Angle-Sensitive Photonic Crystals for Simultaneous Detection and Photocatalytic Degradation of Hazardous Diazo Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the TiO2 Coated 2D-PhC
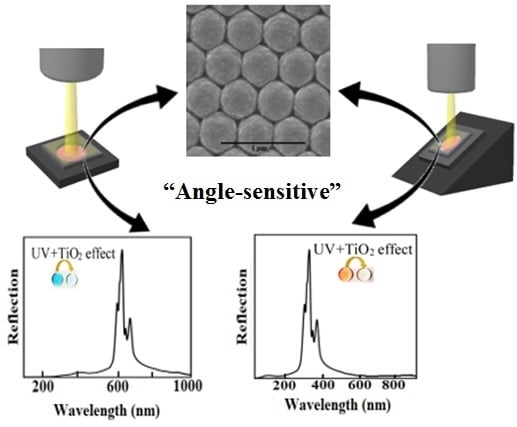
2.2. Reflectance Spectroscopy for Detection of CR and AB-10B
2.3. Photocatalytic Degradation of CR and AB-10B
3. Results
3.1. Production of TiO2 Coated 2D-PhC
3.2. Detection of CR and AB-10B
3.3. Photocatalytic Degradation of CR and AB-10B
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patel, B.R.; Kerman, K. Calorimetric and spectroscopic detection of the interaction between a diazo dye and human serum albumin. Analyst 2018, 143, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, E.M.; Oliveira, A.S.; Pavesi, T.; Maia, C.G.; Ferreira, L.F.V.; Moreira, J.C. Use of titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules 2011, 16, 10370–10386. [Google Scholar] [CrossRef] [PubMed]
- Alves de Lima, R.O.; Bazo, A.P.; Salvadori, D.M.F.; Rech, C.M.; de Palma Oliveira, D.; de Aragão Umbuzeiro, G. Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 626, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, J.; Ashraf, S.M.; Riaz, U. Highly efficient photocatalytic degradation of Amido Black 10b dye using polycarbazole-decorated TiO2 nanohybrids. ACS Omega 2017, 2, 8354–8365. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Yang, S.; Chingin, K.; Zhu, L.; Zhang, X.; Zhou, Z.; Zhao, Z. Quantitative detection of trace malachite green in aquiculture water samples by extractive electrospray ionization mass spectrometry. Int. J. Environ. Res. Public Health 2016, 13, 814. [Google Scholar] [CrossRef] [Green Version]
- Aki, S.; Maeno, K.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Development of a polymer/TiO2 hybrid two-dimensional photonic crystal for highly sensitive fluorescence-based ion sensing applications. Sens. Actuators B 2018, 269, 257–263. [Google Scholar] [CrossRef]
- Su, H.; Cheng, X.R.; Endo, T.; Kerman, K. Photonic crystals on copolymer film for label-free detection of DNA hybridization. Biosens. Bioelectron. 2018, 103, 158–162. [Google Scholar] [CrossRef]
- Liu, B.; Monshat, H.; Gu, Z.; Lu, M.; Zhao, X. Recent advances in merging photonic crystals and plasmonics for bioanalytical applications. Analyst 2018, 143, 2448–2458. [Google Scholar] [CrossRef]
- Endo, T.; Ozawa, S.; Okuda, N.; Yanagida, Y.; Tanaka, S.; Hatsuzawa, T. Reflectometric detection of influenza virus in human saliva using nanoimprint lithography based flexible two-dimensional photonic crystal biosensor. Sens. Actuators B 2010, 148, 269–276. [Google Scholar] [CrossRef]
- Inan, H.; Poyraz, M.; Inci, F.; Lifson, M.A.; Baday, M.; Cunningham, B.T.; Demirci, U. Photonic crystals: Emerging biosensors and their promise for point-of-care applications. Chem. Soc. Rev. 2017, 43, 366–388. [Google Scholar] [CrossRef]
- Baker, J.E.; Sriram, R.; Miller, B.L. Two-dimensional photonic crystals for sensitive microscale chemical and biochemical sensing. Lab Chip 2015, 15, 971–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawawi, W.; Zaharudin, R.; Zuliahani, A.; Shukri, D.; Azis, T.; Razali, Z. Immobilized TiO2-polyethylene glycol: Effects of aeration and pH of methylene blue dye. Appl. Sci. 2017, 7, 508. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.R.; Maeno, K.; Ganesh, H.V.S.; Endo, T.; Kerman, K. TiO2 coated 2D photonic crystals for reflectometric determination of malachite green. Microchim. Acta 2019, 186, 844. [Google Scholar] [CrossRef] [PubMed]
- Aono, K.; Aki, S.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Development of optical biosensor based on photonic crystal made of TiO2 using liquid phase deposition. Jpn. J. Appl. Phys. 2016, 55, 08RE01. [Google Scholar] [CrossRef]
- Owusu-Apenten, R.K. Food Protein Analysis: Quantitative Effects on Processing; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Iwunze, M.O. Aqueous photophysical parameters of Congo red. Spectrosc. Lett. 2010, 43, 16–21. [Google Scholar] [CrossRef]
- Khaledian, H.R.; Zolfaghari, P.; Elhami, V.; Aghbolaghy, M.; Khorram, S.; Karimi, A.; Khataee, A. Modification of immobilized titanium dioxide nanostructures by argon plasma for photocatalytic removal of organic dyes. Molecules 2019, 24, 383. [Google Scholar] [CrossRef] [Green Version]
- Solanki, V.; Majumder, S.; Mishra, I.; Dash, P.; Singh, C.; Kanjilal, D.; Varma, S. Enhanced anomalous photo-absorption from TiO2 nanostructures. J. Appl. Phys. 2014, 115, 124306. [Google Scholar] [CrossRef]
- Solanki, V.; Majumder, S.; Mishra, I.; Joshi, S.R.; Kanjilal, D.; Varma, S. Size dependent optical properties of TiO2 nanostructures. Radiat. Eff. Defects Solids 2013, 168, 518–524. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Lai, C.F.; Wang, Y.C.; Wu, C.L.; Zeng, J.Y.; Lin, C.F. Preparation of a colloidal photonic crystal containing CuO nanoparticles with tunable structural colors. RSC Adv. 2015, 5, 105200–105205. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Lutkenhaus, J.L.; Chu, L.; Tang, B.; Li, S.; Ma, W. All nanoparticle-based P(MMA-AA)/TiO2 one-dimensional photonic crystal films with tunable structural colors. J. Mater. Chem. C 2017, 5, 8266–8272. [Google Scholar] [CrossRef]
- Endo, T.; Sato, M.; Kajita, H.; Okuda, N.; Tanaka, S.; Hisamoto, H. Printed two-dimensional photonic crystals for single-step label-free biosensing of insulin under wet conditions. Lab Chip 2012, 12, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kajita, H.; Kawaguchi, Y.; Kosaka, T.; Himi, T. Label-free optical detection of C-reactive protein by nanoimprint lithography based 2D-photonic crystal film. Biotechnol. J. 2016, 11, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Von Freymann, G.; Koch, W.; Meisel, D.C.; Wegener, M.; Diem, M.; Garcia-Martin, A.; Pereira, S.; Busch, K.; Schilling, J.; Wehrspohn, R.B.; et al. Diffraction properties of two-dimensional photonic crystals. Appl. Phys. Lett. 2003, 83, 614–616. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, S.; Wang, G.; Zhao, J.; Guo, Z. A novel bifunctional molecularly imprinted polymer for determination of Congo red in food. RSC Adv. 2015, 5, 22811–22817. [Google Scholar] [CrossRef]
- Claux, B.; Vittori, O. Bismuth film electrode as an alternative for mercury electrodes: Determination of azo dyes and application for detection in food stuffs. Electroanalysis 2007, 19, 2243–2246. [Google Scholar] [CrossRef]
- Xi, K.; Sharma, S.K.; Taylor, G.T.; Muenow, D.W. Determination of low concentrations of the azo-dye complex of nitrite in fresh water and seawater using surface-enhanced resonance Raman spectroscopy (SERRS). Appl. Spectrosc. 1992, 46, 819–826. [Google Scholar] [CrossRef]
- Pérez-Urquiza, M.; Ferrer, R.; Beltrán, J.L. Determination of sulfonated azo dyes in river water samples by capillary zone electrophoresis. J. Chromatogr. A 2000, 883, 277–283. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Ibarra, I.S.; Miranda, J.M.; Barrado, E.; Santos, E.M. Magnetic solid phase extraction based on fullerene and activated carbon adsorbents for determination of azo dyes in water samples by capillary electrophoresis. Anal. Methods 2016, 8, 8466–8473. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Malladi, R.S.; Nargund, S.L.; Shukla, S.S.; Aminabhavi, T.M. Electrochemical detection and degradation of textile dye Congo red at graphene oxide modified electrode. Microchem. J. 2019, 146, 387–392. [Google Scholar] [CrossRef]
- Dixit, A.; Mungray, A.K.; Chakraborty, M. Photochemical oxidation of phenol and chlorophenol by UV/H2O2/TiO2 process: A kinetic study. Int. J. Chem. Eng. Appl. 2010, 1, 2010–2021. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Collins, G.; Armstrong, E.; McNulty, D.; O’Hanlon, S.; Geaney, H.; O’Dwyer, C. 2D and 3D photonic crystal materials for photocatalysis and electrochemical energy storage and conversion. Sci. Technol. Adv. Mater. 2016, 17, 563–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zeng, F.; Pang, F.; Ge, J. Substantial Enhancement toward the photocatalytic activity of CdS quantum dots by photonic crystal-supporting films. ACS Appl. Mater. Interfaces 2018, 10, 42241–42248. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Meng, S.; Chen, J.; Wang, J.; Xian, J.; Shao, Y.; Fu, X.; Li, D. Titanium dioxide photonic crystals with enhanced photocatalytic activity: Matching photonic band gaps of TiO2 to the absorption peaks of dyes. J. Phys. Chem. C 2013, 117, 21263–21273. [Google Scholar] [CrossRef]







| Sensor Material | Detection | Degradation | Analytical Method | Linear Range (µM) | LOD (µM) | Ref. |
|---|---|---|---|---|---|---|
| MIP@β-CD-MA | Yes | No | SPE | 0.0001–0.006 | 0.0001 | [26] |
| Bismuth film-GCE | Yes | No | Electrochemistry | 0–119 | 5.97 | [27] |
| Bismuth film-GCE | Yes | No | Electrochemistry | 0–83 | 1.65 | [27] |
| Silver hydrosol | Yes | No | SERS | 0–0.03 | 0.00002 | [28] |
| CElect-FS75 CE column | Yes | No | Capillary zone electrophoresis | 0.5–28 | 9.43 | [29] |
| Fe3O4 activated carbon-fullerene adsorbent | Yes | No | MSPE-CE | 11–75 | 3.74 | [30] |
| Graphene oxide-GCE | Yes | Yes | Electrochemistry | 0.01–0.2 | 0.24 | [31] |
| TiO2 coated 2D-PhC | Yes | Yes | Reflection Spectroscopy | CR: 0.2–1 and 1–20 AB-10B: 0.001–1 and 1–20 | CR: 0.0435 AB-10B: 0.0623 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeno, K.; Patel, B.R.; Endo, T.; Kerman, K. Angle-Sensitive Photonic Crystals for Simultaneous Detection and Photocatalytic Degradation of Hazardous Diazo Compounds. Micromachines 2020, 11, 93. https://doi.org/10.3390/mi11010093
Maeno K, Patel BR, Endo T, Kerman K. Angle-Sensitive Photonic Crystals for Simultaneous Detection and Photocatalytic Degradation of Hazardous Diazo Compounds. Micromachines. 2020; 11(1):93. https://doi.org/10.3390/mi11010093
Chicago/Turabian StyleMaeno, Kenichi, Bhargav R. Patel, Tatsuro Endo, and Kagan Kerman. 2020. "Angle-Sensitive Photonic Crystals for Simultaneous Detection and Photocatalytic Degradation of Hazardous Diazo Compounds" Micromachines 11, no. 1: 93. https://doi.org/10.3390/mi11010093