Monitoring Spartina alterniflora Expansion Mode and Dieback Using Multisource High-Resolution Imagery in Yancheng Coastal Wetland, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Preprocessing and DATA acquisition
2.3. Methodologies
2.3.1. Methods for Classifying S. alterniflora and Evaluating Accuracy
- (1)
- Multiscale optimal segmentation
- (2)
- Random Forest Classification Based on Objects
2.3.2. Pattern Recognition in S. alterniflora Expansion
2.3.3. Correlation Analysis between Dieback of S. alterniflora and Main Driving Factors
3. Results
3.1. Accuracy Evaluation of S. alterniflora Maps
3.2. Area and Distribution of S. alterniflora from 2010 to 2020
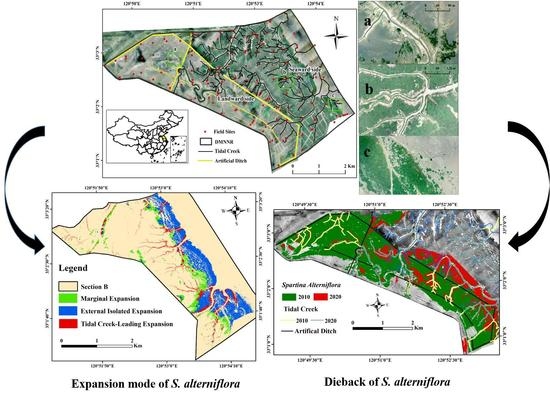
3.3. Expansion mode of S. alterniflora from 2010 to 2020
3.4. Dieback Dynamics of S. alterniflora and Main-Driving-Factors Correlation Analysis
4. Discussion
4.1. Multisource High-Resolution Imagery’s Potential and Reliability in Monitoring S. alterniflora Invasion
4.2. Expansion Mode of S. alterniflora on the Seaward between 2010 and 2020
4.3. The Relationship between Spartina Saltmarsh Dieback and Main Driving Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Li, H.; Li, L.; Man, W.; Jia, M.; Wang, Z.; Lu, C. Monitoring the Invasion of Spartina alterniflora Using Multi-source High-resolution Imagery in the Zhangjiang Estuary, China. Remote Sens. 2017, 9, 539. [Google Scholar] [CrossRef] [Green Version]
- Luan, Z.; Yan, D.; Xue, Y.; Shi, D.; Xu, D.; Liu, B.; Wang, L.; An, Y. Research progress on the ecohydrological mechanisms of Spartina alterniflora invasion in coastal wetlands. J. Agric. Resour. Environ. 2020, 37, 469–476. [Google Scholar] [CrossRef]
- Yan, D.; Li, J.; Yao, X.; Luan, Z. Quantifying the Long-Term Expansion and Dieback of Spartina Alterniflora Using Google Earth Engine and Object-Based Hierarchical Random Forest Classification. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 9781–9793. [Google Scholar] [CrossRef]
- Liu, M. Remote Sensing Analysis of Spartina Alterniflora in the Coastal Areas of China during 1990 to 2015. Ph.D. Thesis, Chinese Academy of Sciences (Northeast Institute of Geography and Agroecology), Changchun, China, 2018. [Google Scholar]
- Liu, X. Spatial Pattern and Changes of Spartina Alterniflora with Different Invasion Ages in Yancheng Coastal Wetlands. Ph.D. Thesis, Nanjing Normal University, Nanjing, China, 2018. [Google Scholar]
- Yan, D.; Li, J.; Yao, X.; Luan, Z. Integrating UAV data for assessing the ecological response of Spartina alterniflora towards inundation and salinity gradients in coastal wetland. Sci. Total Environ. 2022, 814, 152631. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Qin, P.; Liu, J.; Zhou, H. The positive and negative effects of exotic Spartina alterniflora in China. Ecol. Eng. 2009, 35, 444–452. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Qin, P. Impacts of an alien species (Spartina alterniflora) on the macrobenthos community of Jiangsu coastal inter-tidal ecosystem. Ecol. Eng. 2009, 35, 521–528. [Google Scholar] [CrossRef]
- Mao, D.; Liu, M.; Wang, Z.; Li, L.; Man, W.; Jia, M.; Zhang, Y. Rapid Invasion of Spartina Alterniflora in the Coastal Zone of Mainland China: Spatiotemporal Patterns and Human Prevention. Sensors 2019, 19, 2308. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Chen, J.; Jing, C.; Ye, G.; Wu, J.; Huang, Z.; Zhou, C. Monitoring the Invasion of Spartina alterniflora from 1993 to 2014 with Landsat TM and SPOT 6 Satellite Data in Yueqing Bay, China. PLoS ONE 2015, 10, e0135538. [Google Scholar] [CrossRef] [Green Version]
- Grosholz, E.D.; Levin, L.A.; Tyler, A.C.; Neira, C. Changes in Community Structure and Ecosystem Function Following Spartina Alterniflora Invasion of Pacific Estuaries; University of California Press: Oakland, CA, USA, 2009. [Google Scholar]
- Zuo, P.; Zhao, S.; Liu, C.A.; Wang, C.; Liang, Y. Distribution of Spartina spp. along China’s coast. Ecol. Eng. 2012, 40, 160–166. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Gong, H.; Lin, Z.; Lv, S. Appling the One-Class Classification Method of Maxent to Detect an Invasive Plant Spartina alterniflora with Time-Series Analysis. Remote Sens. 2017, 9, 1120. [Google Scholar] [CrossRef] [Green Version]
- Nagendra, H.; Lucas, R.; Honrado, J.P.; Jongman, R.H.G.; Tarantino, C.; Adamo, M.; Mairota, P. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 2013, 33, 45–59. [Google Scholar] [CrossRef]
- Liu, M.; Mao, D.; Wang, Z.; Li, L.; Man, W.; Jia, M.; Ren, C.; Zhang, Y. Rapid Invasion of Spartina alterniflora in the Coastal Zone of Mainland China: New Observations from Landsat OLI Images. Remote Sens. 2018, 10, 1933. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xiao, X.; Qin, Y.; Doughty, R.B.; Dong, J.; Zou, Z. Characterizing the encroachment of juniper forests into sub-humid and semi-arid prairies from 1984 to 2010 using PALSAR and Landsat data. Remote Sens. Environ. 2018, 205, 166–179. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, X.; Wang, X.; Xu, X.; Chen, B.; Wang, J.; Ma, J.; Zhao, B.; Li, B. Quantifying expansion and removal of Spartina alterniflora on Chongming island, China, using time series Landsat images during 1995–2018. Remote Sens. Environ. 2020, 247, 111916. [Google Scholar] [CrossRef]
- Fuller, D.O. Remote detection of invasive Melaleuca trees (Melaleuca quinquenervia) in South Florida with multispectral IKONOS imagery. Int. J. Remote Sens. 2006, 26, 1057–1063. [Google Scholar] [CrossRef]
- Walsh, S.J.; McCleary, A.L.; Mena, C.F.; Shao, Y.; Tuttle, J.P.; González, A.; Atkinson, R. QuickBird and Hyperion data analysis of an invasive plant species in the Galapagos Islands of Ecuador: Implications for control and land use management. Remote Sens. Environ. 2008, 112, 1927–1941. [Google Scholar] [CrossRef]
- Chen, M.; Ke, Y.; Bai, J.; Li, P.; Lyu, M.; Gong, Z.; Zhou, D. Monitoring early stage invasion of exotic Spartina alterniflora using deep-learning super-resolution techniques based on multisource high-resolution satellite imagery: A case study in the Yellow River Delta, China. Int. J. Appl. Earth Obs. Geoinf. 2020, 92, 102180. [Google Scholar] [CrossRef]
- Dai, W.; Li, H.; Gong, Z.; Zhou, Z.; Li, Y.; Wang, L.; Zhang, C.; Pei, H. Self-organization of salt marsh patches on mudflats: Field evidence using the UAV technique. Estuar. Coast. Shelf Sci. 2021, 262, 107608. [Google Scholar] [CrossRef]
- Gardner, R.H.; Urban, D.L. Neutral models for testing landscape hypotheses. Landsc. Ecol. 2007, 22, 15–29. [Google Scholar] [CrossRef]
- Laba, M.; Downs, R.; Smith, S.; Welsh, S.; Neider, C.; White, S.; Richmond, M.; Philpot, W.; Baveye, P. Mapping invasive wetland plants in the Hudson River National Estuarine Research Reserve using quickbird satellite imagery. Remote Sens. Environ. 2008, 112, 286–300. [Google Scholar] [CrossRef]
- Yan, D.; Li, J.; Xie, S.; Liu, Y.; Sheng, Y.; Luan, Z. Examining the expansion of Spartina alterniflora in coastal wetlands using an MCE-CA-Markov model. Front. Mar. Sci. 2022, 9, 964172. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Li, Y.; Liu, L.; Xie, F. Recognition of spatial expansion patterns of invasive Spartina alterniflora and simulation of the resulting landscape-changes. Acta Ecol. Sin. 2018, 38, 5413–5422. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, D.; Gong, H. A new landscape expansion index: Definition and quantification. Acta Ecol. Sin. 2012, 32, 4270–4277. [Google Scholar] [CrossRef] [Green Version]
- Ogburn, M.B.; Alber, M. An investigation of salt marsh dieback in Georgia using field transplants. Estuaries Coasts 2006, 29, 54–62. [Google Scholar] [CrossRef]
- Eom, J.; Choi, J.-K.; Ryu, J.-H.; Woo, H.J.; Won, J.-S.; Jang, S. Tidal channel distribution in relation to surface sedimentary facies based on remotely sensed data. Geosci. J. 2012, 16, 127–137. [Google Scholar] [CrossRef]
- Cui, B.; Cai, Y.; Xie, T.; Ning, Z.; Hua, Y. Ecological effects of wetland hydrological connectivity: Problems ang prospects. J. Beijing Norm. Univ. Nat. Sci. 2016, 52, 738–746. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Q.; Qiu, D.; Shi, W.; Ning, Z.; Cai, Y.; Song, Z.; Cui, B. Hydrological connectivity characteristics and ecological effects of a typical tidal channel system in the Yellow River Delta. J. Beijing Norm. Univ., Nat. Sci. 2018, 54, 17–24. [Google Scholar] [CrossRef]
- Ding, Y. Preference of Feeding on Spartina alterniflora loisel by Mi- deer in Dafeng National Nature Reserve. Chin. J. Wildl. 2009, 30, 118–120. [Google Scholar] [CrossRef]
- Hua, R.; Cui, D.; Liu, J.; Li, S.; Zhao, Y.; Zhang, Y. Winter Diets of Père David’s Deer in Yancheng Wetland, Jiangsu Province, China. Chin. J. Zool. 2020, 55, 1001. [Google Scholar] [CrossRef]
- Jia, Y.; Xie, S.; Yuan, H.; Lu, L. Effects of Wild Elk Activities on Some Ecological Indexes of Spartina alterniflora. J. Anhui Agric. Sci. 2022, 50, 63–65. [Google Scholar] [CrossRef]
- Yao, X.; Yan, D.; Li, J.; Liu, Y.; Sheng, Y.; Xie, S.; Luan, Z. Spatial Distribution of Soil Organic Carbon and Total Nitrogen in a Ramsar Wetland, Dafeng Milu National Nature Reserve. Water 2022, 14, 197. [Google Scholar] [CrossRef]
- Agisoft PhotoScan User Manual: Professional Edition, Version 1.1. Available online: https://docplayer.net/14282795-Agisoft-photoscan-user-manual-professional-edition-version-1-1.html (accessed on 10 May 2022).
- Blaschke, T. Object based image analysis for remote sensing. ISPRS J. Photogramm. Remote Sens. 2010, 65, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.D.; Chen, D. Segmentation for Object-Based Image Analysis (OBIA): A review of algorithms and challenges from remote sensing perspective. ISPRS J. Photogramm. Remote Sens. 2019, 150, 115–134. [Google Scholar] [CrossRef]
- Drǎguţ, L.; Tiede, D.; Levick, S.R. ESP: A tool to estimate scale parameter for multiresolution image segmentation of remotely sensed data. Int. J. Geogr. Inf. Sci. 2010, 24, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L.; Friedman, J.; Stone, C.J.; Olshen, R.A. Classification and Regresssion Trees; Routledge: New York, NY, USA, 1984. [Google Scholar]
- Sheykhmousa, M.; Mahdianpari, M.; Ghanbari, H.; Mohammadimanesh, F.; Ghamisi, P.; Homayouni, S. Support Vector Machine Versus Random Forest for Remote Sensing Image Classification: A Meta-Analysis and Systematic Review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 6308–6325. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Li, B.-L. Fractal geometry applications in description and analysis of patch patterns and patch dynamics. Ecol. Model. 2000, 132, 33–50. [Google Scholar] [CrossRef]
- Cleve, C.; Kelly, M.; Kearns, F.R.; Moritz, M. Classification of the wildland–urban interface: A comparison of pixel- and object-based classifications using high-resolution aerial photography. Comput. Environ. Urban Syst. 2008, 32, 317–326. [Google Scholar] [CrossRef]
- Müllerová, J.; Pergl, J.; Pyšek, P. Remote sensing as a tool for monitoring plant invasions: Testing the effects of data resolution and image classification approach on the detection of a model plant species Heracleum mantegazzianum (giant hogweed). Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 55–65. [Google Scholar] [CrossRef]
- Underwood, E.; Ustin, S.; DiPietro, D. Mapping nonnative plants using hyperspectral imagery. Remote Sens. Environ. 2003, 86, 150–161. [Google Scholar] [CrossRef]
- Mendelssohn, I.A.; Postek, M.T. Elemental analysis of deposits on the roots of spartina alterniflora loisel. Am. J. Bot. 1982, 69, 904–912. [Google Scholar] [CrossRef]
- Hou, M.; Liu, H.; Zhang, H. Effection of tidal creek system on the expansion of the invasive Spartina in the coastal wetland of Yancheng. Acta Ecol. Sin. 2014, 34, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, X.; Chen, Y.; Tan, Z.; Li, S.; Ai, B. A new landscape index for quantifying urban expansion using multi-temporal remotely sensed data. Landsc. Ecol. 2010, 25, 671–682. [Google Scholar] [CrossRef]
- Vandenbruwaene, W.; Meire, P.; Temmerman, S. Formation and evolution of a tidal channel network within a constructed tidal marsh. Geomorphology 2012, 151–152, 114–125. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, D.; Ke, Y.; Wang, Y.; Wang, Q.; Zhang, L. Quantifying the Correlated Spatial Distributions between Tidal Creeks and Coastal Wetland Vegetation in the Yellow River Estuary. Wetlands 2020, 40, 2701–2711. [Google Scholar] [CrossRef]
- Gong, Z.; Mou, K.; Wang, Q.; Qiu, H.; Zhang, C.; Zhou, D. Parameterizing the Yellow River Delta tidal creek morphology using automated extraction from remote sensing images. Sci. Total Environ. 2021, 769, 144572. [Google Scholar] [CrossRef]
- Lu, Z.; Xiao, K.; Wang, F.; Wang, Y.; Yu, Q.; Chen, N. Salt marsh invasion reduces recalcitrant organic carbon pool while increases lateral export of dissolved inorganic carbon in a subtropical mangrove wetland. Geoderma 2023, 437, 116573. [Google Scholar] [CrossRef]
- Noonan, J.M. Evaluating the Potential for Transfer of Heavy Metals Through Trophic Interactions in Spartina alterniflora and Littorina irrorata. Ph.D. Thesis, College of Charleston, Charleston, SC, USA, 2023. [Google Scholar]











| Date | Satellite Sensor | Band | Resolution (m) | Tide Level |
|---|---|---|---|---|
| 9 November 2010 | WorldView-2 | 3 | 0.5 | Low |
| 13 September 2013 | Pléiades 2 | 3 | 0.5 | Middle |
| 27 July 2016 | GaoFen-2 | 4 | 1 | Low |
| 23 December 2018 | Pléiades 2 | 3 | 0.5 | Low |
| 13 August 2020 | UAV Dabai II | 3 | 0.1 | Low |
| Feature Type | Definition or Description |
|---|---|
| Spectral feature | Mean value of all band; Brightness; Standard deviation of all band |
| Spectral index | Green-red ratio index (GRRI) = [41]; Normalized green-red difference index (NGRDI) = [42] |
| Shape feature | Area; Length; Shape index; Density; Compactness Length–width ratio |
| Texture feature | Gray-level co-occurrence matrix (GLCM); Homogeneity; GLCM Mean; GLCM Entropy; GLCM Contrast; GLCM Standard deviation; GLCM Correlation |
| Other | Neighbor distance; Object location |
| Year | Source | Class | PA | UA | OA | Kappa Coefficient |
|---|---|---|---|---|---|---|
| 2010 | WorldView-2 | Spartina | 0.96 | 0.98 | 0.95 | 0.76 |
| Non-Spartina | 0.88 | 0.82 | ||||
| 2013 | Pléiades-2 | Spartina | 0.93 | 0.97 | 0.93 | 0.82 |
| Non-Spartina | 0.92 | 0.82 | ||||
| 2016 | GaoFen-2 | Spartina | 0.96 | 0.97 | 0.95 | 0.85 |
| Non-Spartina | 0.90 | 0.86 | ||||
| 2018 | Pléiades-2 | Spartina | 0.95 | 0.96 | 0.93 | 0.78 |
| Non-Spartina | 0.84 | 0.80 | ||||
| 2020 | UAV Dabai II | Spartina | 0.96 | 0.97 | 0.97 | 0.86 |
| Non-Spartina | 0.91 | 0.87 |
| Year | Area (ha) | Stage | Change of Area (ha) | Annual Change Rate (%) |
|---|---|---|---|---|
| 2010 | 1511.26 | 2010–2013 | −3.02 | −0.07 |
| 2013 | 1508.24 | 2013–2016 | −0.26 | −0.006 |
| 2016 | 1507.97 | 2016–2018 | −162.98 | −5.40 |
| 2018 | 1344.99 | 2018–2020 | −434.75 | −16.16 |
| 2020 | 910.25 | 2010–2020 | −601.02 | −3.98 |
| Expansion Pattern | LEI Interval Distribution | Number of Patches | Proportion of Total Number/% | Area/ha |
|---|---|---|---|---|
| External Isolated Expansion | LEI = 1 and PFD < 1.4 | 7628 | 65.16% | 166.88 |
| Tidal-Creek-Leading Expansion | LEI = 1 and PFD ≥ 1.4 | 1243 | 10.62% | 41.32 |
| Marginal Expansion | LEI (−1,1) | 2835 | 24.22% | 71.27 |
| Year | Area (ha) | The Number of Tidal Creek | The Number of E. davidianus | The Length of the Artificial Ditch (km) |
|---|---|---|---|---|
| 2010 | 985.72 | 90 | 156 | 0 |
| 2013 | 905.50 | 74 | 215 | 2.31 |
| 2016 | 840.51 | 60 | 325 | 7.01 |
| 2018 | 711.20 | 18 | 905 | 17.41 |
| 2020 | 133.36 | 18 | 1820 | 17.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, D.; Luan, Z.; Li, J.; Xie, S.; Wang, Y. Monitoring Spartina alterniflora Expansion Mode and Dieback Using Multisource High-Resolution Imagery in Yancheng Coastal Wetland, China. Remote Sens. 2023, 15, 3853. https://doi.org/10.3390/rs15153853
Yan D, Luan Z, Li J, Xie S, Wang Y. Monitoring Spartina alterniflora Expansion Mode and Dieback Using Multisource High-Resolution Imagery in Yancheng Coastal Wetland, China. Remote Sensing. 2023; 15(15):3853. https://doi.org/10.3390/rs15153853
Chicago/Turabian StyleYan, Dandan, Zhaoqing Luan, Jingtai Li, Siying Xie, and Yu Wang. 2023. "Monitoring Spartina alterniflora Expansion Mode and Dieback Using Multisource High-Resolution Imagery in Yancheng Coastal Wetland, China" Remote Sensing 15, no. 15: 3853. https://doi.org/10.3390/rs15153853