Use of an Active Canopy Sensor Mounted on an Unmanned Aerial Vehicle to Monitor the Growth and Nitrogen Status of Winter Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
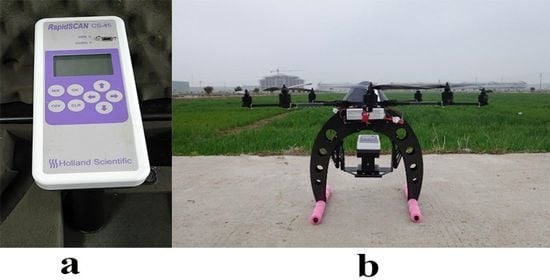
2.2. Spectral Data Collection
2.3. Plant Sampling and Measurements
2.4. Statistical Analyses
3. Results
3.1. Variability of Wheat Growth and Nitrogen Status Indicators
3.2. Dynamic Changes in Vegetation Indices
3.3. Relationships between Crop Growth Indicators and Vegetation Indices
3.4. Relationships between N Nutrition Indicators and Vegetation Indices
3.5. Model Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Yuan, Z.; Yang, T.; Lu, Z.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Chlorophyll meter–based nitrogen fertilizer optimization algorithm and nitrogen nutrition index for in-season fertilization of paddy rice. Agron. J. 2020, 112, 288–300. [Google Scholar] [CrossRef]
- Singh, B.; Singh, V.; Singh, Y.; Thind, H.S.; Kumar, A.; Choudhary, O.P.; Gupta, R.K.; Vashistha, M. Site-Specific Fertilizer Nitrogen Management Using Optical Sensor in Irrigated Wheat in the Northwestern India. Agric. Res. 2017, 96, 159–168. [Google Scholar]
- Battude, M.; Al Bitar, A.; Morin, D.; Cros, J.; Huc, M.; Sicre, C.M.; Le Dantec, V.; Demarez, V. Estimating maize biomass and yield over large areas using high spatial and temporal resolution Sentinel-2 like remote sensing data. Remote Sens. Environ. 2016, 184, 668–681. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, J.; Ju, W.; Zhu, G.; Wu, X.; Fan, W.; Li, D.; Zhou, Y. Satellite-derived LAI products exhibit large discrepancies and can lead to substantial uncertainty in simulated carbon and water fluxes. Remote Sens. Environ. 2018, 206, 174–188. [Google Scholar] [CrossRef]
- Sripada, R.P.; Heiniger, R.W.; White, J.G.; Meijer, A.D. Aerial Color Infrared Photography for Determining Early In-Season Nitrogen Requirements in Corn. Agron. J. 2006, 98, 968–977. [Google Scholar] [CrossRef]
- Gong, Y.; Xiao, J.; Hou, J.; Duan, B. Rape yields estimation research based on spectral analysis for UAV image. J. Geomat. 2017, 42, 40–45. [Google Scholar]
- Zhang, J.; Liu, X.; Liang, Y.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Using a Portable Active Sensor to Monitor Growth Parameters and Predict Grain Yield of Winter Wheat. Sensors 2019, 19, 1108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Ge, X.; Shen, P.; Li, W.; Liu, X.; Cao, Q.; Zhu, Y.; Cao, W.; Tian, Y. Predicting Rice Grain Yield Based on Dynamic Changes in Vegetation Indexes during Early to Mid-Growth Stages. Remote Sens. 2019, 11, 387. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.Z.; Feng, X.; Wen, N.; Zheng, T.; Yang, W.D. Monitoring biomass and N accumulation at jointing stage in winter wheat based on SPOT-5 images. Sci. Agric. Sin. 2012, 45, 3049–3057. [Google Scholar]
- Li, F.; Chang, Q.; Shen, J.; Wang, L. Remote sensing estimation of winter wheat leaf nitrogen content based on GF-1 satellite data. Trans. Chin. Soc. Agric. Eng. 2016, 32, 157–164. [Google Scholar]
- Neale, C.M.; Crowther, B.G. An airborne multispectral video/radiometer remote sensing system: Development and calibration. Remote Sens. Environ. 1994, 49, 187–194. [Google Scholar] [CrossRef]
- Padilla, F.; Maas, S.; González-Dugo, M.P.; Mansilla, F.; Rajan, N.; Gavilán, P.; Dominguez, J. Monitoring regional wheat yield in Southern Spain using the GRAMI model and satellite imagery. Field Crop. Res. 2012, 130, 145–154. [Google Scholar] [CrossRef]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Feng, W.; Yao, X.; Zhu, Y.; Tian, Y.C.; Cao, W.X. Monitoring leaf nitrogen status with hyperspectral reflectance in wheat. Eur. J. Agron. 2008, 28, 394–404. [Google Scholar] [CrossRef]
- Plaza, A.; Benediktsson, J.A.; Boardman, J.W.; Brazile, J.; Bruzzone, L.; Camps-Valls, G.; Chanussot, J.; Fauvel, M.; Gamba, P.; Gualtieri, A.; et al. Recent advances in techniques for hyperspectral image processing. Remote Sens. Environ. 2009, 113, S110–S122. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Liu, L.; Huang, W.; Zhao, C.; Wang, C. Prediction of grain protein content in winter wheat (Triticum aestivum L.) using plant pigment ratio (PPR). Field Crop. Res. 2004, 90, 311–321. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with Crop Circle multispectral active canopy sensor. Field Crop. Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Xia, T.; Miao, Y.; Wu, D.; Hui, S.; Khosla, R.; Mi, G. Active Optical Sensing of Spring Maize for In-Season Diagnosis of Nitrogen Status Based on Nitrogen Nutrition Index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Miao, Y.; Zhang, F.; Cui, Z.; Li, R.; Chen, X.; Zhang, H.; Schroder, J.; Raun, W.R.; Jia, L. In-Season Optical Sensing Improves Nitrogen-Use Efficiency for Winter Wheat. Soil Sci. Soc. Am. J. 2009, 73, 1566–1574. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Qi, B.; Zhao, J.-M.; Zhang, X.-Y.; Wang, S.-G.; Zhao, T.-J.; Gai, J.-Y. Prediction for Soybean Grain Yield Using Active Sensor GreenSeeker. Acta Agron. Sin. 2014, 40, 657. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Li, F.; Liu, B.; Yue, S.; Cheng, S.; Ustin, S.L.; Khosla, R. Active canopy sensing of winter wheat nitrogen status: An evaluation of two sensor systems. Comput. Electron. Agric. 2015, 112, 54–67. [Google Scholar] [CrossRef]
- Shaver, T.; Khosla, R.; Westfall, D. Evaluation of Two Crop Canopy Sensors for Nitrogen Recommendations in Irrigated Maize. J. Plant Nutr. 2014, 37, 406–419. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Shen, J.; Yuan, F.; Cheng, S.; Cui, Z. Evaluating Two Crop Circle Active Canopy Sensors for In-Season Diagnosis of Winter Wheat Nitrogen Status. Agronomy 2018, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Miao, Y.; Huang, Y.; Shi, W.; Hu, X.; Wang, X.; Wan, J. Evaluating an unmanned aerial vehicle-based remote sensing system for estimation of rice nitrogen status. In Proceedings of the 2015 Fourth International Conference on Agro-Geoinformatics (Agro-geoinformatics), Istanbul, Turkey, 20–24 July 2015; pp. 198–203. [Google Scholar]
- Tan, C.W.; Ying, D.U.; Lu, T.; Jian, Z.; Ming, L.; Yan, W.W.; Fei, C. Comparison of the methods for predicting wheat yield based on satellite remote sensing data at anthesis. Sci. Agric. Sin. 2017, 50, 3101–3109. [Google Scholar]
- Erdle, K.; Mistele, B.; Schmidhalter, U. Comparison of active and passive spectral sensors in discriminating biomass parameters and nitrogen status in wheat cultivars. Field Crop. Res. 2011, 124, 74–84. [Google Scholar] [CrossRef]
- Dong, T.; Liu, J.; Shang, J.; Qian, B.; Ma, B.; Kovacs, J.M.; Walters, D.; Jiao, X.; Geng, X.; Shi, Y. Assessment of red-edge vegetation indices for crop leaf area index estimation. Remote Sens. Environ. 2019, 222, 133–143. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Meza, C.; Rivera, J.P.; Alonso, L.; Moreno, J.F. A red-edge spectral index for remote sensing estimation of green LAI over agroecosystems. Eur. J. Agron. 2013, 46, 42–52. [Google Scholar] [CrossRef]
- White, J.W.; Andrade-Sanchez, P.; Gore, M.A.; Bronson, K.F.; Coffelt, T.A.; Conley, M.M.; Feldmann, K.A.; French, A.N.; Heun, J.T.; Hunsaker, D.J.; et al. Field-based phenomics for plant genetics research. Field Crop. Res. 2012, 133, 101–112. [Google Scholar] [CrossRef]
- Krienke, B.; Ferguson, R.B.; Schlemmer, M.; Holland, K.; Marx, D.; Eskridge, K. Using an unmanned aerial vehicle to evaluate nitrogen variability and height effect with an active crop canopy sensor. Precis. Agric. 2017, 18, 900–915. [Google Scholar] [CrossRef] [Green Version]
- Lamb, D.W.; Schneider, D.; Stanley, J.N. Combination active optical and passive thermal infrared sensor for low-level airborne crop sensing. Precis. Agric. 2014, 15, 523–531. [Google Scholar] [CrossRef]
- Lamb, D.W.; Schneider, D.; Trotter, M.G.; Schaefer, M.; Yule, I. Extended-altitude, aerial mapping of crop NDVI using an active optical sensor: A case study using a Raptor™ sensor over wheat. Comput. Electron. Agric. 2011, 77, 69–73. [Google Scholar] [CrossRef]
- Tian, M.; Ban, S.; Chang, Q.; You, M.; Dan, L.; Li, W.; Wang, S. Use of hyperspectral images from UAV-based imaging spectroradiometer to estimate cotton leaf area index. Trans. Chin. Soc. Agric. Eng. 2016, 32, 102–108. [Google Scholar]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Swain, K.C.; Jayasuriya, H.P.; Salokhe, V.M. Suitability of low-altitude remote sensing images for estimating nitrogen treatment variations in rice cropping for precision agriculture adoption. J. Appl. Remote Sens. 2007, 1, 013547. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, G.; Liu, J.; Zhang, X.; Bo, X.; Wang, Y.; Zhao, C.; Gai, J. Estimation of soybean breeding yield based on optimization of spatial scale of UAV hyperspectral image. Trans. Chin. Soc. Agric. Eng. 2017, 33, 110–116. [Google Scholar]
- Zheng, H.; Cheng, T.; Li, D.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Combining Unmanned Aerial Vehicle (UAV)-Based Multispectral Imagery and Ground-Based Hyperspectral Data for Plant Nitrogen Concentration Estimation in Rice. Front. Plant Sci. 2018, 9, 936. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, H.; Wang, P. Quantitative modelling for leaf nitrogen content of winter wheat using UAV-based hyperspectral data. Int. J. Remote Sens. 2016, 38, 2117–2134. [Google Scholar] [CrossRef]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating Biomass of Barley Using Crop Surface Models (CSMs) Derived from UAV-Based RGB Imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef] [Green Version]
- Lamb, D.W.; Trotter, M.G.; Schneider, D.A. Ultra low-level airborne (ULLA) sensing of crop canopy reflectance: A case study using a CropCircle sensor. Comput. Electron. Agric. 2009, 69, 86–91. [Google Scholar] [CrossRef]
- Large, E.C. Growth Stages in Cereals Illustration of the Feekes Scale. Plant Pathol. 1954, 3, 128–129. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident detection of crop water stress, nitrogen status, and canopy density using ground based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Richardson, A.J.; Wiegand, C.L. Distinguishing vegetation from soil background information. Photogramm. Eng. Remote Sens. 1978, 43, 1541–1552. [Google Scholar]
- Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Jasper, J.; Reusch, S.; Link, A. Active sensing of the N status of wheat using optimized wavelength combination: Impact of seed rate, variety and growth stage. In Precision Agriculture 09, Proceedings of the Papers from the 7th European Conference on Precision Agriculture, Wageningen, The Netherlands, 6–8 July 2009; Van Henten, E.J., Goense, D., Lokhorst, C., Eds.; Wageningen Academic: Wageningen, The Netherlands, 2009. [Google Scholar]
- Pearson, R.L.; Miller, L.D. Remote mapping of standing crop biomass for estimation of productivity of the shortgrass prairie. Remote Sens. Environ. 1972, VIII, 1355. [Google Scholar]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for Remote Quantification of Biophysical Characteristics of Vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Perry, C.R.; Lautenschlager, L.F. Functional equivalence of spectral vegetation indices. Remote Sens. Environ. 1984, 14, 169–182. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Guyot, G.; Baret, F. Utilisation de la Haute Resolution Spectrale pour Suivre L’etat des Couverts Vegetaux. Spectr. Signat. Objects Remote Sens. 1988, 287, 279. [Google Scholar]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, 08403. [Google Scholar] [CrossRef] [Green Version]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis, Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Zhao, B.; Yao, X.; Tian, Y.; Liu, X.-J.; Cao, W.; Zhu, Y. Accumulative nitrogen deficit models of wheat aboveground part based on critical nitrogen concentration. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2012, 23, 3141. [Google Scholar]
- Justes, E. Determination of a Critical Nitrogen Dilution Curve for Winter Wheat Crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Gilles, L.; Mariehélène, J.; François, G. Diagnosis tool for plant and crop N status in vegetative stage. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar]
- Huang, B.T.; Zhou, H. Effect of Nitrogen Fertilizer on Wheat Uptake of Soil N in a Pot Experiment. Adv. Mater. Res. 2012, 485, 225–228. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, B.; Tian, Y.C.; Liu, X.J.; Ni, J.; Cao, W.X.; Zhu, Y. Using leaf dry matter to quantify the critical nitrogen dilution curve for winter wheat cultivated in eastern China. Field Crop. Res. 2014, 159, 33–42. [Google Scholar] [CrossRef]
- Van Niel, T.G.; McVicar, T.R. Current and potential uses of optical remote sensing in rice-based irrigation systems: A review. Aust. J. Agric. Res. 2004, 55, 155–185. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crop. Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Kanke, Y.; Raun, W.R.; Solie, J.; Stone, M.; Taylor, R. Red Edge As A Potential Index for Detecting Differences in Plant Nitrogen Status in Winter Wheat. J. Plant Nutr. 2012, 35, 1526–1541. [Google Scholar] [CrossRef]
- Herrmann, I.; Pimstein, A.; Karnieli, A.; Cohen, Y.; Alchanatis, V.; Bonfil, D. LAI assessment of wheat and potato crops by VENμS and Sentinel-2 bands. Remote Sens. Environ. 2011, 115, 2141–2151. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Clevers, J.; Gitelson, A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and-3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.; Peng, Y.; Gitelson, A.A.; Arkebauer, T.J.; Pimstein, A.; Herrmann, I.; Karnieli, A.; Rundquist, D.C.; Bonfil, D.J. Estimating green LAI in four crops: Potential of determining optimal spectral bands for a universal algorithm. Agric. For. Meteorol. 2014, 192, 140–148. [Google Scholar] [CrossRef]
- Rodríguez, J.R.; Riaño, D.; Carlisle, E.; Ustin, S.L.; Smart, D.R. Evaluation of hyperspectral reflectance indexes to detect grapevine water status in vineyards. Am. J. Enol. Vitic. 2007, 58, 302–317. [Google Scholar]
- Haboudane, D. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- He, Y.; Bo, Y.; Chai, L.; Liu, X.; Li, A. Linking in situ LAI and fine resolution remote sensing data to map reference LAI over cropland and grassland using geostatistical regression method. Int. J. Appl. Earth Obs. Geoinf. 2016, 50, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Leon, C.T.; Shaw, D.R.; Cox, M.S.; Abshire, M.J.; Ward, B.; Iii, M.C.W.; Watson, C. Utility of Remote Sensing in Predicting Crop and Soil Characteristics. Precis. Agric. 2003, 4, 359–384. [Google Scholar] [CrossRef]
- Karale, Y.; Mohite, J.; Jagyasi, B. Crop classification based on multi-temporal satellite remote sensing data for agro-advisory services. In SPIE Asia-Pacific Remote Sensing; SPIE—The International Society for Optical Engineering: Bellingham, WA, USA, 2014; p. 926004. [Google Scholar]
- Jonckheere, I.; Nackaerts, K.; Muys, B.; Van Aardt, J.; Coppin, P. A fractal dimension-based modelling approach for studying the effect of leaf distribution on LAI retrieval in forest canopies. Ecol. Model. 2006, 197, 179–195. [Google Scholar] [CrossRef]
- Kross, A.; McNairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of RapidEye vegetation indices for estimation of leaf area index and biomass in corn and soybean crops. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, K.; Tang, C.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Estimation of Rice Growth Parameters Based on Linear Mixed-Effect Model Using Multispectral Images from Fixed-Wing Unmanned Aerial Vehicles. Remote Sens. 2019, 11, 1371. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Du, Y.; Zhou, J.; Wang, D.; Luo, M.; Zhang, Y.; Guo, W. Analysis of Different Hyperspectral Variables for Diagnosing Leaf Nitrogen Accumulation in Wheat. Front. Plant Sci. 2018, 9, 674. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Cao, Q.; Zhu, Y.; Tang, L.; Rehmani, A.; Cao, W. Non-destructive Assessment of Plant Nitrogen Parameters Using Leaf Chlorophyll Measurements in Rice. Front. Plant Sci. 2016, 7, 1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, P.; Kaur, A.; Nigan, R.; Gill, A.; Singh, J.; Sandhu, S. Spectral indices of wheat cultivars at different growth stages under Punjab conditions. J. Agrometeorol. 2018, 19, 160–165. [Google Scholar]
- Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, X.; Tan, C.; Yu, W.; Gnyp, M.L.; Lenz-Wiedemann, V.I.; Rascher, U.; et al. Satellite Remote Sensing-Based In-Season Diagnosis of Rice Nitrogen Status in Northeast China. Remote Sens. 2015, 7, 10646–10667. [Google Scholar] [CrossRef] [Green Version]
- Longchamps, L.; Khosla, R. Early Detection of Nitrogen Variability in Maize Using Fluorescence. Agron. J. 2014, 106, 511–518. [Google Scholar] [CrossRef]
- Pimstein, A.; Eitel, J.U.; Long, D.S.; Mufradi, I.; Karnieli, A.; Bonfil, D.J. A spectral index to monitor the head-emergence of wheat in semi-arid conditions. Field Crop. Res. 2009, 111, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, M.; Reynolds, M.; Klatt, A.R. Effect of leaf and spike morphological traits on the relationship between spectral reflectance indices and yield in wheat. Int. J. Remote Sens. 2015, 36, 701–718. [Google Scholar] [CrossRef]
- Jiang, J.; Cai, W.; Zheng, H.; Cheng, T.; Tian, Y.; Zhu, Y.; Ehsani, R.; Hu, Y.; Niu, Q.; Gui, L.; et al. Using Digital Cameras on an Unmanned Aerial Vehicle to Derive Optimum Color Vegetation Indices for Leaf Nitrogen Concentration Monitoring in Winter Wheat. Remote Sens. 2019, 11, 2667. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Feng, H.; Xu, L.; Miao, M.; Long, H.; Yue, J.; Li, Z.; Yang, G.; Yang, X.; Fan, L. Estimation of Crop Growth Parameters Using UAV-Based Hyperspectral Remote Sensing Data. Sensors 2020, 20, 1296. [Google Scholar] [CrossRef] [Green Version]
- Aranguren, M.; Castellón, A.; Aizpurua, A. Topdressing nitrogen recommendation in wheat after applying organic manures: The use of field diagnostic tools. Nutr. Cycl. Agroecosyst. 2017, 110, 89–103. [Google Scholar] [CrossRef]
- Lu, J.; Miao, Y.; Shi, W.; Li, J.; Yuan, F. Evaluating different approaches to non-destructive nitrogen status diagnosis of rice using portable RapidSCAN active canopy sensor. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Miller, J.J.; Schepers, J.S.; Shapiro, C.A.; Arneson, N.J.; Eskridge, K.M.; Oliveira, M.C.; Giesler, L.J. Characterizing soybean vigor and productivity using multiple crop canopy sensor readings. Field Crop. Res. 2018, 216, 22–31. [Google Scholar] [CrossRef]
- Bonfil, D.J. Wheat phenomics in the field by RapidScan: NDVI vs. NDRE. Isr. J. Plant Sci. 2016, 9978, 1–14. [Google Scholar] [CrossRef]
- Spockeli, B.A. Integration of RTK GPS and IMU for Accurate UAV Positioning. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2015. [Google Scholar]
- Nebiker, S.; Annen, A.; Scherrer, M.; Oesch, D. A light-weight multispectral sensor for micro UAV: Opportunities for very high resolution airborne remote sensing. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2008, 37, 1193–1200. [Google Scholar]
- Colomina, I.; Molina, P. Unmanned aerial systems for photogrammetry and remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2014, 92, 79–97. [Google Scholar] [CrossRef] [Green Version]
- Giletto, C.M.; Echeverría, H.E. Critical Nitrogen Dilution Curve for Processing Potato in Argentinean Humid Pampas. Am. J. Potato Res. 2011, 89, 102–110. [Google Scholar] [CrossRef]






| Experiment No. | Location | Mean Temperature (°C) | Precipitation (mm) | Cultivar | N Rate (kg ha−1) | Sampling Stage (Date) | Soil Classification |
|---|---|---|---|---|---|---|---|
| Experiment 1 2015–2016 | Sihong (33.37°N, 118.26°E) | 16.2 | 910 | Xumai30 Huaimai20 | 0 (N0) 90 (N1) 180 (N2) 270 (N3) 360 (N4) | Feekes stages 6.0 (23-March) Feekes stages 7.0 (5-April) Feekes stages 9.0 (10-April) Feekes stages 10.0 (15-April) Feekes stages 10.3 (22-April) Feekes stages 10.5.2 (26-April) Feekes stages 11.1 (4-May) | Lime concretion black soil Soil pH = 6.56 OM = 26.30 g kg−1 Total N = 2.91 g kg−1 Available P = 43.12 mg g−1 Available K = 89.23 mg g−1 |
| Experiment 2 2016–2017 | Rugao (32.27°N, 120.75°E) | 16.7 | 1050 | Yangmai15 Yangmai16 | 0 (N0) 150 (N1) 300 (N2) | Feekes stages 7.0 (16-March) Feekes stages 9.0 (27-March) Feekes stages 10.5.2 (22-April) | Yellow-brown soils Soil pH = 6.40 OM = 23.55 g kg−1 Total N = 1.55 g kg−1 Available P = 44.80 mg g−1 Available K= 110.50 mg·g−1 |
| Experiment 3 2016–2017 | Sihong (33.37°N, 118.26°E) | 16.2 | 910 | Xumai30 Huaimai20 | 0 (N0) 90 (N1) 180 (N2) 270 (N3) 360 (N4) | Feekes stages 7.0 (13-April) Feekes stages 10.0 (19-April) Feekes stages 10.3 (25-April) Feekes stages 10.5.2 (30-April) | Lime concretion black soil Soil pH = 6.56 OM = 25.98 g kg−1 Total N = 2.80 g kg−1 Available P = 45.45 mg g−1 Available K = 91.66 mg g−1 |
| Experiment 4 2018–2019 | Xinghua (33.08°N, 119.98°E) | 17.3 | 900 | Zhenmai12 Yangmai23 Ningmai13 | 0 (N0) 90 (N1) 180 (N2) 270 (N3) 360 (N4) | Feekes stages 6.0 (15-March) Feekes stages 9.0 (29-March) Feekes stages 10.3 (14-April) Feekes stages 10.5.2 (21-April) | Yellow-brown soils Soil pH = 6.61 OM = 21.26 g kg−1 Total N = 1.71 g kg−1 Available P = 41.06 mg g−1 Available K = 108.61 mg g−1 |
| Index | Formula | Reference |
|---|---|---|
| Normalized difference vegetation index (NDVI) | (NIR − R)/(NIR + R) | [42] |
| Normalized difference red edge (NDRE) | (NIR − RE)/(NIR + RE) | [43] |
| Red edge soil-adjusted vegetation index (RESAVI) | 1.5 × [(NIR − RE)/(NIR + RE + 0.5)] | [5] |
| Difference vegetation index (DVI) | NIR − R | [44] |
| Soil-adjusted vegetation index (SAVI) | (1 + L)(NIR − R)/(NIR + R + L); L = 0.5 | [45] |
| Red edge ratio vegetation index (RERVI) | NIR/RE | [46] |
| Perpendicular vegetation index (PVI) | (NIR + 1.05R − 0.03)/SQRT(1 + 1.052) | [44] |
| Red edge difference vegetation index (REDVI) | NIR − RE | [42] |
| Ratio vegetation index (RVI) | NIR/R | [47] |
| Red edge wide dynamic range vegetation index (REWDRVI) | (a × NIR − RE)/(a × NIR + RE); a = 0.12 | [48] |
| Optimized vegetation index 1 (VIopt1) | 100 × (lnNIR − lnRE) | [46] |
| Transformed vegetation index (TVI) | SQRT((NIR − R)/(NIR + R) + 0.5) | [49] |
| Optimized soil-adjusted vegetation index (OSAVI) | (NIR − R)/(NIR + R + 0.16) | [50] |
| Reflection in red edge (RRE) | (NIR + R)/2 | [51] |
| Red edge re-normalized different vegetation index (RERDVI) | (NIR − RE)/SQRT(NIR + RE) | [52] |
| Red edge chlorophyll index (CIRE) | NIR/RE − 1 | [53] |
| Canopy chlorophyll content index (CCCI) | (NDRE − NDREmin)/(NDREmax − NDREmin) | [43] |
| Parameter | Growth Stage | Calibration Data | Validation Data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min. | Max. | Mean | SD | CV (%) | N | Min. | Max. | Mean | SD | CV (%) | ||
| LAI | Feekes stages 6.0–10.0 | 252 | 0.33 | 5.89 | 2.36 | 1.28 | 52.24 | 90 | 1.56 | 4.88 | 2.93 | 0.85 | 29.01 |
| Feekes stages 10.3–11.1 | 186 | 0.26 | 7.51 | 2.99 | 1.86 | 62.21 | 90 | 1.44 | 6.84 | 3.47 | 1.10 | 31.70 | |
| All growth stages | 438 | 0.26 | 7.51 | 2.63 | 1.58 | 60.08 | 180 | 1.44 | 6.84 | 3.20 | 1.02 | 31.88 | |
| LDM | Feekes stages 6.0–10.0 | 252 | 0.15 | 2.83 | 1.01 | 0.54 | 53.46 | 90 | 0.57 | 2.10 | 1.19 | 0.35 | 29.41 |
| Feekes stages 10.3–11.1 | 186 | 0.14 | 2.28 | 0.99 | 0.49 | 49.49 | 90 | 0.58 | 2.62 | 1.38 | 0.42 | 30.43 | |
| All growth stages | 438 | 0.14 | 2.83 | 1.00 | 0.51 | 51.00 | 180 | 0.57 | 2.62 | 1.29 | 0.40 | 31.01 | |
| PDM | Feekes stages 6.0–10.0 | 252 | 0.53 | 7.91 | 2.93 | 1.65 | 56.31 | 90 | 1.35 | 5.08 | 2.93 | 0.88 | 30.03 |
| Feekes stages 10.3–11.1 | 186 | 0.97 | 11.54 | 6.09 | 2.16 | 35.46 | 90 | 3.23 | 12.69 | 7.72 | 1.95 | 25.26 | |
| All growth stages | 438 | 0.53 | 11.54 | 4.27 | 2.44 | 57.14 | 180 | 1.35 | 12.69 | 5.32 | 2.84 | 53.38 | |
| LNA | Feekes stages 6.0–10.0 | 252 | 1.49 | 107.81 | 29.30 | 21.33 | 72.79 | 90 | 13.61 | 88.83 | 41.31 | 16.58 | 40.14 |
| Feekes stages 10.3–11.1 | 186 | 1.90 | 100.94 | 32.08 | 21.37 | 66.61 | 90 | 14.53 | 77.57 | 42.84 | 16.96 | 39.59 | |
| All growth stages | 438 | 1.49 | 107.81 | 30.48 | 21.37 | 70.11 | 180 | 13.61 | 88.83 | 42.08 | 16.74 | 39.78 | |
| PNA | Feekes stages 6.0–10.0 | 252 | 5.45 | 207.86 | 52.93 | 37.07 | 70.04 | 90 | 23.07 | 133.26 | 64.52 | 23.53 | 36.47 |
| Feekes stages 10.3–11.1 | 186 | 8.83 | 246.63 | 88.57 | 55.00 | 62.09 | 90 | 31.54 | 170.27 | 93.12 | 33.97 | 36.48 | |
| All growth stages | 438 | 5.45 | 246.63 | 68.06 | 48.80 | 71.70 | 180 | 23.07 | 170.27 | 78.82 | 32.47 | 41.20 | |
| NNI | Feekes stages 6.0–10.0 | 252 | 0.18 | 1.57 | 0.64 | 0.26 | 40.63 | 90 | 0.45 | 1.26 | 0.82 | 0.21 | 25.61 |
| Feekes stages 10.3–11.1 | 186 | 0.21 | 1.64 | 0.72 | 0.32 | 44.44 | 90 | 0.38 | 1.06 | 0.69 | 0.18 | 26.09 | |
| All growth stages | 438 | 0.18 | 1.64 | 0.67 | 0.29 | 43.28 | 180 | 0.38 | 1.26 | 0.75 | 0.21 | 28.00 | |
| Growth Stages | LAI | LDM | PDM | |||
|---|---|---|---|---|---|---|
| Index | R² | Index | R² | Index | R2 | |
| Feekes stages 6.0–10.0 | RERVI | 0.75 | DVI | 0.61 | DVI | 0.64 |
| CIRE | 0.75 | RERVI | 0.60 | RERVI | 0.63 | |
| REWDRVI | 0.75 | CIRE | 0.60 | CIRE | 0.63 | |
| REDVI | 0.74 | REDVI | 0.60 | REDVI | 0.63 | |
| RERDVI | 0.74 | REWDRVI | 0.60 | REWDRVI | 0.63 | |
| DVI | 0.73 | RERDVI | 0.59 | RERDVI | 0.62 | |
| Feekes stages 10.3–11.1 | RERVI | 0.88 | DVI | 0.78 | DVI | 0.73 |
| CIRE | 0.88 | RVI | 0.76 | RVI | 0.71 | |
| REWDRVI | 0.88 | RERVI | 0.71 | SAVI | 0.69 | |
| DVI | 0.87 | CIRE | 0.71 | OSAVI | 0.69 | |
| REDVI | 0.87 | REWDRVI | 0.71 | NDVI | 0.69 | |
| RERDVI | 0.87 | SAVI | 0.70 | TVI | 0.68 | |
| All growth stages | RERVI | 0.78 | DVI | 0.61 | DVI | 0.63 |
| CIRE | 0.78 | RERVI | 0.61 | RVI | 0.60 | |
| DVI | 0.78 | CIRE | 0.61 | SAVI | 0.55 | |
| REWDRVI | 0.78 | REDVI | 0.60 | OSAVI | 0.54 | |
| REDVI | 0.78 | REWDRVI | 0.60 | NDVI | 0.54 | |
| RERDVI | 0.77 | RERDVI | 0.59 | RERDVI | 0.54 | |
| Growth Stages | LNA | PNA | NNI | |||
|---|---|---|---|---|---|---|
| Index | R2 | Index | R2 | Index | R2 | |
| Feekes stages 6.0–10.0 | DVI | 0.66 | DVI | 0.65 | DVI | 0.52 |
| RERVI | 0.64 | RERVI | 0.63 | RERVI | 0.50 | |
| CIRE | 0.64 | CIRE | 0.63 | CIRE | 0.50 | |
| REDVI | 0.63 | REDVI | 0.62 | REWDRVI | 0.49 | |
| REWDRVI | 0.63 | REWDRVI | 0.62 | REDVI | 0.49 | |
| RERDVI | 0.61 | RERDVI | 0.60 | RERDVI | 0.48 | |
| Feekes stages 10.3–11.1 | DVI | 0.72 | DVI | 0.82 | DVI | 0.81 |
| RVI | 0.72 | REDVI | 0.76 | RERVI | 0.75 | |
| SAVI | 0.66 | RERVI | 0.76 | CIRE | 0.75 | |
| RERVI | 0.65 | CIRE | 0.76 | REDVI | 0.75 | |
| CIRE | 0.65 | REWDRVI | 0.75 | REWDRVI | 0.75 | |
| OSAVI | 0.65 | RVI | 0.75 | RERDVI | 0.75 | |
| All growth stages | DVI | 0.65 | DVI | 0.73 | DVI | 0.63 |
| RERVI | 0.63 | RERVI | 0.67 | RERVI | 0.62 | |
| CIRE | 0.63 | CIRE | 0.67 | CIRE | 0.62 | |
| REDVI | 0.62 | RVI | 0.67 | REDVI | 0.60 | |
| REWDRVI | 0.62 | REDVI | 0.66 | REWDRVI | 0.59 | |
| RERDVI | 0.61 | REWDRVI | 0.65 | RERDVI | 0.58 | |
| Growth Stages | LAI | LDM | PDM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | R2 | RMSE | Bias | Index | R2 | RMSE (t ha−1) | Bias (t ha−1) | Index | R2 | RMSE (t ha−1) | Bias (t ha−1) | |
| Feekes stages 6.0–10.0 | DVI | 0.68 | 0.72 | −0.54 | DVI | 0.72 | 0.23 | −0.13 | RERVI | 0.61 | 0.69 | −0.42 |
| RERVI | 0.67 | 1.02 | −0.92 | RERVI | 0.72 | 0.36 | −0.31 | CIRE | 0.61 | 0.69 | −0.42 | |
| CIRE | 0.67 | 1.02 | −0.92 | CIRE | 0.72 | 0.36 | −0.31 | DVI | 0.60 | 0.69 | −0.42 | |
| RERDVI | 0.67 | 1.05 | −0.94 | RERDVI | 0.72 | 0.38 | −0.33 | RERDVI | 0.60 | 0.70 | −0.43 | |
| REWDRVI | 0.67 | 1.06 | −0.94 | REWDRVI | 0.72 | 0.38 | −0.33 | REWDRVI | 0.60 | 0.70 | −0.43 | |
| REDVI | 0.67 | 1.08 | −0.97 | REDVI | 0.72 | 0.39 | −0.34 | REDVI | 0.60 | 0.72 | −0.46 | |
| Feekes stages 10.3–11.1 | DVI | 0.69 | 0.92 | −0.60 | DVI | 0.69 | 0.48 | −0.42 | DVI | 0.43 | 1.56 | −0.12 |
| REDVI | 0.69 | 1.47 | −1.32 | RERVI | 0.69 | 0.63 | −0.59 | TVI | 0.43 | 1.57 | 0.10 | |
| RERVI | 0.69 | 1.49 | −1.36 | CIRE | 0.69 | 0.63 | −0.59 | SAVI | 0.43 | 1.61 | 0.14 | |
| CIRE | 0.69 | 1.49 | −1.36 | REWDRVI | 0.69 | 0.63 | −0.59 | NDVI | 0.43 | 1.61 | 0.16 | |
| REWDRVI | 0.69 | 1.50 | −1.36 | SAVI | 0.65 | 0.65 | 0.37 | OSAVI | 0.42 | 2.84 | 1.51 | |
| RERDVI | 0.69 | 1.52 | −1.36 | RVI | 0.65 | 0.66 | 0.38 | RVI | 0.31 | 3.34 | 1.66 | |
| All growth stages | DVI | 0.70 | 0.71 | −0.42 | DVI | 0.72 | 0.34 | −0.26 | DVI | 0.32 | 2.52 | −0.89 |
| RERVI | 0.70 | 1.19 | −1.06 | RERVI | 0.72 | 0.48 | −0.42 | SAVI | 0.31 | 2.53 | 0.81 | |
| CIRE | 0.70 | 1.19 | −1.06 | CIRE | 0.72 | 0.48 | −0.42 | NDVI | 0.30 | 2.53 | 0.82 | |
| REWDRVI | 0.70 | 1.21 | −1.08 | RERDVI | 0.72 | 0.49 | −0.43 | RERDVI | 0.30 | 2.93 | −1.70 | |
| RERDVI | 0.70 | 1.21 | −1.08 | REWDRVI | 0.72 | 0.49 | −0.43 | OSAVI | 0.29 | 3.50 | 2.52 | |
| REDVI | 0.70 | 1.24 | −1.11 | REDVI | 0.72 | 0.50 | −0.44 | RVI | 0.29 | 4.05 | 2.34 | |
| Growth Stages | LNA | PNA | NNI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | R2 | RMSE (kg ha−1) | Bias (kg ha−1) | Index | R2 | RMSE (kg ha−1) | Bias (kg ha−1) | Index | R2 | RMSE | Bias | |
| Feekes stages 6.0–10.0 | DVI | 0.74 | 12.86 | −9.50 | DVI | 0.72 | 14.50 | −7.58 | DVI | 0.56 | 0.26 | −0.21 |
| RERVI | 0.74 | 19.61 | −17.16 | RERVI | 0.72 | 23.98 | −20.46 | RERDVI | 0.54 | 0.28 | −0.23 | |
| CIRE | 0.74 | 19.61 | −17.16 | CIRE | 0.72 | 23.98 | −20.46 | RERVI | 0.54 | 0.29 | −0.24 | |
| RERDVI | 0.74 | 19.90 | −17.52 | RERDVI | 0.72 | 24.61 | −21.05 | CIRE | 0.54 | 0.29 | −0.24 | |
| REWDRVI | 0.74 | 20.14 | −17.68 | REWDRVI | 0.72 | 24.97 | −21.34 | REWDRVI | 0.53 | 0.29 | −0.24 | |
| REDVI | 0.74 | 20.56 | −18.06 | REDVI | 0.71 | 25.61 | −21.99 | REDVI | 0.53 | 0.29 | −0.25 | |
| Feekes stages 10.3–11.1 | RERVI | 0.75 | 11.58 | −9.42 | DVI | 0.68 | 22.79 | −8.45 | RERVI | 0.78 | 0.13 | −0.10 |
| CIRE | 0.75 | 11.58 | −9.42 | RERVI | 0.67 | 34.50 | −28.29 | CIRE | 0.78 | 0.13 | −0.10 | |
| DVI | 0.73 | 12.05 | −9.56 | CIRE | 0.67 | 34.50 | −28.29 | REWDRVI | 0.78 | 0.13 | −0.10 | |
| SAVI | 0.68 | 12.32 | 6.30 | REWDRVI | 0.67 | 34.54 | −28.29 | DVI | 0.78 | 0.13 | −0.10 | |
| NDVI | 0.68 | 12.47 | 6.55 | REDVI | 0.67 | 35.33 | −29.36 | REDVI | 0.78 | 0.14 | −0.11 | |
| OSAVI | 0.67 | 29.18 | 26.53 | RVI | 0.66 | 38.64 | 22.13 | RERDVI | 0.78 | 0.15 | −0.12 | |
| All growth stages | DVI | 0.71 | 14.01 | −10.47 | DVI | 0.69 | 19.98 | −7.90 | RERVI | 0.41 | 0.22 | −0.16 |
| RERVI | 0.71 | 19.60 | −17.24 | RERVI | 0.69 | 30.87 | −24.92 | CIRE | 0.41 | 0.22 | −0.16 | |
| CIRE | 0.71 | 19.60 | −17.24 | CIRE | 0.69 | 30.87 | −24.92 | REWDRVI | 0.41 | 0.22 | −0.16 | |
| RERDVI | 0.71 | 20.07 | −17.63 | REWDRVI | 0.69 | 31.08 | −25.14 | RERDVI | 0.41 | 0.22 | −0.16 | |
| REWDRVI | 0.71 | 20.24 | −17.73 | REDVI | 0.69 | 31.95 | −26.09 | DVI | 0.41 | 0.23 | −0.16 | |
| REDVI | 0.71 | 20.61 | −18.06 | RVI | 0.63 | 38.62 | 21.98 | REDVI | 0.41 | 0.27 | −0.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Zhang, Z.; Cao, Q.; Liang, Y.; Krienke, B.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Use of an Active Canopy Sensor Mounted on an Unmanned Aerial Vehicle to Monitor the Growth and Nitrogen Status of Winter Wheat. Remote Sens. 2020, 12, 3684. https://doi.org/10.3390/rs12223684
Jiang J, Zhang Z, Cao Q, Liang Y, Krienke B, Tian Y, Zhu Y, Cao W, Liu X. Use of an Active Canopy Sensor Mounted on an Unmanned Aerial Vehicle to Monitor the Growth and Nitrogen Status of Winter Wheat. Remote Sensing. 2020; 12(22):3684. https://doi.org/10.3390/rs12223684
Chicago/Turabian StyleJiang, Jie, Zeyu Zhang, Qiang Cao, Yan Liang, Brian Krienke, Yongchao Tian, Yan Zhu, Weixing Cao, and Xiaojun Liu. 2020. "Use of an Active Canopy Sensor Mounted on an Unmanned Aerial Vehicle to Monitor the Growth and Nitrogen Status of Winter Wheat" Remote Sensing 12, no. 22: 3684. https://doi.org/10.3390/rs12223684