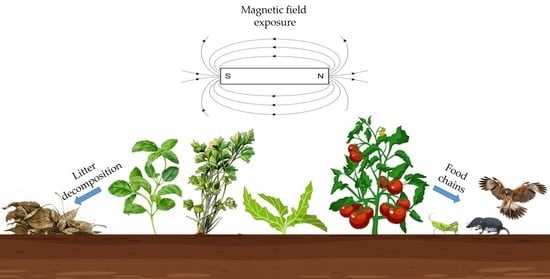
Effects of Weak Magnetic Fields on Plant Chemical Composition and Its Ecological Implications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MF | Magnetic Field |
| RR | Response Ratio |
| PTE | Potentially Toxic Element |
| NBI | Nitrogen Balance Index |
References
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 2022, 19, 20220325. [Google Scholar] [CrossRef] [PubMed]
- Malkemper, E.; Tscheulin, T.; Vanbergen, A.; Vian, A.; Balian, E.; Goudeseune, L. The Impacts of Artificial Electromagnetic Radiation on Wildlife (Flora and Fauna). Current Knowledge Overview: A Background; Technical report, A report of the EKLIPSE project; EKLIPSE Mechanism: Glasgow, Scotland, 2018. [Google Scholar]
- Lyu, W.; Song, Q.; Shi, J.; Wang, H.; Wang, B.; Hu, X. Weak magnetic field affected microbial communities and function in the A/O/A sequencing batch reactors for enhanced aerobic granulation. Sep. Purif. Technol. 2021, 266, 118537. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Ranjitha Kumari, B. Pulsed magnetic field: A contemporary approach offers to enhance plant growth and yield of soybean. Plant Physiol. Biochem. 2012, 51, 139–144. [Google Scholar] [CrossRef]
- Nyakane, N.E.; Markus, E.D.; Sedibe, M.M. The effects of magnetic fields on plants growth: A comprehensive review. ETP Int. J. Food Eng. 2019, 5, 79–87. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.; Menegatti, R.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic field (MF) applications in plants: An overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Dhawi, F. Why magnetic fields are used to enhance a plant’s growth and productivity? Annu. Res. Rev. Biol. 2014, 4, 886–896. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef]
- Bukhari, S.; Tanveer, M.; Mustafa, G.; Zia-Ud-Den, N. Magnetic field stimulation effect on germination and antioxidant activities of presown hybrid seeds of sunflower and its seedlings. J. Food Qual. 2021, 5594183, 1–9. [Google Scholar] [CrossRef]
- Luo, J.; He, W.; Xing, X.; Wu, J.; Gu, X. The phytoremediation efficiency of Eucalyptus globulus treated by static magnetic fields before sowing. Chemosphere 2019, 226, 891–897. [Google Scholar] [CrossRef]
- Luo, J.; He, W.; Yang, D.; Wu, J.; Sophie. Gu, X. Magnetic field enhance decontamination efficiency of Noccaea caerulescens and reduce leaching of non-hyperaccumulated metals. J. Hazard. Mater. 2019, 368, 141–148. [Google Scholar] [CrossRef]
- Litti, Y.; Kovalev, D.; Kovalev, A.; Katraeva, I.; Russkova, Y.; Nozhevnikova, A. Increasing the efficiency of organic waste conversion into biogas by mechanical pretreatment in an electromagnetic mill. J. Phys. Conf. Ser. 2018, 1111, 012013. [Google Scholar] [CrossRef]
- Ahamed, M.; Elzaawely, A.; Bayoumi, Y. Effect of magnetic field on seed germination, growth and yield of sweet pepper (Capsicum annuum L.). Asian J. Crop. Sci. 2013, 5, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Asadi Samani, M.; Pourakbar, L.; Azimi, N. Magnetic field effects on seed germination and activities of some enzymes in cumin. Life Sci. J. 2013, 10, 323–328. [Google Scholar]
- Martinez, E.; Florez, M.; Carbonell, M.V. Stimulatory effect of the magnetic treatment on the germination of cereal seeds. Int. J. Environ. Agric. Biotechnol. 2017, 2, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, F.; Niknam, V.; Ghanati, F.; Masroor, F.; Noorbakhsh, S. Biological effects of weak electromagnetic field on healthy and infected lime (Citrus aurantifolia) trees with phytoplasma. Sci. World J. 2012, 2012, 716929. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.P.; Chen, D.; Liu, Q. Exposure to a magnetic field or laser radiation ameliorates effects of Pb and Cd on physiology and growth of young wheat seedlings. J. Photochem. Photobiol. B Biol. 2017, 169, 171–177. [Google Scholar] [CrossRef]
- Radhakrishnan, R. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants 2019, 25, 1107–1119. [Google Scholar] [CrossRef]
- Ulgen, C.; Yildirim, A.; Sahin, G.; Turker, A. Do magnetic field applications affect in vitro regeneration, growth, phenolic profiles, antioxidant potential and defense enzyme activities (SOD, CAT and PAL) in lemon balm (Melissa officinalis L.)? Ind. Crop. Prod. 2021, 169, 113624. [Google Scholar] [CrossRef]
- Stange, B.; Rowland, R.; Rapley, B.; Podd, J. ELF magnetic fields increase amino acid uptake into Vicia faba L. roots and alter ion movement across the plasma membrane. Bioelectromagnetics 2002, 23, 347–354. [Google Scholar] [CrossRef]
- Atak, Ç.; Çelik, Ö.; Alikamanoğlu, S.; Rzakoulieva, A. Stimulation of regeneration by magnetic field in soybean (Glycine max L. Merrill) tissue cultures. J. Cell Mol. Biol. 2003, 2, 113–119. [Google Scholar]
- Moon, J.D.; Chung, H.S. Acceleration of germination of tomato seed by applying AC electric and magnetic fields. J. Electrost. 2000, 48, 103–114. [Google Scholar] [CrossRef]
- Takimoto, K.; Yaguchi, H.; Miyakoshi, J. Extremely low frequency magnetic fields suppress the reduction of germination rate of Arabidopsis thaliana seeds kept in saturated humidity. Biosci. Biotechnol. Biochem. 2001, 65, 2552–2554. [Google Scholar] [CrossRef] [PubMed]
- Eşitken, A.; Turan, M. Alternating magnetic field effects on yield and plant nutrient element composition of strawberry (Fragaria x ananassa cv. camarosa). Acta Agric. Scand. Sect. B Soil and Plant Sci. 2004, 54, 135–139. [Google Scholar] [CrossRef]
- McCann, J.; Dietrich, F.; Rafferty, C. The genotoxic potential of electric and magnetic fields: An update. Mutat. Res. Rev. Mutat. Res. 1998, 411, 45–86. [Google Scholar] [CrossRef]
- Gall, J.; Boyd, R.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.; Zeng, L.; Xiao, W.; Huang, Z.; Geng, X.; Tan, B. Effect of litter substrate quality and soil nutrients on forest litter decomposition: A review. Acta Ecol. Sin. 2013, 33, 102–108. [Google Scholar] [CrossRef]
- Baldantoni, D.; Morra, L.; Zaccardelli, M.; Alfani, A. Cadmium accumulation in leaves of leafy vegetables. Ecotoxicol. Environ. Saf. 2016, 123, 89–94. [Google Scholar] [CrossRef]
- Baldantoni, D.; Bellino, A.; Cicatelli, A.; Castiglione, S. Influence of the choice of cultivar and soil fertilization on PTE concentrations in Lactuca sativa L. in the framework of the regenerative agriculture revolution. Land 2021, 10. [Google Scholar] [CrossRef]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef]
- Baldantoni, D.; Bellino, A.; Alfani, A. Soil compost amendment enhances tomato (Solanum lycopersicum L.) quality. J. Sci. Food Agric. 2016, 96, 4082–4088. [Google Scholar] [CrossRef] [PubMed]
- Chulliat, A.; Alken, P.; Nair, M. The US/UK World Magnetic Model for 2020–2025: Technical Report; National Centers for Environmental Information, NOAA: Asheville, NC, USA, 2020. [Google Scholar] [CrossRef]
- Baldantoni, D.; Ligrone, R.; Alfani, A. Macro- and trace-element concentrations in leaves and roots of Phragmites australis in a volcanic lake in Southern Italy. J. Geochem. Explor. 2009, 101, 166–174. [Google Scholar] [CrossRef]
- NIST. SRM 1547—Peach Leaves; Technical Report, National Institute of Standards and Technology; U.S. Department of Commerce: Gaithersburg, MD, USA, 2022. [Google Scholar]
- Chow, P.; Landhäusser, S. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Bakbergenuly, I.; Hoaglin, D.; Kulinskaya, E. Estimation in meta-analyses of response ratios. BMC Med Res. Methodol. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.; Gurevitch, J.; Curtis, P. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Lajeunesse, M. Bias and correction for the log response ratio in ecological meta-analysis. Ecology 2015, 96, 2056–2063. [Google Scholar] [CrossRef] [Green Version]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Abdollahi, F.; Amiri, H.; Niknam, V.; Ghanati, F.; Mahdigholi, K. Effects of static magnetic fields on the antioxidant system of almond seeds. Russ. J. Plant Physiol. 2019, 66, 299–307. [Google Scholar] [CrossRef]
- Abou El-Yazied, A.; El-Gizawy, A.; Khalf, S.; El-Satar, A.; Shalaby, O. Effect of magnetic field treatments for seeds and irrigation water as well as N, P and K levels on productivity of tomato plants. J. Appl. Sci. Res. 2012, 8, 2088–2099. [Google Scholar]
- Dhawi, F.; Al-Khayri, J. The effect of magnetic resonance imaging on date palm (Phoenix dactylifera L.) elemental composition. Commun. Biometry Crop. Sci. 2009, 4, 14–20. [Google Scholar]
- Aendo, P.; Netvichian, R.; Thiendedsakul, P.; Khaodhiar, S.; Tulayakul, P. Carcinogenic risk of Pb, Cd, Ni, and Cr and critical ecological risk of Cd and Cu in soil and groundwater around the municipal solid waste open dump in central Thailand. J. Environ. Public Health 2022, 3062215. [Google Scholar] [CrossRef] [PubMed]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. Soil Components and Human Health; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- El-Ramady, H.; Hajdú, P.; Töros, G.; Badgar, K.; Llanaj, X.; Kiss, A.; Abdalla, N.; Omara, A.E.D.; Elsakhawy, T.; Elbasiouny, H.; et al. Plant nutrition for human health: A pictorial review on plant bioactive compounds for sustainable agriculture. Sustainability 2022, 14, 8329. [Google Scholar] [CrossRef]
- Stein, A.J. Global impacts of human mineral malnutrition. Plant Soil 2009, 335, 133–154. [Google Scholar] [CrossRef]
- Chen, Y.P.; He, J.M.; Li, R. Effects of magnetic fields pretreatment of mungbean seeds on sprout yield and quality. Afr. J. Biotechnol. 2012, 11, 8932–8937. [Google Scholar]
- Răcuciu, M.; Creanga, D.E.; Amorariei, C. Biochemical changes induced by low frequency magnetic field exposure of vegetal organisms. Rom. J. Phys. 2007, 52, 601–606. [Google Scholar]
- Răcuciu, M.; Creangǎ, D.; Cǎlugǎru, G. The influence of extremely low frequency magnetic field on tree seedlings. Rom. Rep. Phys. 2008, 53, 361–367. [Google Scholar]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, J.; Qiu, X.; Wei, F.; Xu, X. Decomposing litter and associated microbial activity responses to nitrogen deposition in two subtropical forests containing nitrogen-fixing or non-nitrogen-fixing tree species. Sci. Rep. 2018, 8, 12934. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Long, C.; Zhang, Q.; Cheng, X. Stronger effect of litter quality than micro-organisms on leaf and root litter C and N loss at different decomposition stages following a subtropical land use change. Funct. Ecol. 2022, 36, 896–907. [Google Scholar] [CrossRef]


| Species | Life Stage | Exposure Time | Sampling Time |
|---|---|---|---|
| L. sativa | seed | 4 h/day for 0, 40, 110 and 150 days | 70 and 150 days |
| P. crispum | plant | 0, 1, 2, 3, 6, 9 h/day for 10 days | 15 days |
| O. basilicum | plant | 0, 1, 2, 3, 6, 9 h/day for 10 days | 15 days |
| S. lycopersicum | seed | 8 h/day for 0 and 10 days | 90 days |
| April | May | June | July | August | |
|---|---|---|---|---|---|
| Daylight hours | 13.3 | 14.4 | 15.0 | 14.6 | 13.7 |
| Minimum temperature (°C) | 13.7 | 19.2 | 24.7 | 27.1 | 27.5 |
| Maximum temperature (°C) | 17.1 | 22.2 | 28.4 | 30.1 | 30.9 |
| Rainy days | 5 | 1 | 7 | 1 | 0 |
| Relative humidity (%) | 59.7 | 60.1 | 53.0 | 53.0 | 50.3 |
| Wind speed (Km/h) | 12.2 | 12.5 | 11.0 | 12.1 | 12.7 |
| Value | Unit | |
|---|---|---|
| Skeleton | 43 ± 6 | g/Kg |
| Coarse sand | 132 ± 4 | g/Kg |
| Fine sand | 71 ± 2 | g/Kg |
| Coarse silt | 36 ± 1 | g/Kg |
| Fine silt | 452 ± 15 | g/Kg |
| Clay | 309 ± 10 | g/Kg |
| pH | 8.1 ± 1.5 | – |
| Electrical conductivity | 44.2 ± 3.0 | mS/m |
| Total CaCO | 17 ± 6 | g/Kg |
| Active CaCO | 5 ± 2 | g/Kg |
| Organic matter | 18 ± 4 | g/Kg |
| Organic C | 10.4 ± 2.0 | g/Kg |
| Total N | 1.64 ± 0.20 | g/Kg |
| Extractable P | 67 ± 13 | mg/Kg P |
| Exchangeable Ca | 3862.9 ± 229.0 | mg/Kg Ca |
| Exchangeable Mg | 459.6 ± 27.0 | mg/Kg Mg |
| Exchangeable Na | 76.1 ± 7.0 | mg/Kg Na |
| Exchangeable K | 538.0 ± 20.1 | mg/Kg K |
| Cation exchange capacity | 25 | meq/100g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellino, A.; Bisceglia, B.; Baldantoni, D. Effects of Weak Magnetic Fields on Plant Chemical Composition and Its Ecological Implications. Sustainability 2023, 15, 3918. https://doi.org/10.3390/su15053918
Bellino A, Bisceglia B, Baldantoni D. Effects of Weak Magnetic Fields on Plant Chemical Composition and Its Ecological Implications. Sustainability. 2023; 15(5):3918. https://doi.org/10.3390/su15053918
Chicago/Turabian StyleBellino, Alessandro, Bruno Bisceglia, and Daniela Baldantoni. 2023. "Effects of Weak Magnetic Fields on Plant Chemical Composition and Its Ecological Implications" Sustainability 15, no. 5: 3918. https://doi.org/10.3390/su15053918