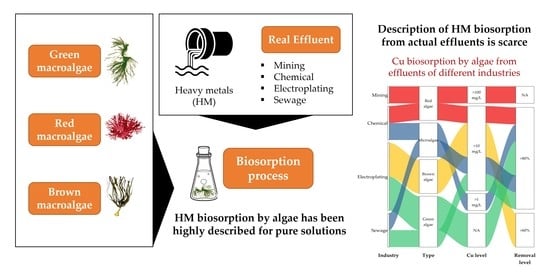
Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents
Abstract
:1. Treatment Techniques for Heavy Metals Contaminated Effluents
2. Biosorption Process
3. Marine Algae as Biosorbents
4. Heavy Metals Biosorption by Algae
4.1. Brown Seaweeds
4.2. Red Seaweeds
4.3. Green Seaweeds
5. New Challenges: Biosorption of Copper from Mining and Industrial Effluents Using Seaweeds
5.1. Mining
5.2. Chemical and Electroplating
5.3. Sewage
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Chen, C. Biosorption of Heavy Metals by Saccharomyces Cerevisiae: A Review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Taib, S.M.; Md Din, M.F.; Dahalan, F.A.; Kamyab, H. Comprehensive Review on Phytotechnology: Heavy Metals Removal by Diverse Aquatic Plants Species from Wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.G.; Mesci, B. Use of Pistachio Shells as an Adsorbent for the Removal of Zinc(II) Ion. CLEAN-Soil Air Water 2011, 39, 475–481. [Google Scholar] [CrossRef]
- Jiménez-Cedillo, M.J.; Olguín, M.T.; Fall, C.; Colin-Cruz, A. As(III) and As(V) Sorption on Iron-Modified Non-Pyrolyzed and Pyrolyzed Biomass from Petroselinum Crispum (Parsley). J. Environ. Manag. 2013, 117, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of Heavy Metals by Microorganisms: Evaluation of Different Underlying Mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Balasubramanian, P. Characteristics, Performances, Equilibrium and Kinetic Modeling Aspects of Heavy Metal Removal Using Algae. Bioresour. Technol. Rep. 2019, 5, 261–279. [Google Scholar] [CrossRef]
- Arumugam, N.; Chelliapan, S.; Kamyab, H.; Thirugnana, S.; Othman, N.; Nasri, N. Treatment of Wastewater Using Seaweed: A Review. Int. J. Environ. Res. Public Health 2018, 15, 2851. [Google Scholar] [CrossRef] [Green Version]
- Diaby, N.; Dold, B.; Pfeifer, H.-R.; Holliger, C.; Johnson, D.B.; Hallberg, K.B. Microbial Communities in a Porphyry Copper Tailings Impoundment and Their Impact on the Geochemical Dynamics of the Mine Waste. Environ. Microbiol. 2007, 9, 298–307. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Kuan, S.H. A Review of Sustainable Development in the Chilean Mining Sector: Past, Present and Future. Int. J. Min. Reclam. Environ. 2017, 31, 137–165. [Google Scholar] [CrossRef]
- Krishna, R.S.; Mishra, J.; Meher, S.; Das, S.K.; Mustakim, S.M.; Singh, S.K. Industrial Solid Waste Management through Sustainable Green Technology: Case Study Insights from Steel and Mining Industry in Keonjhar, India. Mater. Today Proc. 2020, 33, 5243–5249. [Google Scholar] [CrossRef]
- Ordóñez, J.I.; Wong-Pinto, L.; Cortés, S. Biotecnología Aplicada a La Valorización de Relaves Mineros. In Economía Circular en Procesos Mineros; Cisternas, L., Gálvez, E., Rivas, M., Valderrama, J., Eds.; RIL Editores: Santiago, Chile, 2021; pp. 63–91. [Google Scholar]
- Reyes, A.; Cuevas, J.; Fuentes, B.; Fernández, E.; Arce, W.; Guerrero, M.; Letelier, M.V. Distribution of Potentially Toxic Elements in Soils Surrounding Abandoned Mining Waste Located in Taltal, Northern Chile. J. Geochem. Explor. 2021, 220, 106653. [Google Scholar] [CrossRef]
- Khalil, A.; Hanich, L.; Bannari, A.; Zouhri, L.; Pourret, O.; Hakkou, R. Assessment of Soil Contamination around an Abandoned Mine in a Semi-Arid Environment Using Geochemistry and Geostatistics: Pre-Work of Geochemical Process Modeling with Numerical Models. J. Geochem. Explor. 2013, 125, 117–129. [Google Scholar] [CrossRef]
- Solgi, E.; Parmah, J. Analysis and Assessment of Nickel and Chromium Pollution in Soils around Baghejar Chromite Mine of Sabzevar Ophiolite Belt, Northeastern Iran. Trans. Nonferrous Met. Soc. China 2015, 25, 2380–2387. [Google Scholar] [CrossRef]
- Liu, G.; Tao, L.; Liu, X.; Hou, J.; Wang, A.; Li, R. Heavy Metal Speciation and Pollution of Agricultural Soils along Jishui River in Non-Ferrous Metal Mine Area in Jiangxi Province, China. J. Geochem. Explor. 2013, 132, 156–163. [Google Scholar] [CrossRef]
- CSIRO Turning Mining Wastewater into Rainwater. Available online: www.csiro.au/en/news/news-releases/2014/turning-mining-wastewater-into-rainwater (accessed on 2 March 2023).
- Grupo Vento Tratamiento de Efluentes Industriales En Minería. Available online: Evaporadoresindustriales.grupovento.com/tratamiento-de-efluentes-industriales-en-mineria (accessed on 2 March 2023).
- WaterCanada Overcoming the Mine Water Management Challenge. Available online: www.watercanada.net/feature/overcoming-the-mine-water-management-challenge (accessed on 2 March 2023).
- Ministerio de Minería Chile Pampa Austral Tailing Reservoir, Atacama Region. Plan Nacional de Depósitos de Relaves Para Una Minería Sostenible. Available online: www.minmineria.cl/media/2021/05/Plan_Nacional_de_Despositos_de_Relaves_para_una_Mineria_Sostenible_2021.pdf (accessed on 3 March 2023).
- Dodson, J.R.; Hunt, A.J.; Parker, H.L.; Yang, Y.; Clark, J.H. Elemental Sustainability: Towards the Total Recovery of Scarce Metals. Chem. Eng. Process. Process Intensif. 2012, 51, 69–78. [Google Scholar] [CrossRef]
- Brown, S.S.; Kodama, Y. Toxicology of Metals, Clinical and Experimental Research; Ellis Horwood Ltd.: New York, NY, USA, 1987. [Google Scholar]
- Li, Y.; Cha, C.; Lv, X.J.; Liu, J.; He, J.; Pang, Q.; Meng, L.; Kuang, H.; Fan, R. Association between 10 Urinary Heavy Metal Exposure and Attention Deficit Hyperactivity Disorder for Children. Environ. Sci. Pollut. Res. 2020, 27, 31233–31242. [Google Scholar] [CrossRef] [PubMed]
- Balladares, E.; Jerez, O.; Parada, F.; Baltierra, L.; Hernández, C.; Araneda, E.; Parra, V. Neutralization and Co-Precipitation of Heavy Metals by Lime Addition to Effluent from Acid Plant in a Copper Smelter. Miner. Eng. 2018, 122, 122–129. [Google Scholar] [CrossRef]
- Carneiro Brandão Pereira, T.; Batista dos Santos, K.; Lautert-Dutra, W.; de Souza Teodoro, L.; de Almeida, V.O.; Weiler, J.; Homrich Schneider, I.A.; Reis Bogo, M. Acid Mine Drainage (AMD) Treatment by Neutralization: Evaluation of Physical-Chemical Performance and Ecotoxicological Effects on Zebrafish (Danio Rerio) Development. Chemosphere 2020, 253, 126665. [Google Scholar] [CrossRef]
- Ordóñez, J.I.; Mercado, A.; Wong-Pinto, L.; Cortés, S.I. Bioremediation of Mining Waste and Other Copper-Containing Effluents by Biosorption. In Bioremediation of Toxic Metal(loid)s; Malik, A., Kidwai, M.K., Garg, V.K., Eds.; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9781032135779. [Google Scholar]
- Leong, Y.K.; Chang, J.-S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Piervandi, Z.; Khodadadi Darban, A.; Mousavi, S.M.; Abdollahy, M.; Asadollahfardi, G.; Funari, V.; Dinelli, E.; Webster, R.D.; Sillanpää, M. Effect of Biogenic Jarosite on the Bio-Immobilization of Toxic Elements from Sulfide Tailings. Chemosphere 2020, 258, 127288. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Hanus-Fajerska, E.; Gambuś, F.; Muszyńska, E.; Czech, T. Phytostabilization of Zn-Pb Ore Flotation Tailings with Dianthus Carthusianorum and Biscutella Laevigata after Amending with Mineral Fertilizers or Sewage Sludge. J. Environ. Manag. 2017, 189, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Bai, X.; Zhang, X.; Zhang, J.; Gao, X.; Yu, W.W. Multicolor Light-Emitting Diodes with MoS 2 Quantum Dots. Part. Part. Syst. Charact. 2019, 36, 2–6. [Google Scholar] [CrossRef]
- Wong-Pinto, L.; Menzies, A.; Ordóñez, J.I. Bionanomining: Biotechnological Synthesis of Metal Nanoparticles from Mining Waste—Opportunity for Sustainable Management of Mining Environmental Liabilities. Appl. Microbiol. Biotechnol. 2020, 104, 1859–1869. [Google Scholar] [CrossRef]
- Wong-Pinto, L.-S.; Mercado, A.; Chong, G.; Salazar, P.; Ordóñez, J.I. Biosynthesis of Copper Nanoparticles from Copper Tailings Ore—An Approach to the ‘Bionanomining’. J. Clean. Prod. 2021, 315, 128107. [Google Scholar] [CrossRef]
- Pollmann, K.; Kutschke, S.; Matys, S.; Raff, J.; Hlawacek, G.; Lederer, F.L. Bio-Recycling of Metals: Recycling of Technical Products Using Biological Applications. Biotechnol. Adv. 2018, 36, 1048–1062. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the Art for the Biosorption Process—A Review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef] [Green Version]
- Gadd, G.M. Biosorption: Critical Review of Scientific Rationale, Environmental Importance and Significance for Pollution Treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Crist, R.H.; Martin, J.R.; Crist, D.R. Interaction of Metal Ions with Acid Sites of Biosorbents Peat Moss and Vaucheria and Model Substances Alginic and Humic Acids. Environ. Sci. Technol. 1999, 33, 2252–2256. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption of Heavy Metals; CRC Press: Boca Raton, FL, USA, 1990; ISBN 9780849349171. [Google Scholar]
- Davis, T.A.; Volesky, B.; Mucci, A. A Review of the Biochemistry of Heavy Metal Biosorption by Brown Algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an Efficient Method for Removing Heavy Metals from Industrial Effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Cortés, S.; Soto, E.E.; Ordóñez, J.I. Recovery of Copper from Leached Tailing Solutions by Biosorption. Minerals 2020, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Baysal, Z.; Çinar, E.; Bulut, Y.; Alkan, H.; Dogru, M. Equilibrium and Thermodynamic Studies on Biosorption of Pb(II) onto Candida Albicans Biomass. J. Hazard. Mater. 2009, 161, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Mahadevan, A.; Vijayaraghavan, K.; Pavagadhi, S.; Balasubramanian, R. Green Recovery of Gold through Biosorption, Biocrystallization, and Pyro-Crystallization. Ind. Eng. Chem. Res. 2010, 49, 7129–7135. [Google Scholar] [CrossRef]
- Selatnia, A.; Madani, A.; Bakhti, M.Z.; Kertous, L.; Mansouri, Y.; Yous, R. Biosorption of Ni2+ from Aqueous Solution by a NaOH-Treated Bacterial Dead Streptomyces Rimosus Biomass. Miner. Eng. 2004, 17, 903–911. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial Biosorbents and Biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Tsezos, M. Biosorption of Metals. The Experience Accumulated and the Outlook for Technology Development. Hydrometallurgy 2001, 59, 241–243. [Google Scholar] [CrossRef]
- Atiku, H.; Mohamed, R.; Al-Gheethi, A.; Wurochekke, A.; Kassim, A.H.M. Harvesting of Microalgae Biomass from the Phycoremediation Process of Greywater. Environ. Sci. Pollut. Res. 2016, 23, 24624–24641. [Google Scholar] [CrossRef]
- Volesky, B. Detoxification of Metal-Bearing Effluents: Biosorption for the next Century. Hydrometallurgy 2001, 59, 203–216. [Google Scholar] [CrossRef]
- Volesky, B.; Holan, Z.R. Biosorption of Heavy Metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Biosorption with Algae: A Statistical Review. Crit. Rev. Biotechnol. 2006, 26, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Comparative Study of Biosorption of Heavy Metals Using Different Types of Algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, J.P. A Comprehensive Review on Biosorption of Heavy Metals by Algal Biomass: Materials, Performances, Chemistry, and Modeling Simulation Tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Raize, O.; Argaman, Y.; Yannai, S. Mechanisms of Biosorption of Different Heavy Metals by Brown Marine Macroalgae. Biotechnol. Bioeng. 2004, 87, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Figueira, M.M.; Volesky, B.; Mathieu, H.J. Instrumental Analysis Study of Iron Species Biosorption by Sargassum Biomass. Environ. Sci. Technol. 1999, 33, 1840–1846. [Google Scholar] [CrossRef]
- Fourest, E.; Canal, C.; Roux, J.-C. Improvement of Heavy Metal Biosorption by Mycelial Dead Biomasses (Rhizopus Arrhizus, Mucor Miehei and Penicillium Chrysogenum): PH Control and Cationic Activation. FEMS Microbiol. Rev. 1994, 14, 325–332. [Google Scholar] [CrossRef]
- Khoo, K.-M.; Ting, Y.-P. Biosorption of Gold by Immobilized Fungal Biomass. Biochem. Eng. J. 2001, 8, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mata, Y.N.; Torres, E.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Gold(III) Biosorption and Bioreduction with the Brown Alga Fucus Vesiculosus. J. Hazard. Mater. 2009, 166, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Brinza, L.; Dring, M.J.; Gavrilescu, M. Marine Micro and Macro Algal Species as Biosorbents for Heavy Metals. Environ. Eng. Manag. J. 2007, 6, 237–251. [Google Scholar] [CrossRef]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Characterization of the Biosorption of Cadmium, Lead and Copper with the Brown Alga Fucus Vesiculosus. J. Hazard. Mater. 2008, 158, 316–323. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.-P.; Chen, J.P.; Hong, L. Sorption of Lead, Copper, Cadmium, Zinc, and Nickel by Marine Algal Biomass: Characterization of Biosorptive Capacity and Investigation of Mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Adamu, C.I.; Nganje, T.N.; Edet, A. Heavy Metal Contamination and Health Risk Assessment Associated with Abandoned Barite Mines in Cross River State, Southeastern Nigeria. Environ. Nanotechnol. Monit. Manag. 2015, 3, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.S.; Kandil, N.F.E.S.; Ibraheem, I.B.M. Biosorption of Pb2+ and Cr3+ Ions from Aqueous Solution by Two Brown Marine Macroalgae: An Equilibrium and Kinetic Study. Desalin. WATER Treat. 2020, 206, 250–262. [Google Scholar] [CrossRef]
- Plaza Cazón, J.; Bernardelli, C.; Viera, M.; Donati, E.; Guibal, E. Zinc and Cadmium Biosorption by Untreated and Calcium-Treated Macrocystis Pyrifera in a Batch System. Bioresour. Technol. 2012, 116, 195–203. [Google Scholar] [CrossRef]
- Plaza Cazón, J.; Viera, M.; Donati, E.; Guibal, E. Biosorption of Mercury by Macrocystis Pyrifera and Undaria Pinnatifida: Influence of Zinc, Cadmium and Nickel. J. Environ. Sci. 2011, 23, 1778–1786. [Google Scholar] [CrossRef]
- Luna, A.S.; Costa, A.L.H.; da Costa, A.C.A.; Henriques, C.A. Competitive Biosorption of Cadmium(II) and Zinc(II) Ions from Binary Systems by Sargassum Filipendula. Bioresour. Technol. 2010, 101, 5104–5111. [Google Scholar] [CrossRef]
- Perumal, S.V.; Joshi, U.M.; Karthikeyan, S.; Balasubramanian, R. Biosorption of Lead(II) and Copper(II) from Stormwater by Brown Seaweed Sargassum Sp.: Batch and Column Studies. Water Sci. Technol. 2007, 56, 277–285. [Google Scholar] [CrossRef]
- Cid, H.A.; Flores, M.I.; Pizarro, J.F.; Castillo, X.A.; Barros, D.E.; Moreno-Piraján, J.C.; Ortiz, C.A. Mechanisms of Cu2+ Biosorption on Lessonia Nigrescens Dead Biomass: Functional Groups Interactions and Morphological Characterization. J. Environ. Chem. Eng. 2018, 6, 2696–2704. [Google Scholar] [CrossRef]
- Chaisuksant, Y. Biosorption of Cadmium (II) and Copper (II) by Pretreated Biomass of Marine Alga Gracilaria Fisheri. Environ. Technol. 2003, 24, 1501–1508. [Google Scholar] [CrossRef]
- Pandya, K.Y.; Patel, R.V.; Jasrai, R.T.; Brahmbhatt, N. Biosorption of Cr, Ni & Cu from Industrial Dye Effluents onto Kappaphycus Alvarezii: Assessment of Sorption Isotherms and Kinetics. Int. J. Eng. Res. Gen. Sci. 2017, 5, 137–148. [Google Scholar]
- Sarı, A.; Tuzen, M. Biosorption of Cadmium(II) from Aqueous Solution by Red Algae (Ceramium Virgatum): Equilibrium, Kinetic and Thermodynamic Studies. J. Hazard. Mater. 2008, 157, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Lucaci, A.R.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Equilibrium and Kinetics Studies of Metal Ions Biosorption on Alginate Extracted from Marine Red Algae Biomass (Callithamnion Corymbosum sp.). Polymers 2020, 12, 1888. [Google Scholar] [CrossRef]
- Fawzy, M.A. Biosorption of Copper Ions from Aqueous Solution by Codium Vermilara: Optimization, Kinetic, Isotherm and Thermodynamic Studies. Adv. Powder Technol. 2020, 31, 3724–3735. [Google Scholar] [CrossRef]
- Indhumathi, P.; Sathiyaraj, S.; Koelmel, J.P.; Shoba, S.U.; Jayabalakrishnan, C.; Saravanabhavan, M. The Efficient Removal of Heavy Metal Ions from Industry Effluents Using Waste Biomass as Low-Cost Adsorbent: Thermodynamic and Kinetic Models. Z. Für Phys. Chem. 2018, 232, 527–543. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibullah-Al-Mamun, M.; Islam, M.K. Heavy Metal Pollution in Surface Water and Sediment: A Preliminary Assessment of an Urban River in a Developing Country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Cossich, E.S.; Tavares, C.R.G.; da Silva, E.A. Biosorption of Nickel(II) and Copper(II) Ions from Synthetic and Real Effluents by Alginate-Based Biosorbent Produced from Seaweed sargassum sp. Environ. Sci. Pollut. Res. 2019, 26, 11100–11112. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, J.; Mishra, V. Biosorption-Cum-Bioaccumulation of Heavy Metals from Industrial Effluent by Brown Algae: Deep Insight. In Microbial Genomics in Sustainable Agroecosystems; Tripathi, V., Kumar, P., Tripathi, P., Kishore, A., Eds.; Springer: Singapore, 2019; pp. 249–270. [Google Scholar]
- Giese, E.C. Biosorption as Green Technology for the Recovery and Separation of Rare Earth Elements. World J. Microbiol. Biotechnol. 2020, 36, 52–62. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of Rare Earth Metals through Biosorption: An Overview. J. Rare Earths 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Golnaraghi Ghomi, A.; Asasian-Kolur, N.; Sharifian, S.; Golnaraghi, A. Biosorpion for Sustainable Recovery of Precious Metals from Wastewater. J. Environ. Chem. Eng. 2020, 8, 103996. [Google Scholar] [CrossRef]
- Castro, L.; Bonilla, L.A.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Continuous Metal Biosorption Applied to Industrial Effluents: A Comparative Study Using an Agricultural by-Product and a Marine Alga. Environ. Earth Sci. 2017, 76, 491. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Cossich, E.S.; Tavares, C.R.G.; da Silva, E.A. Biosorption of Nickel and Copper Ions from Synthetic Solution and Electroplating Effluent Using Fixed Bed Column of Immobilized Brown Algae. J. Water Process Eng. 2019, 32, 100904. [Google Scholar] [CrossRef]
- Chan, A.; Salsali, H.; McBean, E. Heavy Metal Removal (Copper and Zinc) in Secondary Effluent from Wastewater Treatment Plants by Microalgae. ACS Sustain. Chem. Eng. 2014, 2, 130–137. [Google Scholar] [CrossRef]
- Kottangodan, N.; Das, C.; Ram, A.; Meena, R.M.; Ramaiah, N. Phycoremediation of Hazardous Mixed Industrial Effluent by a Marine Strain of Phormidium sp. CLEAN Soil Air Water 2019, 47, 1800264. [Google Scholar] [CrossRef]





| Property | Specification | Refs. |
|---|---|---|
| Cost | Low. Most sorbents are made from waste or renewable materials. | [4,5,40] |
| Storage | Easy. Dried materials can be stabilized for years. | [41] |
| Selectivity | Medium. The selectivity can be chemically improved by modifying biosorbent surfaces. | [7,32] |
| Sorbent recovery and reusability | High. Biosorbents are frequently reused for many cycles. | [4,5,7] |
| Advantages | Disadvantages |
|---|---|
| Biomass is renewable and low cost of obtaining | If dead biomass is used, energy is needed for drying |
| Biomass can be used dead (no nutrients or oxygen required) | Microalgae have limited applicability in batch systems |
| Biomass can be regenerated (reusability) | Microalgae biomasses need to be immobilized |
| Selective for many heavy metals | |
| High uptake capacity | |
| Immobilization is not mandatory (macroalgae biomasses) | |
| No generation of residual sludge | |
| Few chemicals needed for desorption and regeneration |
| Type | Species | Metal | Refs. |
|---|---|---|---|
| Brown | Hydroclathrus clathratus | Pb, Cr | [61] |
| Cystoseira barbata | Pb, Cr | [61] | |
| Macrocystis pyrifera | Zn, Cd, Ni | [62,63] | |
| Fucus vesiculosus | Cd, Pb, Cu | [58] | |
| Sargassum filipendula | Cd, Zn | [64] | |
| Undaria pinnatifida | Hg | [63] | |
| Sargassum sp. | Pb, Cu | [65] | |
| Lessonia nigrescens | Cu | [66] | |
| Red | Gracilaria chilensis | Cu | [39] |
| Gracilaria fisheri | Cd, Cu | [67] | |
| Kappaphycus alvarezii | Cr, Ni, Cu | [68] | |
| Ceramium virgatum | Cd | [69] | |
| Callithamnion corymbosum | Cu, Co, Zn | [70] | |
| Green | Codium vermilara | Cu | [71] |
| Chlorella vulgaris | Cu | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordóñez, J.I.; Cortés, S.; Maluenda, P.; Soto, I. Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents. Sustainability 2023, 15, 5521. https://doi.org/10.3390/su15065521
Ordóñez JI, Cortés S, Maluenda P, Soto I. Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents. Sustainability. 2023; 15(6):5521. https://doi.org/10.3390/su15065521
Chicago/Turabian StyleOrdóñez, Javier I., Sonia Cortés, Pablo Maluenda, and Ignacio Soto. 2023. "Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents" Sustainability 15, no. 6: 5521. https://doi.org/10.3390/su15065521