigma Aldrich (St. Louis, MO, USA). Absorbance data were normalized to a range of (0, 1).
2.7. Mechanical Testing
Mechanical testing was performed via tensile testing using a TA.XT
Plus Texture Analyser and Exponent Connect software version 6 (Texture Technologies, Hamilton, MA, USA) at the Van Vlack Laboratory at the University of Michigan. Tensile bars were made according to ASTM D412 but scaled to fit the build area of the Asiga printer. A dimensioned drawing of the scaled tensile bars can be found in
Figure S2. Resin samples were fabricated by printing tensile bars directly onto the build platform, removing the printed part, and washing in IPA before post-curing in an Asiga Flash-type DR-301C UV exposure chamber (Asiga, Alexandria, NSW 2015). Sylgard 184 (purchased from Ellsworth Adhesives, Germantown, WI) samples were formed by filling an acrylic mold (
Figure S2) with 10:1 polymer to crosslinker mixture and baked for 1 h at 80 °C. Acrylic mold was formed by cutting out tensile bars from a 3.175 mm thick acrylic sheet (Professional Plastics, Inc., Fullerton, CA) using the Zing 16 laser engraver (Epilog Laser, Golden, CO, USA) and CorelDRAW 2017 software version 19 (Corel Corporation, Ottawa, ON, Canada). All tests were performed with at least
n = 5. One-way ANOVA with a significance level of 0.05 and post hoc Tukey tests were performed using an online statistics calculator [
23].
2.8. Gas Permeability
Gas permeability was tested using a custom, 3D-printed fixture (
Figure S3), which permitted the application of a fixed pressure to one side of a thin film membrane and the measurement of oxygen concentration via a Milwaukee MW600 PRO Dissolved Oxygen Meter (Milwaukee Instruments Inc., Rocky Mount, NC, USA) in a fixed volume of DI water on the other side of the membrane. The custom fixture was drawn in SOLIDWORKS and printed using Asiga PlasGREY resin (Proto Products, Ashland, TN, USA) on the Asiga MAX X27 UV. PDMS resin-based films were printed on the Asiga MAX X27 UV printer. Sylgard 184 films were formed using an SCS G3P-12 Spin Coater (Specialty Coating Systems Inc., Indianapolis, IN, USA) with a 10:1 polymer to crosslinker mixture and baked for 1 h at 80 °C. All films were 100 µm thick and assembled into the membrane holder with the dissolved oxygen meter probe submerged in DI water. Oxygen was fed to the film with a constant pressure of 2 psi, and dissolved oxygen was measured for 90 min.
2.9. Percent Transmission
Transmission measurements (n = 3) were taken from 300 to 800 nm using a Varian Cary 50 Bio UV-Vis spectrophotometer (Aligent Technologies, Santa Clara, CA, USA). 100 µm films of the 3D printing resins were printed on the Asiga MAX X27 UV printer on uncoated glass slides to reduce surface roughness and ensure film transparency. IPA-soaked samples were soaked overnight for 18 h. Sylgard 184 films were formed using an SCS G3P-12 Spin Coater (Specialty Coating Systems Inc., Indianapolis, IN, USA), with a 10:1 polymer to crosslinker mixture and baked for 1 h at 80 °C. Results were normalized to the spectrum of the instrument’s light source.
4. Discussion
Given the widespread use of PDMS in the fabrication of microfluidics, creating a high-resolution photocurable PDMS resin was a significant motive in the development of this formulation. Further, the Asiga MAX X27 UV printer was a desirable system for microfabrication for its high-resolution and open material system.
To achieve the high-resolution printing performance required to print truly microfluidic devices, the unique combination of a photoabsorber, Sudan I, and photosensitizer, and ITX was implemented in this formula. We found that the combination of these two compounds improved resolution over the formulas with photoabsorber alone and photosensitizer alone. From
Figure 2b, the Sudan + ITX resin has the smallest penetration depth,
ha = 22.6 µm versus
ha = 40.9 µm for Sudan I (no ITX) and
ha = 130.8 µm for ITX (no Sudan I). The resolution capabilities of this Sudan I + ITX resin demonstrates a twofold increase over the absorber-only formula and a fivefold increase over the photosensitizer-only formula.
The curing efficiency of the resin was greatly enhanced by the combination of these two compounds, as shown by the Dc, where Sudan + ITX requires the smallest dose, Dc = 7.4 mW/cm2, for polymerization to form a non-flowable material. This greatly enhanced the curing efficiency over the Sudan-I-only formula and marginally improved curing efficiency compared with the ITX-only formula. Generally, it can be concluded that Sudan I is the main driver in resolution, and ITX dictates curing efficiency during polymerization.
The printability of this resin was first studied using a theoretical dose curve model to predict the optimal layer thickness for printing and corresponding channel heights given the penetration depth of Sudan I + ITX. An in-depth explanation of the math and theory of these dose curves can be found in a study by Gong et al., who developed this model [
22]. The dose curves in
Figure 3a were plotted to mimic and validate the printed test channel structure, as shown in
Figure 4. As the plot verifies, a 20 µm layer is thick enough to prevent bleeding of light into the preceding layers so that a dose of 1 is never reached, forming a channel.
While the printer is capable of 1µm resolution in the z plane and 27 µm in the XY plane, the actual printing resolution is determined by the material and the system combined. From the results of the math model and the test channel print in
Figure 4, we printed with a 20 µm layer thickness, producing channels that were resolved within 2 µm of the expected 60 µm height and membranes within 1µm of the expected 20 µm thickness (see
Table S2). The minimum feature size in the XY plane for which the channel could be cleared of uncured resin was 540 µm for 60 µm tall channels. However, channels as small as 30 µm tall and 270 µm wide appear to be resolved in the XY plane (
Figure 3b, third channel from the right), but we were unable to clear them of resin. We believe that had taller channels been printed with this width, then channels would have been properly formed and cleared.
The smallest printable channel height for this formula was 60 µm with 20 µm membranes, as shown in
Figure 4. Potentially smaller channels and membranes could be printed given the high resolution of this resin. However, the large viscosity of the resin restricts the printability of smaller feature sizes due to high separation forces. For comparison, the viscosity of the commercially available GR1 resin is 700 cP whereas the Sudan + ITX resin presented in this paper has a much larger viscosity of 5960 cP (
Figure S6). Separation forces on the printed part is a common mode of failure for SLA systems, where the viscous forces of the resin create a suction-cupping effect on the face of the part and will peel or tear layers away during the build [
25]. Intuitively, the larger the viscosity of the resin is, the more challenging these separation forces will be to overcome.
Further, the high viscosity of this material makes it difficult to remove unpolymerized resin from the micron-scale channels. The capillary forces within the channel can be overcome with the use of solvents to dilute the resin and mechanical stimulation such as sonication or the application of pressurized air, but this puts the printed part at risk of failure while it is still in the semi-reacted “green” state—the state of the part before post-curing to polymerize any unreacted groups after printing [
26,
27]. Applying too much force when trying to remove the uncured resin can result in the separation of layers or fracture of the thin membranes. In the case of the microfluidic channel junction printed in
Figure 5, larger channels (240 µm tall) were printed for ease of removing the uncured resin and to avoid damaging the part. The next steps in the development of this material will include the addition of a diluent to reduce the viscosity of the material, thereby improving the printability and ease of post-processing.
As demonstrated in
Figure 6, the mechanical properties of this material are brittle compared with Sylgard 184, whereas Young’s modulus is much larger for both custom formulas, and elongation at break is much smaller. The inclusion of the photoabsorber and photosensitizer have little effect on the brittleness of the material, revealing that the bulk mechanical properties are driven by the polymer, RMS-083. For microfluidic applications where a low modulus is required, e.g., pneumatic pump, this material would not be suitable. However, in one regard, the larger Young’s modulus of this material aided in overcoming the large separation forces experienced during printing from the high viscosity.
In addition to the mechanical properties of this resin, oxygen permeability and optical clarity were investigated to validate this resin for other desirable features of PDMS.
Figure 7a validates the permeability of this cured resin to oxygen given the increase in dissolved oxygen content in DI water over time. The Sudan I + ITX and no absorber films exhibited larger dissolved oxygen content compared with Sylgard 184, validating its functionality as a gas-permeable material.
In similarity to Sylgard 184 and the no-absorber films in
Figure 7b, the Sudan + ITX resin maintains the visual acuity of the text, and the colors of the background image are still distinguishable despite the orangish-hue present from the photoabsorber Sudan I. In
Figure 7c, transmission in the colored region of the spectrum reaches 83% at 695 nm and allows >75% transmission in the 500–800 nm range. The lower transmission in the UV range of the spectrum can be attributed to the photoabsorbing compounds still present in the polymer and could be addressed by soaking in IPA for a longer time or using another solvent to remove additional extractables, further improving transparency.