Spatiotemporal Distribution and Main Influencing Factors of Grasshopper Potential Habitats in Two Steppe Types of Inner Mongolia, China
Abstract
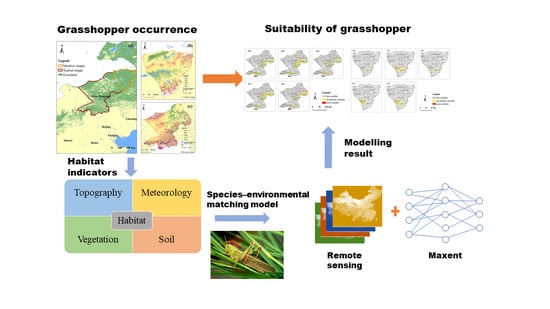
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Acquisition and Processing
2.2.1. Satellite Data
2.2.2. Meteorological and Other Geospatial Data
2.2.3. Survey Data
2.3. Methods
2.3.1. Determination of Grasshopper Developmental Stage
2.3.2. Select Influence Factors
2.3.3. Extraction Method of GPH
3. Results
3.1. Spatial Distribution Characteristics of GPHs between Two Steppe Types
3.2. Temporal Variation Characteristics of GPH
3.3. Main Influencing Factors in the Meadow and Typical Steppes
4. Discussion
4.1. Effeciency of the MaxEnt Model Coupled with Remote Sensing Technology
4.2. Reasons for the Main Influencing Factors Differing between the Two Steppe Types
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olfert, O.; Weiss, R.M.; Giffen, D.; Vankosky, M.A. Modeling Ecological Dynamics of a Major Agricultural Pest Insect (Melanoplus sanguinipes; Orthoptera: Acrididae): A Cohort-Based Approach Incorporating the Effects of Weather on Grasshopper Development and Abundance. J. Econ. Entomol. 2021, 114, 122–130. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and Grasshopper Management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef]
- Onsager, J.A.; Olfert, O. What Tools have Potential for Pets Management of Acrididae? A North American Perspective; Lockwood, J.A., Latchininsky, A.V., Sergee, M.J., Eds.; Grasshoppers and Grassland Health; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Belovsky, G.E.; Slade, J.B. Grasshoppers affect grassland ecosystem functioning: Spatial and temporal variation. Basic Appl. Ecol. 2018, 26, 24–34. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, L.L.; Dong, Y.Y.; Huang, W.J.; Shi, Y.; Ren, Y.; Ren, B.Y. Migratory Locust Habitat Analysis With PB-AHP Model Using Time-Series Satellite Images. IEEE Access 2020, 8, 166813–166823. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, L.L.; Huang, W.J.; Dong, Y.Y.; Geng, Y. Study on the Forming Mechanism of the High-Density Spot of Locust Coupled with Habitat Dynamic Changes and Meteorological Conditions Based on Time-Series Remote Sensing Images. Agronomy 2022, 12, 1610. [Google Scholar] [CrossRef]
- Zhao, L.L.; Huang, W.J.; Chen, J.S.; Dong, Y.Y.; Ren, B.Y.; Geng, Y. Land use/cover changes in the Oriental migratory locust area of China: Implications for ecological control and monitoring of locust area. Agric. Ecosyst. Environ. 2020, 303, 107110. [Google Scholar] [CrossRef]
- Wang, D.L.; Nkurunziza, V.; Barber, N.A.; Zhu, H.; Wang, J.T. Introduced ecological engineers drive behavioral changes of grasshoppers, consequently linking to its abundance in two grassland plant communities. Community Ecol. 2021, 195, 1007–1018. [Google Scholar]
- Helbing, F.; Fartmann, T.; Löffler, F.; Poniatowski, D. Effects of local climate, landscape structure and habitat quality on leafhopper assemblages of acidic grasslands. Agric. Ecosyst. Environ. 2017, 246, 94–101. [Google Scholar] [CrossRef]
- Wu, T.j.; Hao, S.G.; Kang, L. Effects of Soil Temperature and Moisture on the Development and Survival of Grasshopper Eggs in Inner Mongolian Grasslands. Front. Ecol. Evol. 2021, 9, 727911. [Google Scholar] [CrossRef]
- Poniatowski, D.; Stuhldreher, G.; Löffler, F.; Fartmann, T. Patch occupancy of grassland specialists: Habitat quality matters more than habitat connectivity. Biol. Conserv. 2018, 225, 237–244. [Google Scholar] [CrossRef]
- Platts, P.J.; Mason, S.C.; Palmer, G.; Hill, J.K.; Oliver, T.H.; Powney, G.D.; Fox, R.; Thomas, C.D. Habitat availability explains variation in climate-driven range shifts across multiple taxonomic groups. Sci. Rep. 2019, 9, 15039. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.H.; Kong, W.P.; Eerdengqimuge; Ye, H.C.; Sun, Z.X.; Wang, N.; Du, B.; Zhou, Y.T.; Wei, J.; Huang, W.J. Detecting Key Factors of Grasshopper Occurrence in Typical Steppe and Meadow Steppe by Integrating Machine Learning Model and Remote Sensing Data. Insects 2022, 13, 894. [Google Scholar] [CrossRef]
- Hawryło, P.; Bednarz, B.; Wężyk, P.; Szostak, M. Estimating defoliation of Scots pine stands using machine learning methods and vegetation indices of Sentinel-2. Eur. J. Remote Sens. 2018, 51, 194–204. [Google Scholar] [CrossRef]
- Bhattarai, R.; Rahimzadeh-Bajgiran, P.; Weiskittel, A.; MacLean, D.A. Sentinel-2 based prediction of spruce budworm defoliation using red-edge spectral vegetation indices. Remote Sens. Lett. 2020, 11, 777–786. [Google Scholar] [CrossRef]
- Tappan, G.G.; Moore, D.G.; Knausenberger, W.I. Monitoring grasshopper and locust habitats in Sahelian Africa using GIS and remote sensing technology†. Int. J. Geogr. Inf. Syst. 1991, 5, 123–135. [Google Scholar] [CrossRef]
- Jia, H.M.; Lang, C.B.; Oliva, D.; Song, W.L.; Peng, X.X. Hybrid Grasshopper Optimization Algorithm and Differential Evolution for Multilevel Satellite Image Segmentation. Remote Sens. 2019, 11, 1134. [Google Scholar] [CrossRef]
- Klein, I.; Oppelt, N.; Kuenzer, C. Application of Remote Sensing Data for Locust Research and Management—A Review. Insects 2021, 12, 233. [Google Scholar] [CrossRef]
- Theron, K.J.; Pryke, J.S.; Samways, M.J. Identifying managerial legacies within conservation corridors using remote sensing and grasshoppers as bioindicators. Ecol. Appl. 2021, 32, e02496. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Peterson, A.T.; Loiselle, B.A.; NCEAS Predicting Species Distributions Working Group. The influence of spatial errors in species occurrence data used in distribution models. J. Appl. Ecol. 2008, 45, 239–247. [Google Scholar] [CrossRef]
- Kimathi, E.; Tonnang, H.Z.; Subramanian, S.; Cressman, K.; Abdel-Rahman, E.M.; Tesfayohannes, M. Prediction of breeding regions for the desert locust Schistocerca gregaria in East Africa. Sci. Rep. 2020, 10, 11937. [Google Scholar] [CrossRef]
- Dai, J.; Roberts, D.A.; Stow, D.A.; An, L.; Hall, S.J.; Yabiku, S.T.; Kyriakidis, P.C. Mapping understory invasive plant species with field and remotely sensed data in Chitwan, Nepal. Remote Sens. Environ. 2020, 250, 112037. [Google Scholar] [CrossRef]
- Huang, Y.R.; Dong, Y.Y.; Huang, W.J.; Ren, B.Y.; Deng, Q.; Shi, Y.; Bai, J.; Ren, Y.; Geng, Y.; Ma, H.Q. Overwintering Distribution of Fall Armyworm (Spodoptera frugiperda) in Yunnan, China, and Influencing Environmental Factors. Insects 2020, 11, 805. [Google Scholar] [CrossRef]
- Saha, A.; Rahman, S.; Alam, S. Modeling current and future potential distributions of desert locust Schistocerca gregaria (Forskål) under climate change scenarios using MaxEnt. J. Asia-Pac. Biodivers. 2021, 14, 399–409. [Google Scholar] [CrossRef]
- Li, L.L.; Zhao, C.Z.; Zhao, X.W.; Wang, D.W.; Li, Y. Pattern of plant communities’ influence to grasshopper abundance distribution in heterogeneous landscapes at the upper reaches of Heihe River, Qilian Mountains, China. Environ. Sci. Pollut. Res. 2022, 29, 13177–13187. [Google Scholar] [CrossRef]
- Rafuse, D.J. A MaxEnt Predictive Model for Hunter-Gatherer Sites in the Southern Pampas, Argentina. Open Quat. 2021, 7, 6. [Google Scholar] [CrossRef]
- Wysiecki, M.L.; Arturi, M.; Torrusio, S.; Cigliano, M. Influence of weather variables and plant communities on grasshopper density in the Southern Pampas, Argentina. J. Insect Sci. 2011, 11, 109. [Google Scholar]
- Matenaar, D.; Bazelet, C.S.; Hochkirch, A. Simple tools for the evaluation of protected areas for the conservation of grasshoppers. Biol. Conserv. 2015, 192, 192–199. [Google Scholar] [CrossRef]
- Wang, D.; Ba, L. Ecology of meadow steppe in northeast China. Rangel. J. 2008, 30, 247–254. [Google Scholar] [CrossRef]
- Giese, M.; Brueck, H.; Gao, Y.Z.; Lin, S.; Steffens, M.; Kögel-Knabner, I.; Glindemann, T.; Susenbeth, A.; Taube, F.; Butterbach-Bahl, K.; et al. N balance and cycling of Inner Mongolia typical steppe: A comprehensive case study of grazing effects. Ecol. Monogr. 2013, 83, 195–219. [Google Scholar] [CrossRef]
- Dai, H.Y.; Wang, H.M. Influence of rainfall events on soil moisture in a typical steppe of Xilingol. Phys. Chem. Earth 2021, 121, 102964. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.Y.; He, B.; Gexigeduren; Xin, Z.Y.; Lin, H. Spatiotemporal heterogeneity of the potential occurrence of Oedaleus decorus asiaticus in Inner Mongolia steppe habitats. J. Arid. Environ. 2015, 116, 33–43. [Google Scholar] [CrossRef]
- Ma, W.; Fang, J.; Yang, Y.; Mohammat, A. Biomass carbon stocks and their changes in northern China’s grasslands during 1982–2006. Sci. China Life Sci. 2010, 53, 841–850. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Luo, G.; Ding, J.L.; Chen, X.F. Detecting soil salinity with arid fraction integrated index and salinity index in feature space using Landsat TM imagery. J. Arid. Land 2013, 5, 340–353. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Hao, S.G.; Kang, L. Postdiapause Development and Hatching Rate of Three Grasshopper Species (Orthoptera: Acrididae) in Inner Mongolia. Environ. Entomol. 2004, 33, 1528–1534. [Google Scholar] [CrossRef]
- Bai, Y.M.; Liu, L.; Gao, S.H. Research on Grasshopper Meteorological Monitoring and Prediction; China Meteorological Press: Beijing, China, 2013; p. 160. [Google Scholar]
- Powell, L.R.; Berg, A.A.; Johnson, D.L.; Warland, J.S. Relationships of pest grasshopper populations in Alberta, Canada to soil moisture and climate variables. Agric. For. Meteorol. 2007, 144, 73–84. [Google Scholar] [CrossRef]
- Ni, S.X.; Wang, J.C.; Jiang, J.J.; Zha, Y. Rangeland Grasshoppers in Relation to Soils in the Qinghai Lake Region, China. Pedosphere 2007, 17, 84–89. [Google Scholar] [CrossRef]
- Cronin, J.B.; Hing, R.D.; McNair, P.J. Reliability and Validity of a Linear Position Transducer for Measuring Jump Performance. J. Strength Cond. Res. 2004, 18, 590–593. [Google Scholar]
- Xiao, C.; Ye, J.; Esteves, R.M.; Rong, C. Using Spearman’s correlation coefficients for exploratory data analysis on big dataset. Concurr. Comput. Pract. Exp. 2016, 28, 3866–3878. [Google Scholar] [CrossRef]
- Bujang, M.A.; Baharum, N. A simplified guide to determination of sample size requirements for estimating the value of intraclass correlation coefficient: A review. Arch. Orofac. Sci. 2017, 12, 1–11. [Google Scholar]
- Norris, D. Model thresholds are more important than presence location type: Understanding the distribution of lowland tapir (Tapirus terrestris) in a continuous Atlantic forest of southeast Brazil. Trop. Conserv. Sci. 2014, 7, 529–547. [Google Scholar]
- Lombardo, L.; Bachofer, F.; Cama, M.; Märker, M.; Rotigliano, E. Exploiting Maximum Entropy method and ASTER data for assessing debris flow and debris slide susceptibility for the Giampilieri catchment (north-eastern Sicily, Italy). Earth Surf. Process. Landf. 2016, 41, 1776–1789. [Google Scholar] [CrossRef]
- Bosso, L.; Russo, D.; Febbraro, M.D.; Cristinzio, G.; Zoina, A. Potential distribution of Xylella fastidiosa in Italy: A maximum entropy mode. Phypotathologia Mediterr. 2016, 55, 62–72. [Google Scholar]
- Young, N.; Carter, L.; Evangelista, P. A MaxEnt Model v3.3.3e Tutorial (ArcGIS v10). Natural Resource Ecology Laboratory, Colorado State University and the National Institute of Invasive Species Science: Fort Collins, CO, USA, 2011. [Google Scholar]
- Du, B.B.; Wei, J.; Lin, K.J.; Lu, L.H.; Ding, X.L.; Ye, H.C.; Huang, W.J.; Wang, N. Spatial and Temporal Variability of Grassland Grasshopper Habitat Suitability and Its Main Influencing Factors. Remote Sens. 2022, 14, 3910. [Google Scholar] [CrossRef]
- Wan, G.-Z.; Wang, L.; Jin, L.; Chen, J. Evaluation of environmental factors affecting the quality of Codonopsis pilosula based on chromatographic fingerprint and MaxEnt model. Ind. Crops Prod. 2021, 170, 113783. [Google Scholar] [CrossRef]
- Burt, P.J.; Colvin, J.; Smith, S.M. Remote sensing of rainfall by satellite as an aid to Oedaleus senegalensis (Orthoptera: Acrididae) control in the Sahel. Bull. Entomol. Res. 1995, 85, 455–462. [Google Scholar]
- Gage, S.H.; Mukerji, M.K. A Perspective of Grasshopper Population Distribution in Saskatchewan and Interrelationship with Weather. Environ. Entomol. 1977, 6, 469–479. [Google Scholar]
- Branson, D.H. Influence of a Large Late Summer Precipitation Event on Food Limitation and Grasshopper Population Dynamics in a Northern Great Plains Grassland. Environ. Entomol. 2008, 37, 686–695. [Google Scholar]
- Pang, B.P.; Li, N.; Zhou, X.R. Supercooling Capacity and Cold Hardiness of Band-Winged Grasshopper eggs (Orthoptera: Acrididae). J. Insect Sci. 2014, 14, 289. [Google Scholar] [CrossRef]
- Cease, A.J.; Harrison, J.F.; Hao, S.; Niren, D.C.; Zhang, G.; Kang, L.; Elser, J.J. Nutritional imbalance suppresses migratory phenotypes of the Mongolian locust (Oedaleus asiaticus). R. Soc. Open Sci. 2017, 4, 161039. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, L.L.; Huang, W.J.; Dong, Y.Y.; Ma, H.Q.; Guo, A.T.; Ren, Y.; Xing, N.C.; Huang, Y.R.; Sun, R.Q.; et al. A Landscape-Based Habitat Suitability Model (LHS Model) for Oriental Migratory Locust Area Extraction at Large Scales: A Case Study along the Middle and Lower Reaches of the Yellow River. Remote Sens. 2022, 14, 1058. [Google Scholar] [CrossRef]
- Groeters, F.R. The adaptive role of facultative embryonic diapause in the grasshopper Caledia captiva (Orthoptera: Acrididae) in southeastern Australia. Ecography 2006, 17, 221–228. [Google Scholar]








| Category | Environmental Variables | Detailed Description of Environmental Variables | Spatial Resolution |
|---|---|---|---|
| Topography | Elevation Slope Aspect | 90 m 90 m 90 m | |
| Meteorology | Land surface temperature | Minimum land surface temperature in the egg stage (EMinLST) Minimum land surface temperature in the nymph stage (NMinLST) Mean land surface temperature in the nymph stage(NMeanLST) Mean land surface temperature in the adult stage(AMeanLST) | 1 km |
| Precipitation | Precipitation in the egg stage (EPre) Precipitation in the nymph stage (NPre) Precipitation in the adult stage (APre) | 0.1° | |
| Soil temperature | Soil temperature in the egg stage (EST) Soil temperature in the nymph stage (NST) Soil temperature in the adult stage (AST) | 1 km | |
| Vegetation | Vegetation type | 1 km | |
| Aboveground biomass | Aboveground biomass in the nymph stage (NAB) Aboveground biomass in the adult stage (AAB) | 1 km | |
| Soil | Soil type | 1 km | |
| Soil salinity index | Soil salinity in the egg stage (ESI) Soil salinity in the nymph stage (NSI) Soil salinity in the adult stage (ASI) | 1 km |
| Year | Area of Meadow Steppe (km2) | Area of Typical Steppe (km2) | ||||
|---|---|---|---|---|---|---|
| Most Suitable | Moderately Suitable | Less Suitable | Most Suitable | Moderately Suitable | Less Suitable | |
| 2018 | 44 | 407 | 32,853 | 1091 | 8829 | 110,098 |
| 2019 | 101 | 1135 | 32,068 | 1055 | 12,460 | 106,503 |
| 2020 | 64 | 691 | 32,549 | 686 | 10,341 | 108,991 |
| 2021 | 192 | 1218 | 31,894 | 672 | 10,854 | 7491 |
| 2022 | 102 | 1622 | 31,580 | 1192 | 7491 | 111,335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Lu, L.; Dong, Y.; Huang, W.; Zhang, B.; Du, B.; Ding, C.; Ye, H.; Wang, K.; Huang, Y.; et al. Spatiotemporal Distribution and Main Influencing Factors of Grasshopper Potential Habitats in Two Steppe Types of Inner Mongolia, China. Remote Sens. 2023, 15, 866. https://doi.org/10.3390/rs15030866
Guo J, Lu L, Dong Y, Huang W, Zhang B, Du B, Ding C, Ye H, Wang K, Huang Y, et al. Spatiotemporal Distribution and Main Influencing Factors of Grasshopper Potential Habitats in Two Steppe Types of Inner Mongolia, China. Remote Sensing. 2023; 15(3):866. https://doi.org/10.3390/rs15030866
Chicago/Turabian StyleGuo, Jing, Longhui Lu, Yingying Dong, Wenjiang Huang, Bing Zhang, Bobo Du, Chao Ding, Huichun Ye, Kun Wang, Yanru Huang, and et al. 2023. "Spatiotemporal Distribution and Main Influencing Factors of Grasshopper Potential Habitats in Two Steppe Types of Inner Mongolia, China" Remote Sensing 15, no. 3: 866. https://doi.org/10.3390/rs15030866