Hydrological Drivers for the Spatial Distribution of Wetland Herbaceous Communities in Poyang Lake
Abstract
:1. Introduction
2. Materials and Methods
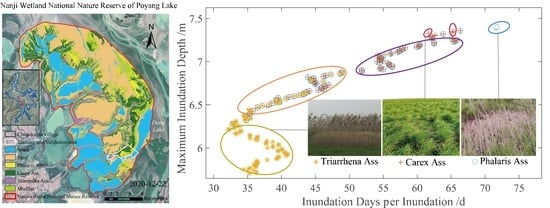
2.1. Poyang Lake and Nanji Wetland National Nature Reserve
2.2. Data Acquisition
2.2.1. Field Sampling Data
2.2.2. Remote Sensing Data
2.2.3. Hydrological and Topographic Data
2.3. Methodology
2.3.1. Support-Vector Classification
2.3.2. Evolutionary-Algorithm-Based SVM Parameter Optimization
- Start the search process based on the initial population. Each individual in the population contains a floating-point number (0 to 1) representing each input variable to indicate whether it participates in the model construction or not, and also prepares a floating-point number for each support-vector classification parameter;
- Apply genetic operators (i.e., selection, crossover, and mutation) to generate the offspring population;
- Evaluate each individual in the offspring population according to the following steps:
- (a)
- Divide the individual into two parts: one for variable indication information and the other for model parameters;
- (b)
- If the floating-point number is greater than 0.5, the corresponding variable is selected to build the model; otherwise, it is abandoned;
- (c)
- Train the support-vector classification model using n-fold cross-validation to avoid overfitting;
- (d)
- Use the cross-validation’s root-mean-square error as the fitness value of the individual;
- Check whether the generation has reached the maximum;
- If yes, end the search and return the best individual; otherwise, go back to Step (2).
2.3.3. Canonical Correspondence Analysis
3. Results
3.1. Wetland Plant Community Classification
3.2. Hydrological Drivers for the Spatial Distribution of Wetland Herbaceous Communities
4. Discussion
5. Conclusions
- (1)
- Support-vector classification with an input/parameter-synchronized optimization is beneficial for constructing an accurate wetland herbaceous vegetation classification model. The optimized model improved the classification accuracy by ~8% compared with the classic SVM.
- (2)
- Significant interspecific differences were found in terms of the hydrological niche. Carex Ass was the most adaptable to the duration of inundation, had the widest distribution range, and had a larger hydrological niche amplitude. Triarrhena Ass was the least capable and had the smallest hydrological niche amplitude. The main reasons for the interspecific differences were the different survival strategies of species in the face of inundation, such as dormancy and biological traits.
- (3)
- The analytical framework was successfully applied to identify key indicators characterizing plant communities’ distribution and quantifying the hydrological niches/optima of the communities in the Poyang Lake wetland.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Indices | Formula (Sentinel-2) | References |
|---|---|---|
| DVI-Difference Vegetation Index | [95] | |
| RVI-Ratio Vegetation Index | [96] | |
| NDVI- Normalized Difference Vegetation Index | [97] | |
| EVI—Enhanced Vegetation Index | [98] | |
| MSAVI—Modified Soil Adjusted Vegetation Index | [99] | |
| RDVI—Renormalized Difference Vegetation Index | [100] | |
| HJVI—Huan Jing Vegetation Index | [101] | |
| ARVI -Atmospherically Resistant Vegetation Index | [102] | |
| VDVI—Visible-band Difference Vegetation Index | [103] | |
| NGRDI—Normalized Green and Red Difference Vegetation Index | [104] | |
| NGBDI—Normalized Green and Bule Difference Vegetation Index | [105] | |
| NDVIre1—Normalized Difference Vegetation Index red-edge 1 | [106] | |
| NDVIre1n—Normalized Difference Vegetation Index red-edge 1 narrow | [107] | |
| NDVIre2—Normalized Difference Vegetation Index red-edge 2 | [106] | |
| NDVIre2n—Normalized Difference Vegetation Index red-edge 2 narrow | [107] | |
| NDVIre3—Normalized Difference Vegetation Index red-edge 3 | [106] | |
| NDVIre3n—Normalized Difference Vegetation Index red-edge 3 narrow | [107] | |
| PSRI—Plant Senescence Reflectance Index | [108] | |
| CIre—ChlorophyerII Index red-edge | [109] | |
| NDre1—Normalized Difference red-edge1 | [106] |
| Subject | Parameter | Value |
| Genetic Algorithm | Maximum number of function calls | 4000 |
| Normalized Geometric Selection | Selecting the best individual probability | 0.05 |
| Simple Crossover | - | - |
| Arithmetic Crossover | - | - |
| Heuristic Crossover | Number of retries | 10 |
| Uniform Variation | - | - |
| Non-Uniform Variation | Shape parameters | 3 |
| Multiple Non-Uniform Variation | Shape parameters | 3 |
| Boundary Variation | - | - |
| Search Boundaries | C lower boundary | 1 |
| C upper boundary | 1 × 1012 | |
| γ lower boundary | 1 × 10−2 | |
| γ upper boundary | 1 × 104 |
| Water | Sand | Phalaris Ass | Carex Ass | Triarrhena Ass | Mudflat | UA(%) | |
|---|---|---|---|---|---|---|---|
| Water | 3721/2724 | 21/30 | 0/0 | 0/0 | 0/0 | 0/0 | 99.44/99.20 |
| Sand | 13/22 | 2671/2789 | 0/0 | 0/0 | 0/0 | 2/10 | 99.44/98.87 |
| Phalaris Ass | 0/0 | 0/0 | 2516/2470 | 50/81 | 15/9 | 17/38 | 96.84/95.07 |
| Carex Ass | 0/0 | 0/0 | 70/69 | 5693/5806 | 4/3 | 4/7 | 98.65/98.66 |
| Triarrhena Ass | 0/0 | 0/0 | 20/27 | 0/2 | 1584/1637 | 0/0 | 98.75/98.26 |
| Mudflat | 0/0 | 12/15 | 20/38 | 1/2 | 0/0 | 2625/2788 | 98.76/98.07 |
| PA(%) | 99.65/99.41 | 98.78/98.41 | 95.81/94.85 | 99.11/98.56 | 98.81/99.27 | 99.13/98.07 | OA = 98.69/98.20 |

References
- Casanova, M.T.; Brock, M.A. How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? Plant Ecol. 2000, 147, 237–250. [Google Scholar] [CrossRef]
- Bunn, S.E.; Arthington, A.H. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Keddy, P.A. Wetland Ecology: Principles and Conservation, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Wilcox, D.A.; Nichols, S.J. The effects of water-level fluctuations on vegetation in a Lake Huron wetland. Wetlands 2008, 28, 487–501. [Google Scholar] [CrossRef]
- Todd, M.J.; Muneepeerakul, R.; Pumo, D.; Azaele, S.; Miralles-Wilhelm, F.; Rinaldo, A.; Rodriguez-Iturbe, I. Hydrological drivers of wetland vegetation community distribution within Everglades National Park, Florida. Adv. Water Resour. 2010, 33, 1279–1289. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, X.-C.; Werner, A.D.; Li, Y.-L.; Yao, J.; Li, X.-H.; Xu, C.-Y. An investigation of enhanced recessions in Poyang Lake: Comparison of Yangtze River and local catchment impacts. J. Hydrol. 2014, 517, 425–434. [Google Scholar] [CrossRef]
- Tang, X.; Li, H.; Xu, X.; Yang, G.; Liu, G.; Li, X.; Chen, D. Changing land use and its impact on the habitat suitability for wintering Anseriformes in China’s Poyang Lake region. Sci. Total Environ. 2016, 557, 296–306. [Google Scholar]
- Liang, D.; Lu, J.; Chen, X.; Liu, C.; Lin, J. An investigation of the hydrological influence on the distribution and transition of wetland cover in a complex lake–floodplain system using time-series remote sensing and hydrodynamic simulation. J. Hydrol. 2020, 587, 125038. [Google Scholar] [CrossRef]
- Wan, R.; Dai, X.; Shankman, D. Vegetation response to hydrological changes in Poyang Lake, China. Wetlands 2018, 39, 99–112. [Google Scholar] [CrossRef]
- Mei, X.; Dai, Z.; Fagherazzi, S.; Chen, J. Dramatic variations in emergent wetland area in China’s largest freshwater lake, Poyang Lake. Adv. Water Resour. 2016, 96, 1–10. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.; Feng, L. Four decades of winter wetland changes in Poyang Lake based on Landsat observations between 1973 and 2013. Remote Sens. Environ. 2015, 156, 426–437. [Google Scholar] [CrossRef]
- Mu, S.; Yang, G.; Xu, X.; Wan, R.; Li, B. Assessing the inundation dynamics and its impacts on habitat suitability in Poyang Lake based on integrating Landsat and MODIS observations. Sci. Total Environ. 2022, 834, 154936. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wan, R.; Yang, G.; Wang, X.; Xu, L. Responses of wetland vegetation in Poyang Lake, China to water-level fluctuations. Hydrobiologia 2016, 773, 35–47. [Google Scholar] [CrossRef]
- Xia, S.; Liu, Y.; Wang, Y.; Chen, B.; Jia, Y.; Liu, G.; Yu, X.; Wen, L. Wintering waterbirds in a large river floodplain: Hydrological connectivity is the key for reconciling development and conservation. Sci. Total Environ. 2016, 573, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Tamisier, A.; Grillas, P. A review of habitat changes in the Camargue: An assessment of the effects of the loss of biological diversity on the wintering waterfowl community. Biol. Conserv. 1994, 70, 39–47. [Google Scholar] [CrossRef]
- Wang, H.F.; Ren, M.X.; López-Pujol, J.; Ross Friedman, C.; Fraser, L.H.; Huang, G.X. Plant species and communities in Poyang Lake, the largest freshwater lake in China. Collect. Bot. 2015, 34, e004. [Google Scholar]
- Hu, Z.P.; Ge, G.; Liu, C.L.; Chen, F.S.; Li, S. Structure of Poyang Lake wetland plants ecosystem and influence of lake water level for the structure. Resour. Environ. Yangtze Basin 2010, 19, 597–605. [Google Scholar]
- Fan, H.; Xu, L.; Wang, X.; Jiang, J.; Feng, W.; You, H. Relationship between vegetation community distribution patterns and environmental factors in typical wetlands of Poyang Lake, China. Wetlands 2019, 39, 75–87. [Google Scholar] [CrossRef]
- Kumari, N.; Srivastava, A.; Kumar, S. Hydrological Analysis Using Observed and Satellite-Based Estimates: Case Study of a Lake Catchment in Raipur, India. J. Indian Soc. Remote Sens. 2022, 50, 115–128. [Google Scholar] [CrossRef]
- Mahdavi, S.; Salehi, B.; Granger, J.; Amani, M.; Brisco, B.; Huang, W. Remote sensing for wetland classification: A comprehensive review. GIScience Remote Sens. 2018, 55, 623–658. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Sheng, C.; Xu, J.; Wu, L. A Review of Wetland Remote Sensing. Multidiscip. Digit. Publ. Inst. 2017, 17, 777. [Google Scholar] [CrossRef]
- Dronova, I. Object-Based Image Analysis in Wetland Research: A Review. Remote Sens. 2015, 7, 6380–6413. [Google Scholar] [CrossRef]
- Huang, C.; Davis, L.S.; Townshend, J.R.G. An assessment of support vector machines for land cover classification. Int. J. Remote Sens. 2002, 23, 725–749. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, Z.; Johnston, C.A.; Hu, S. A Unifying Approach to Classifying Wetlands in the Ontonagon River Basin, Michigan, Using Multi_temporal Landsat_8 OLI Imagery. Can. J. Remote Sens. 2018, 44, 373–389. [Google Scholar] [CrossRef]
- Xu, P.; Niu, Z.; Tang, P. Comparison and assessment of NDVI time series for seasonal wetland classification. Int. J. Digit. Earth 2018, 11, 1103–1131. [Google Scholar] [CrossRef]
- Wang, L.; Dronova, I.; Gong, P.; Yang, W.; Li, Y.; Liu, Q. A new time series vegetation–water index of phenological–hydrological trait across species and functional types for Poyang Lake wetland ecosystem. Remote Sens. Environ. 2012, 125, 49–63. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Z. Object-based Vegetation Mapping in the Kissimmee River Watershed Using HyMap Data and Machine Learning Techniques. Wetlands 2013, 33, 233–244. [Google Scholar] [CrossRef]
- Zhiqiang, T.; Qi, Z.; Mengfan, L.; Yunliang, L.; Xiuli, X.; Jiahu, J. A study of the relationship between wetland vegetation communities and water regimes using a combined remote sensing and hydraulic modeling approach. Hydrol. Res. 2016, 47, 278–292. [Google Scholar] [CrossRef]
- Han, X.; Feng, L.; Hu, C.; Chen, X. Wetland changes of China’s largest freshwater lake and their linkage with the Three Gorges Dam. Remote Sens. Environ. 2018, 204, 799–811. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, L.; Hu, L.; Gong, P. Comparison of classification algorithms and training sample sizes in urban land classification with Landsat thematic mapper imagery. Remote Sens. 2014, 6, 964–983. [Google Scholar] [CrossRef]
- Amani, M.; Brisco, B.; Afshar, M.; Mirmazloumi, S.M.; Mahdavi, S.; Mirzadeh, S.M.J.; Huang, W.; Granger, J. A generalized supervised classification scheme to produce provincial wetland inventory maps: An application of Google Earth Engine for big geo data processing. Big Earth Data 2019, 3, 378–394. [Google Scholar] [CrossRef]
- Ghorbanian, A.; Kakooei, M.; Amani, M.; Mahdavi, S.; Mohammadzadeh, A.; Hasanlou, M. Improved land cover map of Iran using Sentinel imagery within Google Earth Engine and a novel automatic workflow for land cover classification using migrated training samples. ISPRS J. Photogramm. Remote Sens. 2020, 167, 276–288. [Google Scholar] [CrossRef]
- Parmuchi, M.G.; Karszenbaum, H.; Kandus, P. Mapping wetlands using multi-temporal RADARSAT-1 data and a decision-based classifier. Can. J. Remote Sens. 2002, 28, 175–186. [Google Scholar] [CrossRef]
- Slatton, K.C.; Crawford, M.M.; Chang, L.-D. Modeling temporal variations in multipolarized radar scattering from intertidal coastal wetlands. ISPRS J. Photogramm. Remote Sens. 2008, 63, 559–577. [Google Scholar] [CrossRef]
- Hu, T.; Mao, J.; Pan, S.; Dai, L.; Zhang, P.; Xu, D.; Dai, H. Water level management of lakes connected to regulated rivers: An integrated modeling and analytical methodology. J. Hydrol. 2018, 562, 796–808. [Google Scholar] [CrossRef]
- Guan, L.; Wen, L.; Feng, D.; Zhang, H.; Lei, G. Delayed flood recession in central Yangtze floodplains can cause significant food shortages for wintering geese: Results of inundation experiment. Environ. Manag. 2014, 54, 1331–1341. [Google Scholar] [CrossRef]
- Foti, R.; del Jesus, M.; Rinaldo, A.; Rodriguez-Iturbe, I. Hydroperiod regime controls the organization of plant species in wetlands. Proc. Natl. Acad. Sci. USA 2012, 109, 19596–19600. [Google Scholar] [CrossRef]
- David, P.G. Changes in plant communities relative to hydrologic conditions in the Florida Everglades. Wetlands 1996, 16, 15–23. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, P.; Chen, X.; Jiang, T. Hydrological extremes in the Poyang Lake basin, China: Changing properties, causes and impacts. Hydrol. Process. 2011, 25, 3121–3130. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Keddy, P.; Reznicek, A. Great Lakes vegetation dynamics: The role of fluctuating water levels and buried seeds. J. Great Lakes Res. 1986, 12, 25–36. [Google Scholar] [CrossRef]
- Riis, T.; Hawes, I. Relationships between water level fluctuations and vegetation diversity in shallow water of New Zealand lakes. Aquat. Bot. 2002, 74, 133–148. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, J.; Jiang, Y.; Wang, H. Relationship between the hydrological conditions and the distribution of vegetation communities within the Poyang Lake National Nature Reserve, China. Ecol. Inform. 2012, 11, 65–75. [Google Scholar] [CrossRef]
- Silvertown, J.; Dodd, M.E.; Gowing, D.J.G.; Mountford, J.O. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 1999, 400, 61–63. [Google Scholar] [CrossRef]
- Magee, T.K.; Kentula, M.E. Response of wetland plant species to hydrologic conditions. Wetl. Ecol. Manag. 2005, 13, 163–181. [Google Scholar] [CrossRef]
- Ma, S.; Ren, J.; Wu, C.; Cheng, F.; Wang, X.; Li, B.; He, Q. Hydrological control of threshold transitions in vegetation over early-period wetland development. J. Hydrol. 2022, 610, 127931. [Google Scholar] [CrossRef]
- Silvertown, J.; Araya, Y.N.; Gowing, D. Hydrological niches in terrestrial plant communities: A review. J. Ecol. 2015, 103, 93–108. [Google Scholar] [CrossRef]
- Liao, F.; Wang, G.; Yi, L.; Shi, Z.; Cheng, G.; Kong, Q.; Mu, W.; Guo, L.; Cheng, K.; Dong, N.; et al. Applying radium isotopes to estimate groundwater discharge into Poyang Lake, the largest freshwater lake in China. J. Hydrol. 2020, 585, 124782. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, X.; Li, D.; Xu, G.; Guo, Y. Spatial heterogeneity of vegetation extent and the response to water level fluctuations and micro-topography in Poyang Lake, China. Ecol. Indic. 2021, 124, 107420. [Google Scholar] [CrossRef]
- Lai, X.; Huang, Q.; Zhang, Y.; Jiang, J. Impact of lake inflow and the Yangtze River flow alterations on water levels in Poyang Lake, China. Lake Reserv. Manag. 2014, 30, 321–330. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Z.; Liu, Y.; Ji, W.; Ge, G. The function and significance of the Shallow-Lakes in the Poyang Lake wetland ecosystem. Jiangxi Hydraul. Sci. Technol. 2015, 41, 317–323. [Google Scholar]
- Liu, X.; Zhang, Q.; Li, Y.; Tan, Z.; Werner, A.D. Satellite image-based investigation of the seasonal variations in the hydrological connectivity of a large floodplain (Poyang Lake, China). J. Hydrol. 2020, 585, 124810. [Google Scholar] [CrossRef]
- Shen, R.; Lan, Z.; Huang, X.; Chen, Y.; Hu, Q.; Fang, C.; Jin, B.; Chen, J. Soil and plant characteristics during two hydrologically contrasting years at the lakeshore wetland of Poyang Lake, China. J. Soils Sediments 2020, 20, 3368–3379. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, Q.; Li, Y.; Li, M. Hydrological evidence and causes of seasonal low water levels in a large river-lake system: Poyang Lake, China. Hydrol. Res. 2016, 47, 24–39. [Google Scholar] [CrossRef]
- Dronova, I.; Gong, P.; Wang, L.; Zhong, L. Mapping dynamic cover types in a large seasonally flooded wetland using extended principal component analysis and object-based classification. Remote Sens. Environ. 2015, 158, 193–206. [Google Scholar] [CrossRef]
- Shankman, D.; Keim, B.D. Flood risk forecast for China’s Poyang Lake region. Phys. Geogr. 2016, 37, 88–91. [Google Scholar] [CrossRef]
- Liu, X.Z.; Fan, S.B.; Hu, B.H. Comprehensive and Scientific Survey of Jiangxi Nanjishan Wetland Nature Reserve; China Foresry Press: Beijing, China, 2006. [Google Scholar]
- Kettenring, K.; Gardner, G.; Galatowitsch, S.M. Effect of light on seed germination of eight wetland Carex species. Ann. Bot. 2006, 98, 869–874. [Google Scholar] [CrossRef]
- Chen, X.-S.; Deng, Z.-M.; Xie, Y.-H.; Li, F.; Hou, Z.-Y.; Li, X. Belowground bud banks of four dominant macrophytes along a small-scale elevational gradient in Dongting Lake wetlands, China. Aquat. Bot. 2015, 122, 9–14. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.-S.; Zou, Y.-A.; Zhang, P.-Y.; Li, F.; Hou, Z.-Y.; Li, X.; Zeng, J.; Deng, Z.-M.; Zhong, J.-R.; et al. Exploring the relative contribution of flood regimes and climatic factors to Carex phenology in a Yangtze River-connected floodplain wetland. Sci. Total Environ. 2022, 847, 157568. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, Y.; Lei, G.; Liu, Y.; Lei, J.; Yu, X.; Wen, L.; Zhou, Y. Restriction of herbivorous waterbird distributions in the middle and lower Yangtze River floodplain in view of hydrological isolation. Wetlands 2017, 37, 79–88. [Google Scholar] [CrossRef]
- Ming-Qin, S.; Hong, G. Population sizes and group characteristics of Siberian crane (Leucogeranus leucogeranus) and hooded crane (Grus monacha) in Poyang Lake Wetland. Zool. Res. 2014, 35, 373. [Google Scholar]
- ESA. Sentinel-2 User Handbook; ESA Standard Document; ESA-Star Publication: Paris, France, 2015; Volume 64, pp. 1–64. [Google Scholar]
- Kaplan, G.; Avdan, U. Evaluating Sentinel-2 Red-Edge Bands for Wetland Classification. Proceedings 2019, 18, 12. [Google Scholar]
- Rupasinghe, P.A.; Chow-Fraser, P. Identification of most spectrally distinguishable phenological stage of invasive Phramites australis in Lake Erie wetlands (Canada) for accurate mapping using multispectral satellite imagery. Wetl. Ecol. Manag. 2019, 27, 513–538. [Google Scholar] [CrossRef]
- Amani, M.; Kakooei, M.; Ghorbanian, A.; Warren, R.; Mahdavi, S.; Brisco, B.; Moghimi, A.; Bourgeau-Chavez, L.; Toure, S.; Paudel, A.; et al. Forty Years of Wetland Status and Trends Analyses in the Great Lakes Using Landsat Archive Imagery and Google Earth Engine. Remote Sens. 2022, 14, 3778. [Google Scholar] [CrossRef]
- Rosenfield, G.H.; Fitzpatrick-Lins, K. A coefficient of agreement as a measure of thematic classification accuracy. Photogramm. Eng. Remote Sens. 1986, 52, 223–227. [Google Scholar]
- Congalton, R.G. A review of assessing the accuracy of classifications of remotely sensed data. Remote Sens. Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- Gause, G. The influence of ecological factors on the size of population. Am. Nat. 1931, 65, 70–76. [Google Scholar] [CrossRef]
- Mountrakis, G.; Im, J.; Ogole, C. Support vector machines in remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2011, 66, 247–259. [Google Scholar] [CrossRef]
- Vapnik, V.N. Statistical Learning Theory; John Wiley & Sons, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Maryantika, N.; Lin, C. Exploring changes of land use and mangrove distribution in the economic area of Sidoarjo District, East Java using multi-temporal Landsat images(Article). Inf. Process. Agric. 2017, 4, 321–332. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Yu, D.; Yao, R.; Li, C.; He, Q.; Wang, S.; Wang, L. Four decades of wetland changes in Dongting Lake using Landsat observations during 1978–2018. J. Hydrol. 2020, 587, 124954. [Google Scholar] [CrossRef]
- Keramitsoglou, I.; Sarimveis, H.; Kiranoudis, C.T.; Kontoes, C.; Sifakis, N.; Fitoka, E. The performance of pixel window algorithms in the classification of habitats using VHSR imagery. ISPRS J. Photogramm. Remote Sens. 2006, 60, 225–238. [Google Scholar] [CrossRef]
- Wang, M.; Fei, X.; Zhang, Y.; Chen, Z.; Wang, X.; Tsou, J.Y.; Liu, D.; Lu, X. Assessing texture features to classify coastal wetland vegetation from high spatial resolution imagery using completed local binary patterns (CLBP). Remote Sens. 2018, 10, 778. [Google Scholar] [CrossRef]
- Giuliani, G.; Egger, E.; Italiano, J.; Poussin, C.; Richard, J.-P.; Chatenoux, B. Essential variables for environmental monitoring: What are the possible contributions of earth observation data cubes? Data 2020, 5, 100. [Google Scholar] [CrossRef]
- Pillsbury, F.C.; Miller, J.R. Habitat and landscape characteristics underlying anuran community structure along an urban–rural gradient. Ecol. Appl. 2008, 18, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Q.; Tan, Z.; Li, Y.; Wang, X. Effects of water-table depth and soil moisture on plant biomass, diversity, and distribution at a seasonally flooded wetland of Poyang Lake, China. Chin. Geogr. Sci. 2015, 25, 739–756. [Google Scholar] [CrossRef]
- Yang, X.-D.; Dong, X.-H.; Gao, G.; Pan, H.-X.; Wu, J.-L. Relationship between surface sediment diatoms and summer water quality in shallow lakes of the middle and lower reaches of the Yangtze River. J. Integr. Plant Biol. 2005, 47, 153–164. [Google Scholar] [CrossRef]
- Han, Z.; Wang, S.; Liu, X.; Peng, W.; Ge, G.; Huang, A. Ecological thresholds for the dominated wetland plants of Poyang Lake along the gradient of flooding duration. J. Hydraul. Eng. 2019, 50, 252–262. [Google Scholar]
- Coops, H.; Beklioglu, M.; Crisman, T.L. The role of water-level fluctuations in shallow lake ecosystems—Workshop conclusions. Hydrobiologia 2003, 506, 23–27. [Google Scholar] [CrossRef]
- Deegan, B.M.; White, S.D.; Ganf, G.G. The influence of water level fluctuations on the growth of four emergent macrophyte species. Aquat. Bot. 2007, 86, 309–315. [Google Scholar] [CrossRef]
- Schütz, W. Dormancy characteristics and germination timing in two alpine Carex species. Basic Appl. Ecol. 2002, 3, 125–134. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Bögemann, G.M.; VAN DE Steeg, H.M.; Pierik, R.; Blom, C.W.P.M. Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation. New Phytol. 2000, 148, 93–103. [Google Scholar] [CrossRef]
- Lytle, D.A.; Poff, N.L. Adaptation to natural flow regimes. Trends Ecol. Evol. 2004, 19, 94–100. [Google Scholar] [CrossRef]
- Deng, Z.; Xie, Y.; Chen, X.; Li, F.; Hu, C.; Liu, N. Effect of clone size on submergence tolerance and post-submergence growth recovery in Carex brevicuspis (Cyperaceae). J. Limnol. 2017, 76, 424–430. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; Wiley: Hoboken, NJ, USA, 2015; p. 456. [Google Scholar]
- Yuan, S.; Yang, Z.; Liu, X.; Wang, H. Key parameters of water level fluctuations determining the distribution of Carex in shallow lakes. Wetlands 2017, 37, 1005–1014. [Google Scholar] [CrossRef]
- Castelli, R.M.; Chambers, J.C.; Tausch, R.J. Soil-plant relations along a soil-water gradient in Great Basin riparian meadows. Wetlands 2000, 20, 251–266. [Google Scholar]
- Solon, J.; Degórski, M.; Roo-Zielińska, E. Vegetation response to a topographical-soil gradient. Catena 2007, 71, 309–320. [Google Scholar] [CrossRef]
- Palanisamy, B.; Chui, T.F.M. Understanding wetland plant dynamics in response to water table changes through ecohydrological modelling. Ecohydrology 2013, 6, 287–296. [Google Scholar] [CrossRef]
- Koning, C.O. Vegetation patterns resulting from spatial and temporal variability in hydrology, soils, and trampling in an isolated basin marsh, New Hampshire, USA. Wetlands 2005, 25, 239–251. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Cai, Y.; Tan, Z.; Wu, H.; Liu, X.; Yao, J. Hydrodynamic investigation of surface hydrological connectivity and its effects on the water quality of seasonal lakes: Insights from a complex floodplain setting (Poyang Lake, China). Sci. Total Environ. 2019, 660, 245–259. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Liu, X.; Tan, Z.; Yao, J. The role of a seasonal lake groups in the complex Poyang Lake-floodplain system (China): Insights into hydrological behaviors. J. Hydrol. 2019, 578, 124055. [Google Scholar] [CrossRef]
- Richardson, A.J.; Wiegand, C.L. Distinguishing vegetation from soil background information. Photogramm. Eng. Remote Sens. 1977, 43, 1541–1552. [Google Scholar]
- Pearson, R.L.; Miller, L.D. Remote Mapping of Standing Crop Biomass for Estimation of Productivity of the Shortgrass Prairie. Remote Sens. Environ. 1972, VIII, 1355. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Wu, C.; Chen, J.M.; Huang, N. Predicting gross primary production from the enhanced vegetation index and photosynthetically active radiation: Evaluation and calibration. Remote Sens. Environ. 2011, 115, 3424–3435. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A Modified Soil Adjusted Vegetation Index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Q.-Y.; Wu, J.-L.; Zhao, F. Study of environmental vegetation index based on environment satellite CCD data and LAI inversion. Spectrosc. Spectr. Anal. 2011, 31, 2789–2793. [Google Scholar]
- Kaufman, Y.J.; Tanre, D. Atmospherically resistant vegetation index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Wang, S.; Wu, Y. Extraction of vegetation information from visible unmanned aerial vehicle images. Trans. Chin. Soc. Agric. Eng. 2015, 31, 152–159. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Hunt, E.R.; Cavigelli, M.; Daughtry, C.S.; Mcmurtrey, J.E.; Walthall, C.L. Evaluation of Digital Photography from Model Aircraft for Remote Sensing of Crop Biomass and Nitrogen Status. Precis. Agric. 2005, 6, 359–378. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus hippocastanum L. and Acer platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar]
- Fernández-Manso, A.; Fernández-Manso, O.; Quintano, C. SENTINEL-2A red-edge spectral indices suitability for discriminating burn severity. Int. J. Appl. Earth Obs. Geoinf. 2016, 50, 170–175. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, 431–433. [Google Scholar] [CrossRef] [Green Version]







| Community | Phalaris Ass | Carex Ass | Triarrhena Ass |
|---|---|---|---|
| Dominant species | Phragmites australis | Carex cinerascens | Triarrhena lutarioriparia, Phragmites australis |
| Accompanying species | Potentilla limprichtii, Carex ovatispiculata, and Lapsana apogonoides | Potentilla limprichtii spp., Cardamine lyrata spp., and various Carex spp. | Carex cinerascens, Carex argyi, and Polygonum posumbu |
| Coverage | 60~80% | 95~100% | 85~98% |
| Structure | Vertical (two layers) | Horizontal | Vertical (three layers) |
| Phenology | Perennial herb that sprouts from January to February, blooms before the beginning of the flooding season, and fully develops from April to May [29]. It is submerged during the flood and continues to grow after it recedes until the winter ends [29]. | Perennial herb with two growing seasons (late spring and mid-autumn) and sprouts in the early spring. It reaches its maximum coverage in April during the first growing season [58]. Generally, they are flooded in the flood season, and a large number of aboveground parts of Carex die or become dormant. The second growing season begins in early autumn when the floodwaters recede, and germination takes place until the maximum coverage is reached, before completely withering in winter [59,60]. | Blooms from September to October, bears fruit in November, and leaves wither in December [29]. |
| Phalaris Ass | Carex Ass | Triarrhena Ass | |
|---|---|---|---|
| Average annual inundation days (d) | 187~251 (219) | 132~245 (188.2) | 107~130 (119.9) |
| Average inundation days per inundation (d) | 44~65 (54.7) | 24~61 (44.3) | 33~42 (36.5) |
| Annual average inundation depth (m) | 1.54~1.76 (1.65) | 1.50~1.68 (1.58) | 1.43~1.50 (1.45) * |
| Annual maximum inundation depth (m) | 6.58~7.41 (7.00) | 6.13~7.24 (6.69) | 5.70~5.99 (5.87) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Hu, T.; Mao, J.; Montzka, C.; Bol, R.; Wan, S.; Li, J.; Yue, J.; Dai, H. Hydrological Drivers for the Spatial Distribution of Wetland Herbaceous Communities in Poyang Lake. Remote Sens. 2022, 14, 4870. https://doi.org/10.3390/rs14194870
Huang W, Hu T, Mao J, Montzka C, Bol R, Wan S, Li J, Yue J, Dai H. Hydrological Drivers for the Spatial Distribution of Wetland Herbaceous Communities in Poyang Lake. Remote Sensing. 2022; 14(19):4870. https://doi.org/10.3390/rs14194870
Chicago/Turabian StyleHuang, Wenqin, Tengfei Hu, Jingqiao Mao, Carsten Montzka, Roland Bol, Songxian Wan, Jianxin Li, Jin Yue, and Huichao Dai. 2022. "Hydrological Drivers for the Spatial Distribution of Wetland Herbaceous Communities in Poyang Lake" Remote Sensing 14, no. 19: 4870. https://doi.org/10.3390/rs14194870