Light Absorption by Optically Active Components in the Arctic Region (August 2020) and the Possibility of Application to Satellite Products for Water Quality Assessment
Abstract
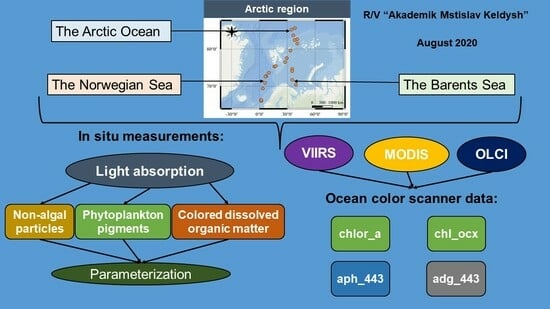
:1. Introduction
2. Materials and Methods
2.1. Water Sampling
2.2. Pigment Analysis
2.3. Absorption Measurements
2.4. Satellite Data
2.5. Statistical Analysis
3. Results
3.1. Pigment Concentration
3.2. Light Absorption by Phytoplankton
3.3. Light Absorption by Non-Algal Particles
3.4. Light Absorption by Colored Dissolved Organic Matter
3.5. Total Non-Water Light Absorption Budget
3.6. Satellite Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morley, S.A.; Abele, D.; Barnes, D.K.; Cardenas, C.A.; Cotte, C.; Gutt, J.; Henley, S.F.; Hofer, J.; Hughes, K.A.; Martin, S.M.; et al. Global drivers on Southern Ocean ecosystems: Changing physical environments and anthropogenic pressures in an Earth system. Front. Mar. Sci. 2020, 7, 547188. [Google Scholar] [CrossRef]
- Walsh, J.E. Intensified Warming of the Arctic: Causes and impacts on middle latitudes. Glob. Planet. Chang. 2014, 117, 52–63. [Google Scholar] [CrossRef]
- Jansen, E.; Christensen, J.H.; Dokken, T.; Nisancioglu, K.H.; Vinther, B.M.; Capron, E.; Guo, C.; Jensen, M.F.; Langen, P.L.; Pedersen, R.A.; et al. Past perspectives on the present era of abrupt Arctic climate change. Nat. Clim. Chang. 2020, 10, 714–721. [Google Scholar] [CrossRef]
- Yu, L.; Zhong, S.; Vihma, T.; Sun, B. Attribution of late summer early autumn Arctic Sea ice decline in recent decades. NPJ Clim. Atmos. Sci. 2021, 4, 3. [Google Scholar] [CrossRef]
- Rantanen, M.; Karpechko, A.Y.; Lipponen, A.; Nordling, K.; Hyvärinen, O.; Ruosteenoja, K.; Vihma, T.; Laaksonen, A. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 2022, 3, 168. [Google Scholar] [CrossRef]
- Stroeve, J.C.; Serreze, M.C.; Holland, M.M.; Kay, J.E.; Malanik, J.; Barrett, A.P. The Arctic’s rapidly shrinking sea ice cover: A research synthesis. Clim. Chang. 2012, 110, 1005–1027. [Google Scholar] [CrossRef]
- Comiso, J.C.; Yang, J.; Honjo, S.; Krishfield, R.A. Detection of change in the Arctic using satellite and in situ data. J. Geophys. Res. 2003, 108, 3384. [Google Scholar] [CrossRef]
- Palm, S.P.; Strey, S.T.; Spinhirne, J.; Markus, T. Influence of Arctic Sea ice extent on polar cloud fraction and vertical structure and implications for regional climate. J. Geophys. Res. 2010, 115, D21209. [Google Scholar] [CrossRef]
- Bélanger, S.; Babin, M.; Tremblay, J.-É. Increasing cloudiness in Arctic damps the increase in phytoplankton primary production due to sea ice receding. Biogeosciences 2013, 10, 4087–4101. [Google Scholar] [CrossRef]
- Tremblay, J.-É.; Gagnon, J. The effects of irradiance and nutrient supply on the productivity of Arctic waters: A perspective on climate change. In Influence of Climate Change on the Changing Arctic and Sub-Arctic Conditions; Springer: Dordrecht, The Netherlands, 2009; pp. 73–93. [Google Scholar]
- Li, W.K.W.; McLaughlin, F.A.; Lovejoy, C.; Carmack, E.C. Smallest algae thrive as the Arctic Ocean freshens. Science 2009, 326, 539. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Secular trends in Arctic Ocean net primary production. J. Geophys. Res. 2011, 116, C09011. [Google Scholar] [CrossRef]
- Chan, F.T.; Stanislawczyk, K.; Sneekes, A.C.; Dvoretsky, A.; Gollasch, S.; Minchin, D.; David, M.; Jelmert, A.; Albretsen, J.; Bailey, S.A. Climate change opens new frontiers for marine species in the Arctic: Current trends and future invasion risks. Glob. Chang. Biol. 2019, 25, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis, 3rd ed.; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Babin, M.; Arrigo, K.; Bélanger, S.; Forget, M.H. Ocean colour remote sensing in polar seas. IOCCG Rep. Ser. 2015, 16, 130. [Google Scholar]
- Melin, F.; Wang, M.; Werdell, J.; Hiroshi, K. Uncertainties in Ocean Colour Remote Sensing. IOCCG Rep. Ser. 2019, 18, 164. [Google Scholar]
- Demidov, A.B.; Kopelevich, O.V.; Mosharov, S.A.; Sheberstov, S.V.; Vazyulya, S.V. Modelling Kara Sea phytoplankton primary production: Development and skill assessment of regional algorithms. J. Sea Res. 2017, 125, 1–17. [Google Scholar] [CrossRef]
- Glukhovets, D.; Kopelevich, O.; Yushmanova, A.; Vazyulya, S.; Sheberstov, S.; Karalli, P.; Sahling, I. Evaluation of the CDOM absorption coefficient in the Arctic seas based on Sentinel-3 OLCI data. Remote Sens. 2020, 12, 3210. [Google Scholar] [CrossRef]
- Glukhovets, D.; Sheberstov, S.; Vazyulya, S.; Yushmanova, A.; Salyuk, P.; Sahling, I.; Aglova, E. Influence of the accuracy of chlorophyll-retrieval algorithms on the estimation of solar radiation absorbed in the Barents Sea. Remote Sens. 2022, 14, 4995. [Google Scholar] [CrossRef]
- Yushmanova, A.V.; Vazyulya, S.V. Validation of satellite algorithms for calculating the absorption coefficient of colored dissolved organic matter in the Barents Sea. Curr. Probl. Remote Sens. Earth Space 2022, 19, 28–39. [Google Scholar] [CrossRef]
- Matsuoka, A.; Huot, Y.; Shimada, K.; Saitoh, S.-I.; Babin, M. Bio-optical characteristics of the western Arctic Ocean: Implications for ocean color algorithms. Can. J. Remote Sens. 2007, 33, 503–518. [Google Scholar] [CrossRef]
- Mitchell, B.G. Predictive Bio-optical relationships for polar oceans and marginal ice zones. J. Mar. Syst. 1992, 3, 91–105. [Google Scholar] [CrossRef]
- Glukhovets, D.I.; Goldin, Y.A. Surface desalinated layer distribution in the Kara Sea determined by shipboard and satellite data. Oceanologia 2020, 62, 364–373. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Kiefer, D. A Chlorophyll α specific absorption and fluorescence excitation spectra for light-limited phytoplankton. Deep Sea Res. Part A Oceanogr. Res. Pap. 1988, 35, 639–663. [Google Scholar] [CrossRef]
- Wang, J.; Cota, G.F.; Ruble, D.A. Absorption and backscattering in the Beaufort and Chukchi Seas. J. Geophys. Res. Oceans 2005, 110, 1–12. [Google Scholar] [CrossRef]
- Mobley, C.D. (Ed.) The Oceanic Optics Book; International Ocean Colour Co-ordinating Group (IOCCG): Dartmouth, NS, Canada, 2022; 924p. [Google Scholar] [CrossRef]
- Kopelevich, O.V.; Burenkov, V.I.; Ershova, S.V.; Sheberstov, S.V.; Evdoshenko, M.A. Application of SeaWiFS Data for Studying Variability of bio-optical characteristics in the Barents, Black and Caspian Seas. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 1063–1091. [Google Scholar] [CrossRef]
- Babin, M.; Stramski, D.; Ferrari, G.M.; Claustre, H.; Bricaud, A.; Obolensky, G.; Hoepffner, N. Variations in the light absorption coefficients of phytoplankton, nonalgal particles, and dissolved organic matter in coastal waters around Europe. J. Geophys. Res. Oceans 2003, 108, C7. [Google Scholar] [CrossRef]
- Churilova, T.; Suslin, V.; Krivenko, O.; Efimova, T.; Moiseeva, N. Spectral approach to assessment of phytoplankton photosynthesis rate in the Black Sea based on satellite information: Methodological aspects of the regional model development. J. Sib. Fed. Univ. Biol. 2016, 9, 367–384. (In Russian) [Google Scholar] [CrossRef]
- Matsuoka, A.; Hill, V.; Huot, Y.; Babin, M.; Bricaud, A. Seasonal variability in the light absorption properties of Western Arctic waters: Parameterization of the individual components of absorption for ocean color applications. J. Geophys. Res. Oceans 2011, 116, C2. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Wang, J.; Cota, J.F. Remote-sensing reflectance in the Beaufort and Chukchi Seas: Observations and models. Appl. Opt. 2003, 42, 2754–2765. [Google Scholar] [CrossRef]
- Brunelle, C.B.; Larouche, P.; Gosselin, M. Variability of phytoplankton light absorption in Canadian Arctic Seas. J. Geophys. Res. Oceans 2012, 117, C9. [Google Scholar] [CrossRef]
- Mustapha, S.B.; Bélanger, S.; Larouche, P. Evaluation of ocean color algorithms in the Southeastern Beaufort Sea, Canadian Arctic: New parameterization using SeaWiFS, MODIS, and MERIS spectral bands. Can. J. Remote Sens. 2012, 38, 535–556. [Google Scholar] [CrossRef]
- Gonçalves-Araujo, R.; Rabe, B.; Peeken, I.; Bracher, A. High colored dissolved organic matter (CDOM) absorption in surface waters of the Central-Eastern Arctic Ocean: Implications for biogeochemistry and ocean color algorithms. PLoS ONE 2018, 13, e0190838. [Google Scholar] [CrossRef]
- Neukermans, G.; Reynolds, R.A.; Stramski, D. Contrasting Inherent optical properties and particle characteristics between an under-ice phytoplankton bloom and open water in the Chukchi Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 105, 59–73. [Google Scholar] [CrossRef]
- Bélanger, S.; Cizmeli, S.A.; Ehn, J.; Matsuoka, A.; Doxaran, D.; Hooker, S.; Babin, M. Light absorption and partitioning in Arctic Ocean surface waters: Impact of multiyear ice melting. Biogeosciences 2013, 10, 6433–6452. [Google Scholar] [CrossRef]
- Sánchez-García, L.; Alling, V.; Pugach, S.; Vonk, J.; van Dongen, B.; Humborg, C.; Dudarev, O.; Semiletov, I.; Gustafsson, Ö. Inventories and behavior of particulate organic carbon in the Laptev and East Siberian Seas. Glob. Biogeochem. Cycles 2011, 25, 1–13. [Google Scholar] [CrossRef]
- Fichot, C.G.; Kaiser, K.; Hooker, S.B.; Amon, R.M.W.; Babin, M.; Bélanger, S.; Walker, S.A.; Benner, R. Pan-Arctic distributions of continental runoff in the Arctic Ocean. Sci. Rep. 2013, 3, 1053. [Google Scholar] [CrossRef] [PubMed]
- Zatsepin, A.G.; Zavialov, P.O.; Kremenetskiy, V.V.; Poyarkov, S.G.; Soloviev, D.M. The upper desalinated layer in the Kara Sea. Oceanology 2010, 50, 657–667. [Google Scholar] [CrossRef]
- Glukhovets, D. An Overview of the special issue on seawater bio-optical characteristics from satellite ocean color data. Remote Sens. 2023, 15, 1094. [Google Scholar] [CrossRef]
- Klyuvitkin, A.A.; Politova, N.V.; Novigatsky, A.N.; Kravchishina, M.D. Studies of the European Arctic on cruise 80 of the R/V Akademik Mstislav Keldysh. Oceanology 2021, 61, 139–141. [Google Scholar] [CrossRef]
- Babin, M.; Morel, A.; Claustre, H.; Bricaud, A.; Kolber, Z.; Falkowski, P.G. Nitrogen- and irradiance-dependent variations of the maximum quantum yield of carbon fixation in eutrophic, mesotrophic and oligotrophic marine systems. Deep Sea Res. Part I Oceanogr. Res. Pap. 1996, 43, 1241–1272. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New Spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Lorenzen, C.J. Determination of chlorophyll and pheo-pigments: Spectrophotometric equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Neeley, A.R.; Mannino, A.; Boss, E.; D’sa, E.J.; Freeman, S.; Fry, E.; Mueller, J.L.; Pegau, S.; Reynolds, R.A.; Roesler, C.; et al. Inherent Optical Property Measurements and Protocols: Absorption Coefficient (vl.0); International Ocean-Colour Coordinating Group: Dartmouth, NS, Canada, 2018; Volume 1. [Google Scholar]
- Kishino, M.; Takahashi, M.; Okami, N.; Ichimura, S. Estination of the spectral absorption coefficients of phytoplankton in the sea. Bull. Mar. Sci. 1985, 37, 634–642. [Google Scholar]
- Mitchell, B.G. Algorithms for determining the absorption coefficient for aquatic particulates using the quantitative filter technique. Ocean. Optics X 1990, 1302, 137–148. [Google Scholar] [CrossRef]
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll a algorithms for oligotrophic oceans: A novel approach based on three-band reflectance difference. J. Geophys. Res. Oceans 2012, 117, C1. [Google Scholar] [CrossRef]
- O’Reilly, J.E. SeaWiFS Postlaunch calibration and validation analyses. NASA Tech. Memo. 2000, 11, 49. [Google Scholar]
- Inherent Optical Properties (IOPs). Available online: https://oceancolor.gsfc.nasa.gov/atbd/giop/ (accessed on 14 April 2023).
- Franz, B.A.; Werdell, P.J. A Generalized framework for modeling of inherent optical properties in remote sensing applications. In Proceedings of the Ocean Optics XX Conference, Anchorage, AK, USA, 27 September–1 October 2010; p. 13. [Google Scholar]
- Churilova, T.; Suslin, V.; Krivenko, O.; Efimova, T.; Moiseeva, N.; Mukhanov, V.; Smirnova, L. Light absorption by phytoplankton in the upper mixed layer of the Black Sea: Seasonality and parametrization. Front. Mar. Sci. 2017, 4, 90. [Google Scholar] [CrossRef]
- Bricaud, A.; Babin, M.; Morel, A.; Claustre, H. Variability in the chlorophyll-specific absorption coefficients of natural phytoplankton: Analysis and parameterization. J. Geophys. Res. 1995, 100, 13321–13332. [Google Scholar] [CrossRef]
- Kopelevich, O.V.; Sahling, I.V.; Vazyulya, S.V.; Glukhovets, D.I.; Sheberstov, S.V.; Burenkov, V.I.; Karalli, P.G.; Yushmanova, A.V. Bio-Optical Characteristics of the Seas, Surrounding the Western Part of Russia, from Data of the Satellite Ocean Color Scanners of 1998–2017; OOO VASH FORMAT: Moscow, Russia, 2018. [Google Scholar]
- Ardyna, M.; Babin, M.; Gosselin, M.; Devred, E.; Bélanger, S.; Matsuoka, A.; Tremblay, J.E. parameterization of vertical chlorophyll a in the Arctic Ocean: Impact of the subsurface chlorophyll maximum on regional, seasonal, and annual primary production estimates. Biogeosciences 2013, 10, 4383–4404. [Google Scholar] [CrossRef]
- Tilzer, M.M.; Elbrächter, M.; Gieskes, W.W.; Beese, B. Light-temperature interactions in the control of photosynthesis in Antarctic phytoplankton. Polar Biol. 1986, 5, 105–111. [Google Scholar] [CrossRef]
- Sosik, H.M.; Mitchell, B.G. Effects of temperature on growth, light absorption, and quantum yield in Dunaliella tertiolecta (chlorophyceae). J. Phycol. 1994, 30, 833–840. [Google Scholar] [CrossRef]
- Berges, J.; Varela, D.; Harrison, P. Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Mar. Ecol. Prog. Ser. 2002, 225, 139–146. [Google Scholar] [CrossRef]
- Lutz, V.; Sathyendranath, S.; Head, E.; Li, W. Variability in pigment composition and optical characteristics of phytoplankton in the Labrador Sea and the Central North Atlantic. Mar. Ecol. Prog. Ser. 2003, 260, 1–18. [Google Scholar] [CrossRef]
- Grant, C.; Louda, J. Microalgal pigment ratios in relation to light intensity: Implications for chemotaxonomy. Aquat. Biol. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Barlow, R.; Aiken, J.; Moore, G.; Holligan, P.; Lavender, S. pigment adaptations in surface phytoplankton along the eastern boundary of the Atlantic Ocean. Mar. Ecol. Prog. Ser. 2004, 281, 13–26. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Stuart, V.; Platt, T.; Bouman, H.; Ulloa, O.; Maass, H. Remote sensing of ocean colour: Towards algorithms for retrieval of pigment composition. Indian J. Mar. Sci. 2005, 34, 333–340. [Google Scholar]
- Fujiki, T. Variability in chlorophyll a specific absorption coefficient in marine phytoplankton as a function of cell size and irradiance. J. Plankton Res. 2002, 24, 859–874. [Google Scholar] [CrossRef]
- Churilova, T.; Suslin, V.; Sosik, H.M.; Efimova, T.; Moiseeva, N.; Moncheva, S.; Mukhanov, V.; Rylkova, O.; Krivenko, O. Phytoplankton light absorption in the deep chlorophyll maximum layer of the Black Sea. Eur. J. Remote Sens. 2019, 52, 123–136. [Google Scholar] [CrossRef]
- Morel, A.; Bricaud, A. Theoretical results concerning light absorption in a discrete medium, and application to specific absorption of phytoplankton. Deep Sea Res. Part A Oceanogr. Res. Pap. 1981, 28, 1375–1393. [Google Scholar] [CrossRef]
- Millán-Núñez, E.; Sieracki, M.E.; Millán-Núñez, R.; Lara-Lara, J.R.; Gaxiola-Castro, G.; Trees, C.C. Specific absorption coefficient and phytoplankton biomass in the southern region of the California current. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 817–826. [Google Scholar] [CrossRef]
- Pautova, L.; Silkin, V.; Kravchishina, M.; Klyuvitkin, A.; Kudryavtseva, E.; Glukhovets, D.; Chultsova, A.; Politova, N. Phytoplankton of the high-latitude Arctic: Intensive growth large diatoms porosira glacialis in the Nansen Basin. J. Mar. Sci. Eng. 2023, 11, 453. [Google Scholar] [CrossRef]
- Kudryavtseva, E.A.; Kravchishina, M.D.; Pautova, L.A.; Rusanov, I.I.; Silkin, V.A.; Glukhovets, D.I.; Torgunova, N.I.; Netsvetaeva, O.P.; Politova, N.V.; Klyuvitkin, A.A.; et al. Size structure of primary producers in the marginal ice zone of the European Arctic in summer. Dokl. Earth Sci. 2022, 507, S313–S318. [Google Scholar] [CrossRef]
- Glukhovets, D.I.; Kopelevich, O.V.; Sahling, I.V.; Artemiev, V.A.; Pautova, L.A.; Lange, E.K.; Kravchishina, M.D. Biooptical characteristics of the surface layer of the Baltic, Norwegian, and Barents Seas in summer 2014–2016 from shipboard and satellite data. Oceanology 2017, 57, 410–418. [Google Scholar] [CrossRef]
- Matsuoka, A.; Babin, M.; Doxaran, D.; Hooker, S.B.; Mitchell, B.G.; Bélanger, S.; Bricaud, A. A Synthesis of light absorption properties of the Arctic Ocean: Application to semianalytical estimates of dissolved organic carbon concentrations from space. Biogeosciences 2014, 11, 3131–3147. [Google Scholar] [CrossRef]
- Nelson, N.B.; Siegel, D.A. The global distribution and dynamics of chromophoric dissolved organic matter. Annu. Rev. Mar. Sci. 2013, 5, 447–476. [Google Scholar] [CrossRef]
- Suslin, V.; Churilova, T. A regional algorithm for separating light absorption by chlorophyll-a and coloured detrital matter in the Black Sea, using 480–560 nm bands from ocean colour scanners. Int. J. Remote Sens. 2016, 37, 4380–4400. [Google Scholar] [CrossRef]
- Suslin, V.; Pryahina, S.; Churilova, T.; Slabakova, V. The Black Sea IOPs based on SeaWiFS data. In Proceedings of the 22nd International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics, Tomsk, Russia, 30 June–3 July 2016; p. 1003531. [Google Scholar]
- Bricaud, A.; Morel, A.; Babin, M.; Allali, K.; Claustre, H. Variations of light absorption by suspended particles with chlorophyll a concentration in oceanic (case 1) waters: Analysis and implications for bio-optical models. J. Geophys. Res. Oceans 1998, 103, 31033–31044. [Google Scholar] [CrossRef]
- Churilova, T.Y.; Buchelnikova, V.A.; Efimova, T.V.; Moiseeva, N.A.; Skorokhod, E.Y. Spectral Bio-Optical Properties and Phytoplankton Productivity in the Black Sea Coastal Waters Near Sevastopol. In Proceedings of the XI All-Russian Conference with International Participation “Current Problems in Optics of Natural Waters”, St. Petersburg, Russia, 29 September–1 October 2021; pp. 88–93. [Google Scholar]
- Mobley, C.D.; Stramski, D.; Bissett, W.P.; Boss, E. Optical modeling of ocean waters: Is the Case 1–Case 2 classification still useful? Oceanography 2004, 17, 60–67. [Google Scholar] [CrossRef]
- Schofield, O.; Arnone, R.A.; Bissett, W.P.; Dickey, T.D.; Davis, C.O.; Finkel, Z.; Oliver, M.; Moline, M.A. Watercolors in the coastal zone: What can we see? Oceanography 2004, 17, 24–31. [Google Scholar] [CrossRef]










| λ | A(λ) | B(λ) | λ | A(λ) | B(λ) | λ | A(λ) | B(λ) | λ | A(λ) | B(λ) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 400 | 0.0367 | 0.7747 | 476 | 0.0438 | 0.9775 | 551 | 0.0076 | 0.8966 | 626 | 0.0071 | 0.8567 |
| 401 | 0.0371 | 0.7780 | 477 | 0.0431 | 0.9819 | 552 | 0.0073 | 0.8947 | 627 | 0.0072 | 0.8585 |
| 402 | 0.0376 | 0.7868 | 478 | 0.0424 | 0.9839 | 553 | 0.0071 | 0.8853 | 628 | 0.0073 | 0.8547 |
| 403 | 0.0381 | 0.7870 | 479 | 0.0415 | 0.9858 | 554 | 0.0069 | 0.8797 | 629 | 0.0074 | 0.8627 |
| 404 | 0.0388 | 0.7940 | 480 | 0.0408 | 0.9888 | 555 | 0.0067 | 0.8826 | 630 | 0.0075 | 0.8632 |
| 405 | 0.0393 | 0.7963 | 481 | 0.0401 | 0.9912 | 556 | 0.0065 | 0.8801 | 631 | 0.0076 | 0.8660 |
| 406 | 0.0400 | 0.8015 | 482 | 0.0394 | 0.9954 | 557 | 0.0063 | 0.8732 | 632 | 0.0077 | 0.8656 |
| 407 | 0.0406 | 0.8089 | 483 | 0.0386 | 0.9954 | 558 | 0.0062 | 0.8647 | 633 | 0.0078 | 0.8718 |
| 408 | 0.0414 | 0.8092 | 484 | 0.0379 | 0.9956 | 559 | 0.0060 | 0.8642 | 634 | 0.0079 | 0.8710 |
| 409 | 0.0421 | 0.8119 | 485 | 0.0371 | 0.9980 | 560 | 0.0059 | 0.8559 | 635 | 0.0080 | 0.8731 |
| 410 | 0.0428 | 0.8133 | 486 | 0.0363 | 0.9995 | 561 | 0.0057 | 0.8523 | 636 | 0.0081 | 0.8775 |
| 411 | 0.0434 | 0.8172 | 487 | 0.0356 | 0.9992 | 562 | 0.0056 | 0.8472 | 637 | 0.0082 | 0.8810 |
| 412 | 0.0438 | 0.8207 | 488 | 0.0348 | 0.9981 | 563 | 0.0055 | 0.8503 | 638 | 0.0083 | 0.8869 |
| 413 | 0.0445 | 0.8207 | 489 | 0.0340 | 0.9978 | 564 | 0.0054 | 0.8543 | 639 | 0.0083 | 0.8876 |
| 414 | 0.0449 | 0.8232 | 490 | 0.0333 | 0.9981 | 565 | 0.0054 | 0.8436 | 640 | 0.0084 | 0.8948 |
| 415 | 0.0453 | 0.8267 | 491 | 0.0325 | 0.9961 | 566 | 0.0053 | 0.8409 | 641 | 0.0084 | 0.8971 |
| 416 | 0.0458 | 0.8269 | 492 | 0.0317 | 0.9956 | 567 | 0.0053 | 0.8455 | 642 | 0.0084 | 0.9046 |
| 417 | 0.0460 | 0.8279 | 493 | 0.0309 | 0.9934 | 568 | 0.0053 | 0.8466 | 643 | 0.0084 | 0.9000 |
| 418 | 0.0464 | 0.8287 | 494 | 0.0302 | 0.9918 | 569 | 0.0053 | 0.8459 | 644 | 0.0084 | 0.9043 |
| 419 | 0.0466 | 0.8323 | 495 | 0.0294 | 0.9897 | 570 | 0.0053 | 0.8482 | 645 | 0.0084 | 0.9105 |
| 420 | 0.0469 | 0.8320 | 496 | 0.0286 | 0.9844 | 571 | 0.0053 | 0.8459 | 646 | 0.0084 | 0.9133 |
| 421 | 0.0472 | 0.8328 | 497 | 0.0279 | 0.9845 | 572 | 0.0053 | 0.8500 | 647 | 0.0084 | 0.9097 |
| 422 | 0.0471 | 0.8271 | 498 | 0.0272 | 0.9823 | 573 | 0.0054 | 0.8485 | 648 | 0.0084 | 0.9082 |
| 423 | 0.0476 | 0.8329 | 499 | 0.0265 | 0.9818 | 574 | 0.0054 | 0.8499 | 649 | 0.0084 | 0.9040 |
| 424 | 0.0479 | 0.8412 | 500 | 0.0258 | 0.9776 | 575 | 0.0055 | 0.8530 | 650 | 0.0085 | 0.8998 |
| 425 | 0.0484 | 0.8402 | 501 | 0.0251 | 0.9776 | 576 | 0.0056 | 0.8544 | 651 | 0.0085 | 0.8943 |
| 426 | 0.0488 | 0.8388 | 502 | 0.0245 | 0.9769 | 577 | 0.0056 | 0.8584 | 652 | 0.0087 | 0.8917 |
| 427 | 0.0495 | 0.8435 | 503 | 0.0239 | 0.9755 | 578 | 0.0057 | 0.8525 | 653 | 0.0088 | 0.8846 |
| 428 | 0.0500 | 0.8442 | 504 | 0.0233 | 0.9716 | 579 | 0.0058 | 0.8576 | 654 | 0.0090 | 0.8799 |
| 429 | 0.0507 | 0.8425 | 505 | 0.0227 | 0.9724 | 580 | 0.0059 | 0.8662 | 655 | 0.0093 | 0.8648 |
| 430 | 0.0513 | 0.8438 | 506 | 0.0222 | 0.9685 | 581 | 0.0060 | 0.8667 | 656 | 0.0097 | 0.8572 |
| 431 | 0.0521 | 0.8480 | 507 | 0.0216 | 0.9711 | 582 | 0.0061 | 0.8673 | 657 | 0.0102 | 0.8560 |
| 432 | 0.0528 | 0.8531 | 508 | 0.0212 | 0.9707 | 583 | 0.0062 | 0.8735 | 658 | 0.0107 | 0.8520 |
| 433 | 0.0535 | 0.8528 | 509 | 0.0207 | 0.9700 | 584 | 0.0063 | 0.8772 | 659 | 0.0114 | 0.8436 |
| 434 | 0.0542 | 0.8502 | 510 | 0.0202 | 0.9691 | 585 | 0.0064 | 0.8840 | 660 | 0.0122 | 0.8432 |
| 435 | 0.0546 | 0.8578 | 511 | 0.0198 | 0.9699 | 586 | 0.0065 | 0.8843 | 661 | 0.0130 | 0.8420 |
| 436 | 0.0550 | 0.8545 | 512 | 0.0193 | 0.9728 | 587 | 0.0066 | 0.8938 | 662 | 0.0140 | 0.8446 |
| 437 | 0.0553 | 0.8598 | 513 | 0.0189 | 0.9744 | 588 | 0.0066 | 0.8961 | 663 | 0.0151 | 0.8465 |
| 438 | 0.0555 | 0.8574 | 514 | 0.0185 | 0.9759 | 589 | 0.0067 | 0.9013 | 664 | 0.0162 | 0.8506 |
| 439 | 0.0555 | 0.8620 | 515 | 0.0181 | 0.9772 | 590 | 0.0067 | 0.9032 | 665 | 0.0174 | 0.8577 |
| 440 | 0.0552 | 0.8653 | 516 | 0.0177 | 0.9732 | 591 | 0.0067 | 0.9058 | 666 | 0.0185 | 0.8638 |
| 441 | 0.0551 | 0.8650 | 517 | 0.0173 | 0.9774 | 592 | 0.0067 | 0.9077 | 667 | 0.0197 | 0.8701 |
| 442 | 0.0546 | 0.8698 | 518 | 0.0170 | 0.9765 | 593 | 0.0067 | 0.9102 | 668 | 0.0208 | 0.8719 |
| 443 | 0.0541 | 0.8725 | 519 | 0.0166 | 0.9785 | 594 | 0.0067 | 0.9128 | 669 | 0.0218 | 0.8825 |
| 444 | 0.0537 | 0.8730 | 520 | 0.0163 | 0.9782 | 595 | 0.0066 | 0.9138 | 670 | 0.0228 | 0.8832 |
| 445 | 0.0530 | 0.8775 | 521 | 0.0159 | 0.9765 | 596 | 0.0065 | 0.9079 | 671 | 0.0235 | 0.8887 |
| 446 | 0.0525 | 0.8846 | 522 | 0.0156 | 0.9752 | 597 | 0.0064 | 0.9076 | 672 | 0.0241 | 0.8919 |
| 447 | 0.0519 | 0.8864 | 523 | 0.0153 | 0.9769 | 598 | 0.0064 | 0.9110 | 673 | 0.0245 | 0.8962 |
| 448 | 0.0516 | 0.8941 | 524 | 0.0149 | 0.9766 | 599 | 0.0063 | 0.9049 | 674 | 0.0246 | 0.9013 |
| 449 | 0.0511 | 0.8959 | 525 | 0.0146 | 0.9789 | 600 | 0.0062 | 0.9035 | 675 | 0.0245 | 0.8996 |
| 450 | 0.0507 | 0.9013 | 526 | 0.0143 | 0.9765 | 601 | 0.0061 | 0.9048 | 676 | 0.0242 | 0.9006 |
| 451 | 0.0504 | 0.9027 | 527 | 0.0140 | 0.9760 | 602 | 0.0061 | 0.8983 | 677 | 0.0237 | 0.9005 |
| 452 | 0.0502 | 0.9108 | 528 | 0.0137 | 0.9732 | 603 | 0.0060 | 0.8945 | 678 | 0.0229 | 0.9029 |
| 453 | 0.0501 | 0.9129 | 529 | 0.0133 | 0.9733 | 604 | 0.0060 | 0.8897 | 679 | 0.0220 | 0.9012 |
| 454 | 0.0499 | 0.9121 | 530 | 0.0131 | 0.9678 | 605 | 0.0059 | 0.8851 | 680 | 0.0207 | 0.9007 |
| 455 | 0.0499 | 0.9204 | 531 | 0.0127 | 0.9635 | 606 | 0.0059 | 0.8809 | 681 | 0.0194 | 0.8911 |
| 456 | 0.0500 | 0.9228 | 532 | 0.0124 | 0.9595 | 607 | 0.0059 | 0.8815 | 682 | 0.0179 | 0.8855 |
| 457 | 0.0499 | 0.9223 | 533 | 0.0121 | 0.9621 | 608 | 0.0060 | 0.8784 | 683 | 0.0163 | 0.8736 |
| 458 | 0.0500 | 0.9254 | 534 | 0.0118 | 0.9572 | 609 | 0.0060 | 0.8784 | 684 | 0.0147 | 0.8636 |
| 459 | 0.0500 | 0.9283 | 535 | 0.0115 | 0.9564 | 610 | 0.0060 | 0.8793 | 685 | 0.0131 | 0.8527 |
| 460 | 0.0500 | 0.9320 | 536 | 0.0113 | 0.9524 | 611 | 0.0061 | 0.8673 | 686 | 0.0116 | 0.8395 |
| 461 | 0.0500 | 0.9335 | 537 | 0.0110 | 0.9481 | 612 | 0.0062 | 0.8686 | 687 | 0.0102 | 0.8256 |
| 462 | 0.0498 | 0.9378 | 538 | 0.0107 | 0.9470 | 613 | 0.0063 | 0.8636 | 688 | 0.0089 | 0.8169 |
| 463 | 0.0498 | 0.9384 | 539 | 0.0105 | 0.9399 | 614 | 0.0063 | 0.8591 | 689 | 0.0078 | 0.8001 |
| 464 | 0.0497 | 0.9392 | 540 | 0.0102 | 0.9353 | 615 | 0.0064 | 0.8621 | 690 | 0.0067 | 0.7887 |
| 465 | 0.0495 | 0.9431 | 541 | 0.0099 | 0.9324 | 616 | 0.0065 | 0.8588 | 691 | 0.0058 | 0.7776 |
| 466 | 0.0492 | 0.9444 | 542 | 0.0097 | 0.9297 | 617 | 0.0065 | 0.8599 | 692 | 0.0050 | 0.7628 |
| 467 | 0.0489 | 0.9464 | 543 | 0.0094 | 0.9289 | 618 | 0.0066 | 0.8585 | 693 | 0.0043 | 0.7521 |
| 468 | 0.0485 | 0.9512 | 544 | 0.0092 | 0.9258 | 619 | 0.0067 | 0.8611 | 694 | 0.0038 | 0.7460 |
| 469 | 0.0481 | 0.9522 | 545 | 0.0090 | 0.9134 | 620 | 0.0068 | 0.8602 | 695 | 0.0033 | 0.7305 |
| 470 | 0.0476 | 0.9590 | 546 | 0.0087 | 0.9146 | 621 | 0.0068 | 0.8579 | 696 | 0.0029 | 0.7268 |
| 471 | 0.0470 | 0.9585 | 547 | 0.0085 | 0.9094 | 622 | 0.0069 | 0.8552 | 697 | 0.0026 | 0.7201 |
| 472 | 0.0465 | 0.9634 | 548 | 0.0083 | 0.9068 | 623 | 0.0069 | 0.8584 | 698 | 0.0023 | 0.7087 |
| 473 | 0.0458 | 0.9665 | 549 | 0.0080 | 0.9003 | 624 | 0.0070 | 0.8563 | 699 | 0.0020 | 0.6984 |
| 474 | 0.0452 | 0.9703 | 550 | 0.0078 | 0.8976 | 625 | 0.0071 | 0.8534 | 700 | 0.0018 | 0.6933 |
| 475 | 0.0445 | 0.9739 |
| λ | A(λ) | B(λ) | λ | A(λ) | B(λ) | λ | A(λ) | B(λ) | λ | A(λ) | B(λ) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 400 | 0.0311 | 0.9975 | 476 | 0.0374 | 0.8571 | 551 | 0.0085 | 0.9651 | 626 | 0.0071 | 1.0237 |
| 401 | 0.0315 | 0.9956 | 477 | 0.0369 | 0.8568 | 552 | 0.0082 | 0.9707 | 627 | 0.0073 | 1.0166 |
| 402 | 0.0321 | 0.9877 | 478 | 0.0365 | 0.8503 | 553 | 0.0080 | 0.9799 | 628 | 0.0073 | 1.0143 |
| 403 | 0.0325 | 0.9881 | 479 | 0.0360 | 0.8481 | 554 | 0.0077 | 0.9857 | 629 | 0.0074 | 1.0114 |
| 404 | 0.0329 | 0.9853 | 480 | 0.0355 | 0.8440 | 555 | 0.0075 | 0.9894 | 630 | 0.0075 | 1.0078 |
| 405 | 0.0335 | 0.9805 | 481 | 0.0350 | 0.8414 | 556 | 0.0073 | 0.9966 | 631 | 0.0077 | 1.0011 |
| 406 | 0.0341 | 0.9755 | 482 | 0.0345 | 0.8367 | 557 | 0.0071 | 1.0016 | 632 | 0.0077 | 0.9992 |
| 407 | 0.0346 | 0.9737 | 483 | 0.0340 | 0.8348 | 558 | 0.0069 | 1.0079 | 633 | 0.0078 | 0.9933 |
| 408 | 0.0352 | 0.9702 | 484 | 0.0335 | 0.8312 | 559 | 0.0066 | 1.0208 | 634 | 0.0080 | 0.9868 |
| 409 | 0.0357 | 0.9677 | 485 | 0.0330 | 0.8283 | 560 | 0.0064 | 1.0277 | 635 | 0.0080 | 0.9870 |
| 410 | 0.0362 | 0.9632 | 486 | 0.0324 | 0.8271 | 561 | 0.0062 | 1.0370 | 636 | 0.0081 | 0.9791 |
| 411 | 0.0367 | 0.9595 | 487 | 0.0318 | 0.8242 | 562 | 0.0061 | 1.0440 | 637 | 0.0082 | 0.9741 |
| 412 | 0.0371 | 0.9578 | 488 | 0.0313 | 0.8216 | 563 | 0.0060 | 1.0493 | 638 | 0.0082 | 0.9697 |
| 413 | 0.0375 | 0.9541 | 489 | 0.0307 | 0.8211 | 564 | 0.0058 | 1.0588 | 639 | 0.0083 | 0.9628 |
| 414 | 0.0378 | 0.9529 | 490 | 0.0301 | 0.8197 | 565 | 0.0057 | 1.0618 | 640 | 0.0083 | 0.9575 |
| 415 | 0.0382 | 0.9480 | 491 | 0.0295 | 0.8184 | 566 | 0.0056 | 1.0672 | 641 | 0.0084 | 0.9497 |
| 416 | 0.0384 | 0.9486 | 492 | 0.0290 | 0.8193 | 567 | 0.0056 | 1.0722 | 642 | 0.0084 | 0.9458 |
| 417 | 0.0387 | 0.9455 | 493 | 0.0284 | 0.8193 | 568 | 0.0055 | 1.0775 | 643 | 0.0083 | 0.9421 |
| 418 | 0.0389 | 0.9437 | 494 | 0.0278 | 0.8203 | 569 | 0.0055 | 1.0832 | 644 | 0.0084 | 0.9368 |
| 419 | 0.0390 | 0.9414 | 495 | 0.0272 | 0.8202 | 570 | 0.0054 | 1.0857 | 645 | 0.0084 | 0.9344 |
| 420 | 0.0392 | 0.9404 | 496 | 0.0266 | 0.8214 | 571 | 0.0054 | 1.0871 | 646 | 0.0083 | 0.9327 |
| 421 | 0.0394 | 0.9343 | 497 | 0.0260 | 0.8229 | 572 | 0.0054 | 1.0901 | 647 | 0.0083 | 0.9305 |
| 422 | 0.0398 | 0.9353 | 498 | 0.0255 | 0.8250 | 573 | 0.0055 | 1.0877 | 648 | 0.0083 | 0.9313 |
| 423 | 0.0398 | 0.9375 | 499 | 0.0250 | 0.8240 | 574 | 0.0055 | 1.0903 | 649 | 0.0083 | 0.9334 |
| 424 | 0.0401 | 0.9327 | 500 | 0.0244 | 0.8269 | 575 | 0.0055 | 1.0929 | 650 | 0.0084 | 0.9354 |
| 425 | 0.0403 | 0.9321 | 501 | 0.0239 | 0.8284 | 576 | 0.0056 | 1.0930 | 651 | 0.0085 | 0.9387 |
| 426 | 0.0407 | 0.9277 | 502 | 0.0234 | 0.8303 | 577 | 0.0056 | 1.0924 | 652 | 0.0086 | 0.9424 |
| 427 | 0.0410 | 0.9273 | 503 | 0.0230 | 0.8310 | 578 | 0.0057 | 1.0891 | 653 | 0.0088 | 0.9470 |
| 428 | 0.0413 | 0.9263 | 504 | 0.0225 | 0.8324 | 579 | 0.0058 | 1.0858 | 654 | 0.0090 | 0.9539 |
| 429 | 0.0417 | 0.9264 | 505 | 0.0220 | 0.8349 | 580 | 0.0059 | 1.0850 | 655 | 0.0093 | 0.9575 |
| 430 | 0.0422 | 0.9234 | 506 | 0.0216 | 0.8360 | 581 | 0.0059 | 1.0842 | 656 | 0.0096 | 0.9611 |
| 431 | 0.0426 | 0.9214 | 507 | 0.0212 | 0.8372 | 582 | 0.0061 | 1.0810 | 657 | 0.0101 | 0.9635 |
| 432 | 0.0434 | 0.9141 | 508 | 0.0208 | 0.8383 | 583 | 0.0062 | 1.0776 | 658 | 0.0107 | 0.9635 |
| 433 | 0.0437 | 0.9155 | 509 | 0.0204 | 0.8411 | 584 | 0.0063 | 1.0727 | 659 | 0.0113 | 0.9639 |
| 434 | 0.0441 | 0.9131 | 510 | 0.0201 | 0.8424 | 585 | 0.0063 | 1.0714 | 660 | 0.0120 | 0.9619 |
| 435 | 0.0445 | 0.9080 | 511 | 0.0197 | 0.8426 | 586 | 0.0064 | 1.0673 | 661 | 0.0128 | 0.9563 |
| 436 | 0.0445 | 0.9150 | 512 | 0.0194 | 0.8425 | 587 | 0.0065 | 1.0628 | 662 | 0.0137 | 0.9513 |
| 437 | 0.0449 | 0.9088 | 513 | 0.0191 | 0.8451 | 588 | 0.0066 | 1.0605 | 663 | 0.0147 | 0.9435 |
| 438 | 0.0448 | 0.9109 | 514 | 0.0188 | 0.8455 | 589 | 0.0066 | 1.0577 | 664 | 0.0158 | 0.9362 |
| 439 | 0.0450 | 0.9070 | 515 | 0.0184 | 0.8469 | 590 | 0.0067 | 1.0537 | 665 | 0.0168 | 0.9283 |
| 440 | 0.0447 | 0.9078 | 516 | 0.0181 | 0.8476 | 591 | 0.0067 | 1.0512 | 666 | 0.0179 | 0.9219 |
| 441 | 0.0446 | 0.9080 | 517 | 0.0178 | 0.8494 | 592 | 0.0067 | 1.0491 | 667 | 0.0190 | 0.9122 |
| 442 | 0.0443 | 0.9068 | 518 | 0.0175 | 0.8509 | 593 | 0.0067 | 1.0467 | 668 | 0.0200 | 0.9054 |
| 443 | 0.0439 | 0.9084 | 519 | 0.0172 | 0.8519 | 594 | 0.0067 | 1.0476 | 669 | 0.0209 | 0.8975 |
| 444 | 0.0435 | 0.9097 | 520 | 0.0170 | 0.8521 | 595 | 0.0066 | 1.0453 | 670 | 0.0216 | 0.8920 |
| 445 | 0.0431 | 0.9112 | 521 | 0.0167 | 0.8542 | 596 | 0.0065 | 1.0474 | 671 | 0.0223 | 0.8878 |
| 446 | 0.0427 | 0.9094 | 522 | 0.0164 | 0.8550 | 597 | 0.0065 | 1.0467 | 672 | 0.0227 | 0.8840 |
| 447 | 0.0423 | 0.9104 | 523 | 0.0161 | 0.8584 | 598 | 0.0064 | 1.0486 | 673 | 0.0231 | 0.8785 |
| 448 | 0.0419 | 0.9114 | 524 | 0.0158 | 0.8602 | 599 | 0.0063 | 1.0499 | 674 | 0.0231 | 0.8784 |
| 449 | 0.0414 | 0.9147 | 525 | 0.0156 | 0.8610 | 600 | 0.0062 | 1.0501 | 675 | 0.0230 | 0.8753 |
| 450 | 0.0411 | 0.9151 | 526 | 0.0153 | 0.8641 | 601 | 0.0061 | 1.0533 | 676 | 0.0227 | 0.8743 |
| 451 | 0.0409 | 0.9150 | 527 | 0.0150 | 0.8663 | 602 | 0.0061 | 1.0526 | 677 | 0.0222 | 0.8740 |
| 452 | 0.0407 | 0.9154 | 528 | 0.0147 | 0.8685 | 603 | 0.0061 | 1.0541 | 678 | 0.0215 | 0.8751 |
| 453 | 0.0406 | 0.9157 | 529 | 0.0144 | 0.8718 | 604 | 0.0060 | 1.0545 | 679 | 0.0207 | 0.8757 |
| 454 | 0.0405 | 0.9173 | 530 | 0.0141 | 0.8739 | 605 | 0.0060 | 1.0545 | 680 | 0.0196 | 0.8810 |
| 455 | 0.0406 | 0.9129 | 531 | 0.0138 | 0.8790 | 606 | 0.0060 | 1.0533 | 681 | 0.0184 | 0.8838 |
| 456 | 0.0404 | 0.9172 | 532 | 0.0136 | 0.8802 | 607 | 0.0060 | 1.0530 | 682 | 0.0171 | 0.8921 |
| 457 | 0.0405 | 0.9132 | 533 | 0.0133 | 0.8839 | 608 | 0.0061 | 1.0536 | 683 | 0.0158 | 0.8991 |
| 458 | 0.0405 | 0.9124 | 534 | 0.0130 | 0.8891 | 609 | 0.0061 | 1.0533 | 684 | 0.0143 | 0.9083 |
| 459 | 0.0405 | 0.9136 | 535 | 0.0127 | 0.8926 | 610 | 0.0062 | 1.0497 | 685 | 0.0130 | 0.9178 |
| 460 | 0.0405 | 0.9117 | 536 | 0.0124 | 0.8967 | 611 | 0.0062 | 1.0471 | 686 | 0.0116 | 0.9315 |
| 461 | 0.0406 | 0.9079 | 537 | 0.0122 | 0.8996 | 612 | 0.0063 | 1.0459 | 687 | 0.0103 | 0.9424 |
| 462 | 0.0406 | 0.9073 | 538 | 0.0119 | 0.9041 | 613 | 0.0064 | 1.0443 | 688 | 0.0091 | 0.9572 |
| 463 | 0.0406 | 0.9039 | 539 | 0.0116 | 0.9096 | 614 | 0.0065 | 1.0408 | 689 | 0.0080 | 0.9731 |
| 464 | 0.0405 | 0.9025 | 540 | 0.0114 | 0.9128 | 615 | 0.0065 | 1.0388 | 690 | 0.0070 | 0.9898 |
| 465 | 0.0405 | 0.8966 | 541 | 0.0111 | 0.9178 | 616 | 0.0066 | 1.0398 | 691 | 0.0061 | 1.0093 |
| 466 | 0.0403 | 0.8964 | 542 | 0.0108 | 0.9224 | 617 | 0.0067 | 1.0364 | 692 | 0.0053 | 1.0270 |
| 467 | 0.0402 | 0.8915 | 543 | 0.0105 | 0.9277 | 618 | 0.0067 | 1.0337 | 693 | 0.0046 | 1.0506 |
| 468 | 0.0400 | 0.8886 | 544 | 0.0103 | 0.9301 | 619 | 0.0068 | 1.0345 | 694 | 0.0040 | 1.0750 |
| 469 | 0.0398 | 0.8845 | 545 | 0.0100 | 0.9367 | 620 | 0.0069 | 1.0287 | 695 | 0.0035 | 1.0980 |
| 470 | 0.0396 | 0.8821 | 546 | 0.0098 | 0.9422 | 621 | 0.0069 | 1.0302 | 696 | 0.0031 | 1.1203 |
| 471 | 0.0393 | 0.8772 | 547 | 0.0095 | 0.9451 | 622 | 0.0069 | 1.0289 | 697 | 0.0027 | 1.1443 |
| 472 | 0.0390 | 0.8728 | 548 | 0.0092 | 0.9505 | 623 | 0.0070 | 1.0299 | 698 | 0.0024 | 1.1767 |
| 473 | 0.0386 | 0.8696 | 549 | 0.0090 | 0.9524 | 624 | 0.0070 | 1.0254 | 699 | 0.0022 | 1.1987 |
| 474 | 0.0382 | 0.8664 | 550 | 0.0087 | 0.9594 | 625 | 0.0071 | 1.0226 | 700 | 0.0019 | 1.2246 |
| 475 | 0.0378 | 0.8622 |
| Statistical Metric | MODIS | VIIRS | OLCI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| chlor_a | chl_ocx | aph_443 | adg_443 | chlor_a | chl_ocx | aph_443 | adg_443 | chlor_a | aph_443 | adg_443 | |
| n | 22 | 22 | 22 | 16 | 21 | 21 | 21 | 21 | 20 | 20 | 20 |
| Bias | 1.0 | 1.0 | 1.4 | 0.20 | 0.66 | 0.74 | 0.58 | 0.34 | 0.88 | 0.89 | 0.33 |
| MAE | 1.5 | 1.5 | 2.0 | 5.0 | 2.2 | 2.0 | 2.7 | 3.0 | 1.6 | 2.0 | 3.1 |
| MdAD | 1.3 | 1.3 | 1.5 | 5.7 | 2.0 | 2.1 | 2.4 | 2.6 | 1.5 | 1.8 | 2.4 |
| RMSE | 0.20 | 0.20 | 0.03 | 0.09 | 0.48 | 0.45 | 0.03 | 0.07 | 0.26 | 0.02 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimova, T.; Churilova, T.; Skorokhod, E.; Suslin, V.; Buchelnikov, A.S.; Glukhovets, D.; Khrapko, A.; Moiseeva, N. Light Absorption by Optically Active Components in the Arctic Region (August 2020) and the Possibility of Application to Satellite Products for Water Quality Assessment. Remote Sens. 2023, 15, 4346. https://doi.org/10.3390/rs15174346
Efimova T, Churilova T, Skorokhod E, Suslin V, Buchelnikov AS, Glukhovets D, Khrapko A, Moiseeva N. Light Absorption by Optically Active Components in the Arctic Region (August 2020) and the Possibility of Application to Satellite Products for Water Quality Assessment. Remote Sensing. 2023; 15(17):4346. https://doi.org/10.3390/rs15174346
Chicago/Turabian StyleEfimova, Tatiana, Tatiana Churilova, Elena Skorokhod, Vyacheslav Suslin, Anatoly S. Buchelnikov, Dmitry Glukhovets, Aleksandr Khrapko, and Natalia Moiseeva. 2023. "Light Absorption by Optically Active Components in the Arctic Region (August 2020) and the Possibility of Application to Satellite Products for Water Quality Assessment" Remote Sensing 15, no. 17: 4346. https://doi.org/10.3390/rs15174346