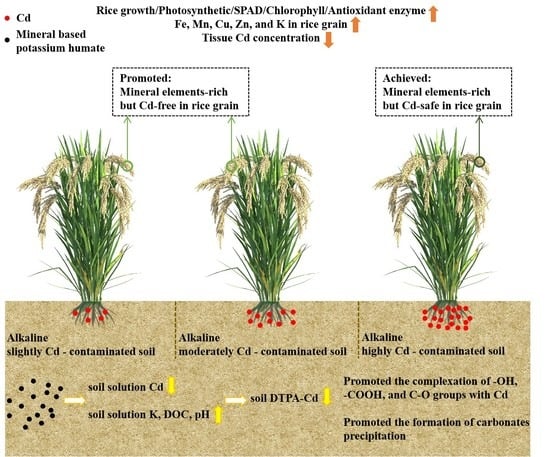
Effects of Mineral-Based Potassium Humate on Cadmium Accumulation in Rice (Oryza sativa L.) under Three Levels of Cadmium-Contaminated Alkaline Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Characterization of Mineral-Based Potassium Humate
2.2. Pot Experiments
2.2.1. Experimental Design
2.2.2. Rice Cultivation
2.3. Collection and Analysis of Soil Solution
2.4. Measurement of SPAD Value, Photosynthetic Ability, and Chlorophyll Fluorescence Parameter
2.5. Antioxidant Enzyme Assays
2.6. Plant Harvest, Soil Sample Collection, and Measurement of Agronomic Traits
2.7. Determination of Cd and Mineral Elements in Rice Organs
2.8. Soil Available Cd and ATR-FTIR Spectroscopic Analyses of Rhizosphere Soil
2.9. Data Analysis
3. Results
3.1. Growth Responses of Rice
3.2. Effect on Soil Solution Chemistry
3.2.1. Change in Soil Solution pH
3.2.2. Change in Soil Solution DOC
3.2.3. Change in Soil Solution K and Cd
3.3. Photosynthetic Parameters, SPAD, and Chlorophyll Fluorescence of Rice Leaves
3.4. Rice Leaves Antioxidant Enzyme Activities
3.5. Effects on Mineral Elements of Brown Rice
3.6. Cadmium Concentrations in Plant Parts and Transport Factors of Cd in Brown Rice
3.6.1. Cd Concentrations in Various Organs of the Plant
3.6.2. Transfer Factors of Cd between Different Organs
3.7. Effect on Soil Available Cd of the Rhizosphere Soil
3.8. FTIR Spectra Analysis of the Rhizosphere Soil
3.9. Stepwise Regression Analysis
4. Discussion
4.1. Effects of Soil Solution Chemistry Characteristics on Brown Rice Cd
4.2. Contribution of Humic Acid The Applications to the Healthy Growth of Rice by Supporting Photosynthesis and Chlorophyll Density and Enzyme Activities
4.3. A Key Mechanism of MBPH Affecting Cd Uptake in Rice
4.4. The Importance of Decreasing Cadmium and Increasing Mineral Elements in the Quality of Brown Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization; Food and Agriculture Organization of the United Nations; Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Seventy-Third 73rd Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Uddin, M.M.; Zakeel, M.C.M.; Zavahir, J.S.; Marikar, F.M.M.T.; Jahan, I. Heavy metal accumulation in rice and aquatic plants used as human food: A general review. Toxics 2021, 9, 360. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Cadmium toxicity and treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Cai, L.Q.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.Z.; Ma, W.J.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R.; Kalita, P. Rice-not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Technol. 2020, 97, 265–285. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Rice breaks ground for cadmium-free cereals. Curr. Opin. Plant Biol. 2013, 16, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.X.; Li, B.R.; Li, J.; Chen, W.; Xu, L. Heavy metals in paddy soil-rice systems of industrial and township areas from subtropical China: Levels, transfer and health risks. J. Geochem. Explor. 2018, 194, 210–217. [Google Scholar] [CrossRef]
- Lee, S.-B.; Kang, D.-W.; Yoo, J.-H.; Park, S.-W.; Oh, K.S.; Lee, J.; Cho, I.K.; Moon, B.-C.; Kim, W.-I.J.O.S.J. Determination of bioconcentration factor of heavy metal (loid)s in rice grown on soils vulnerable to heavy metal (loid)s contamination. Ocean Sci. J. 2017, 50, 106–114. [Google Scholar] [CrossRef]
- Koizumi, N.; Ohashi, F.; Ikeda, M. Lack of correlation between cadmium level in local brown rice and renal failure mortality among the residents: A nation-wide analysis in Japan. Int. Arch. Occup. Environ. Health 2010, 83, 333–339. [Google Scholar] [CrossRef]
- Mng’ong’o, M.; Munishi, L.K.; Ndakidemi, P.A.; Blake, W.; Comber, S.; Hutchinson, T.H. Accumulation and bioconcentration of heavy metals in two phases from agricultural soil to plants in Usangu agroecosystem-Tanzania. Heliyon 2021, 7, e07514. [Google Scholar] [CrossRef]
- Sriprachote, A.; Kanyawongha, P.; Ochiai, K.; Matoh, T. Current situation of cadmium-polluted paddy soil, rice and soybean in the Mae Sot District, Tak Province, Thailand. Soil Sci. Plant Nutr. 2012, 58, 349–359. [Google Scholar] [CrossRef]
- Hussain, B.; Umer, M.J.; Li, J.M.; Ma, Y.B.; Abbas, Y.; Ashraf, M.N.; Tahir, N.; Ullah, A.; Gogoi, N.; Farooq, M. Strategies for reducing cadmium accumulation in rice grains. J. Clean Prod. 2020, 286, 125557. [Google Scholar] [CrossRef]
- Ahmed, N.; Shah, A.R.; Danish, S.; Alharbi, K.; Datta, R. Acidified carbon with variable irrigation sources impact on rice growth and yield under Cd toxic alkaline Soil conditions. Sustainability 2022, 14, 10086. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pandey, S.D.; Misra, V. Stability constants of metal–humic acid complexes and its role in environmental detoxification. Ecotoxicol. Environ. Saf. 2000, 47, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Asli, S.; Neumann, P.M. Rhizosphere humic acid interacts with root cell walls to reduce hydraulic conductivity and plant development. Plant Soil 2010, 336, 313–322. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Anil, A.G.; Romero, R.; Ramamurthy, P.C.; Singh, J. Biodegradation of phorate by bacterial strains in the presence of humic acid and metal ions. J. Basic Microbiol. 2022, 62, 498–507. [Google Scholar] [CrossRef]
- Khan, K.Y.; Ali, B.; Cui, X.Q.; Feng, Y.; Stoffella, P.J.; Tang, L.; Yang, X.E. Effect of humic acid amendment on cadmium bioavailability and accumulation by pak choi (Brassica rapa ssp. chinensis L.) to alleviate dietary toxicity risk. Arch. Agron. Soil Sci. 2017, 63, 1431–1442. [Google Scholar] [CrossRef]
- Zhao, K.Q.; Yang, Y.; Peng, H.; Zhang, L.H.; Zhou, Y.Y.; Zhang, J.C.; Du, C.Y.; Liu, J.W.; Lin, X.; Wang, N.Y.; et al. Silicon fertilizers, humic acid and their impact on physicochemical properties, availability and distribution of heavy metals in soil and soil aggregates. Sci. Total Environ. 2022, 822, 153483. [Google Scholar] [CrossRef]
- Ding, H.J.; Tang, L.; Nie, Y.N.; Ji, H.B. Characteristics and interactions of heavy metals with humic acid in gold mining area soil at a upstream of a metropolitan drinking water source. J. Geochem. Explor. 2019, 200, 266–275. [Google Scholar] [CrossRef]
- Rashid, I.; Murtaza, G.; Dar, A.A.; Wang, Z.Y. The influence of humic and fulvic acids on Cd bioavailability to wheat cultivars grown on sewage irrigated Cd-contaminated soils. Ecotoxicol. Environ. Saf. 2020, 205, 111347. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Shams, M.S.; Khalifa, M.R.; El-Dali, M.A.; Rinklebe, J. Various soil amendments and environmental wastes affect the (im) mobilization and phytoavailability of potentially toxic elements in a sewage effluent irrigated sandy soil. Ecotoxicol. Environ. Saf. 2017, 142, 375–387. [Google Scholar] [CrossRef]
- Dmitrieva, E.; Efimova, E.; Siundiukova, K.; Perelomov, L. Surface properties of humic acids from peat and sapropel of increasing transformation. Environ. Chem. Lett. 2015, 13, 197–202. [Google Scholar] [CrossRef]
- Doskocil, L.; Burdikova-Szewieczkova, J.; Enev, V.; Kalina, L.; Wasserbauer, J. Spectral characterization and comparison of humic acids isolated from some European lignites. Fuel 2018, 213, 123–132. [Google Scholar] [CrossRef]
- Zhou, L.P.; Yuan, L.; Zhao, B.Q.; Li, Y.T.; Lin, Z.A. Structural characteristics of humic acids derived from Chinese weathered coal under different oxidizing conditions. PLoS ONE 2019, 14, e0217469. [Google Scholar] [CrossRef] [PubMed]
- Zara, M.; Ahmad, Z.; Akhtar, J.; Shahzad, K.; Sheikh, N.; Munir, S. Extraction and characterization of humic acid from Pakistani lignite coals. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1159–1166. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J. Phytoextraction of potentially toxic elements by Indian mustard, rapeseed, and sunflower from a contaminated riparian soil. Environ. Geochem. Health 2015, 37, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Tapia, Y.; Bustos, P.; Salazar, O.; Casanova, M.; Castillo, B.; Acuna, E.; Masaguer, A. Phytostabilization of Cu in mine tailings using native plant Carpobrotus aequilaterus and the addition of potassium humates. J. Geochem. Explor. 2017, 183, 102–113. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, Y.N.; Camara, A.Y.; Li, H.F. Effects of the addition and aging of humic acid-based amendments on the solubility of Cd in soil solution and its accumulation in rice. Chemosphere 2018, 196, 303–310. [Google Scholar] [CrossRef]
- Li, B.; Zhu, Q.H.; Zhang, Q.; Zhu, H.H.; Huang, D.Y.; Su, S.M.; Wang, Y.N.; Zeng, X.B. Cadmium and arsenic availability in soil under submerged incubation: The influence of humic substances on iron speciation. Ecotoxicol. Environ. Saf. 2021, 225, 112773. [Google Scholar] [CrossRef]
- Wang, Y.F.; Ying, Y.Q.; Lu, S.G. Si-Ca-K-Mg amendment reduces the phytoavailability and transfer of Cd from acidic soil to rice grain. Environ. Sci. Pollut. Res. 2020, 27, 33248–33258. [Google Scholar] [CrossRef]
- Jorfi, S.; Maleki, R.; Jaafarzadeh, N.; Ahmadi, M. Pollution load index for heavy metals in Mian-Ab plain soil, Khuzestan, Iran. Data Brief 2017, 15, 584–590. [Google Scholar] [CrossRef]
- Wang, M.Y.; Chen, A.K.; Wong, M.H.; Qiu, R.L.; Cheng, H.; Ye, Z.H. Cadmium accumulation in and tolerance of rice (Oryza sativa L.) varieties with different rates of radial oxygen loss. Environ. Pollut. 2011, 159, 1730–1736. [Google Scholar] [CrossRef]
- Li, S.; Li, G.; Huang, X.; Chen, Y.; Lv, C.; Bai, L.; Zhang, K.; He, H.; Dai, J. Cultivar-specific response of rhizosphere bacterial community to uptake of cadmium and mineral elements in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2023, 249, 114403. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M.; Evans, C.D.; Fenner, N.; Freeman, C.; Gough, R.; Jones, T.G.; Lebron, I. UV-visible absorbance spectroscopy as a proxy for peatland dissolved organic carbon (DOC) quantity and quality: Considerations on wavelength and absorbance degradation. Environ. Sci. Process. Impacts 2014, 16, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.T.; Tipping, E.; Koprivnjak, J.F.; Miller, M.P.; Cookson, B.; Hamilton-Taylor, J. Freshwater DOM quantity and quality from a two-component model of UV absorbance. Water Res. 2012, 46, 4532–4542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Hou, H.; Zhao, L.; Sun, Z.J.; Li, H. Protective Effect of foliar application of sulfur on photosynthesis and antioxidative defense system of rice under the stress of Cd. Sci. Total Environ. 2020, 710, 136230. [Google Scholar] [CrossRef]
- Tan, X.F.; Guo, X.; Guo, W.H.; Liu, S.N.; Du, N. Invasive Rhus typhina invests more in height growth and traits associated with light acquisition than do native and non-invasive alien shrub species. Trees Struct. Funct. 2018, 32, 1103–1112. [Google Scholar] [CrossRef]
- Wieland, G.; Neumann, R.; Fau-Backhaus, H.; Backhaus, H. Variation of microbial communities in soil, rhizosphere, and rhizoplane in response to crop species, soil type, and crop development. Appl. Environ. Microbiol. 2001, 67, 5849–5854. [Google Scholar] [CrossRef]
- Li, H.H.; Liu, Y.T.; Tang, S.Y.; Yu, Z.C.; Cai, X.Z.; Xu, S.P.; Chen, Y.H.; Wang, M.K.; Wang, G. Mechanisms for potential Pb immobilization by hydroxyapatite in a soil-rice system. Sci. Total Environ. 2021, 783, 147037. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Chen, J.; Huang, B.; Zhao, Y. Exploring the spatially varying relationships between cadmium accumulations and the main influential factors in the rice-wheat rotation system in a large-scale area. Sci. Total Environ. 2020, 736, 139565. [Google Scholar] [CrossRef] [PubMed]
- Janos, P.; Vavrova, J.; Herzogova, L.; Pilarova, V. Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study. Geoderma 2010, 159, 335–341. [Google Scholar] [CrossRef]
- Huang, Y.; Sheng, H.; Zhou, P.; Zhang, Y.Z. Remediation of Cd-contaminated acidic paddy fields with four-year consecutive liming. Ecotoxicol. Environ. Saf. 2020, 188, 109903. [Google Scholar] [CrossRef]
- Neff, J.C.; Asner, G.P. Dissolved organic carbon in terrestrial ecosystems: Synthesis and a model. Ecosystems 2001, 4, 29–48. [Google Scholar] [CrossRef]
- Smebye, A.; Ailing, V.; Vogt, R.D.; Gadmar, T.C.; Mulder, J.; Cornelissen, G.; Hale, S.E. Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 2016, 142, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Song, X.W.; Lyu, S.; Wang, J.; Sun, K.; Gao, Y.; Wen, X.F. High dissolved organic carbon deposition is buffered by surface soil in a headwater catchment of a subtropical plantation. J. Hydrol. 2022, 607, 127557. [Google Scholar] [CrossRef]
- Kaiser, K.; Zech, W. Rates of dissolved organic matter release and sorption in forest soils. Soil Sci. 1998, 163, 714–725. [Google Scholar] [CrossRef]
- Calace, N.; Massimiani, A.; Petronio, B.M.; Pietroletti, M. Municipal landfill leachate-soil interactions: A kinetic approach. Chemosphere 2001, 44, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, I.H.; Jilani, G.; Zhang, G.P.; Wei, K. Cadmium stress tolerance through potassium nutrition in soybean. Asian J. Chem. 2008, 20, 1099–1108. [Google Scholar]
- Wang, K.; Fu, G.P.; Yu, Y.; Wan, Y.A.; Liu, Z.; Wang, Q.; Zhang, J.S.; Li, H.F. Effects of different potassium fertilizers on cadmium uptake by three crops. Environ. Sci. Pollut. Res. 2019, 26, 27014–27022. [Google Scholar] [CrossRef]
- Naciri, R.; Lahrir, M.; Benadis, C.; Chtouki, M.; Oukarroum, A. Interactive effect of potassium and cadmium on growth, root morphology and chlorophyll a fluorescence in tomato plant. Sci. Rep. 2021, 11, 5384. [Google Scholar] [CrossRef]
- De Anicesio, E.C.A.; Monteiro, F.A. Potassium reduces oxidative stress in tanzania guinea grass under cadmium toxicity. Environ. Sci. Pollut. Res. 2022, 29, 1184–1198. [Google Scholar] [CrossRef]
- Wu, J.W.; Li, R.J.; Lu, Y.; Bai, Z.Q. Sustainable management of cadmium-contaminated soils as affected by exogenous application of nutrients: A review. J. Environ. Manag. 2021, 295, 113081. [Google Scholar] [CrossRef]
- Arif, N.; Sharma, N.C.; Yadav, V.; Ramawat, N.; Dubey, N.K.; Tripathi, D.K.; Chauhan, D.K.; Sahi, S. Understanding heavy metal stress in a rice crop: Toxicity, tolerance mechanisms, and amelioration strategies. J. Plant Biol. 2019, 62, 239–253. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Fan, H.M.; Wang, X.W.; Sun, X.; Li, Y.Y.; Sun, X.Z.; Zheng, C.S. Effects of humic acid derived from sediments on growth, photosynthesis and chloroplast ultrastructure in chrysanthemum. Sci. Hortic. 2014, 177, 118–123. [Google Scholar] [CrossRef]
- Meganid, A.S.; Al-Zahrani, H.S.M.; Selim, M.M.; Arabia, S. Effect of Humic acid application on growth and chlorophyll contents of common bean plants (Phaseolus vulgaris L.) under salinity stress conditions. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 2651–2660. [Google Scholar] [CrossRef]
- Arduini, I.; Masoni, A.; Mariotti, M.; Pampana, S.; Ercoli, L. Cadmium uptake and translocation in durum wheat varieties differing in grain-Cd accumulation. Plant Soil Environ. 2014, 60, 43–49. [Google Scholar] [CrossRef]
- Liu, N.; Huang, X.M.; Sun, L.M.; Li, S.S.; Chen, Y.H.; Cao, X.Y.; Wang, W.X.; Dai, J.L.; Rinnan, R. Screening stably low cadmium and moderately high micronutrients wheat cultivars under three different agricultural environments of China. Chemosphere 2020, 241, 125065. [Google Scholar] [CrossRef] [PubMed]
- Gholami, H.; Samavat, S.; Ardebili, Z.O. The alleviating effects of humic substances on photosynthesis and yield of Plantago ovate in salinity conditions. Int. Res. J. Appl. Basic Sci. 2013, 4, 1683–1686. [Google Scholar]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Bahtiyar, M.; Kucukoduk, M. The humic acid-induced changes in the water status, chlorophyll fluorescence and antioxidant defense systems of wheat leaves with cadmium stress. Ecotoxicol. Environ. Saf. 2018, 155, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, M.; Chen, W.; Jiang, H.; Yue, X. Foliar application of humic acid decreased hazard of cadmium toxicity on the growth of Hybrid Pennisetum. Acta Physiol. Plant. 2020, 42, 129. [Google Scholar] [CrossRef]
- Kromdijk, J.; Glowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Liu, W.X.; Leiser, W.L.; Reif, J.C.; Tucker, M.R.; Losert, D.; Weissmann, S.; Hahn, V.; Maurer, H.P.; Wurschum, T. Multiple-line cross QTL mapping for grain yield and thousand kernel weight in triticale. Plant Breed. 2016, 135, 567–573. [Google Scholar] [CrossRef]
- Haghighi, M.; Kafi, M.; Khoshgoftarmanesh, A. Effect of humic acid application on cadmium accumulation by lettuce leaves. J. Plant Nutr. 2013, 36, 1521–1532. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, Y.N.; Wang, Q.; Li, H.F. Effect of humic acid-based amendments with foliar application of Zn and Se on Cd accumulation in tobacco. Ecotoxicol. Environ. Saf. 2017, 138, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food. Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Chen, Y.J.; Bai, X.; Ye, Z.F. Recent progress in heavy metal ion decontamination based on metal-organic frameworks. Nanomaterials 2020, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.X.; Kang, W.W.; Xing, B.L.; Chen, L.J.; Zhang, C.X. Oxygen-rich and hierarchical porous carbons prepared from coal based humic acid for supercapacitor electrodes. Fuel Process. Technol. 2016, 142, 1–5. [Google Scholar] [CrossRef]
- Hu, G.X.; Tang, B.; Min, X. Synthesis and characterization of alternated (humic acid/Fe3+) (n) multilayer film on alumina fiber. Surf. Coat. Technol. 2012, 206, 3586–3594. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Zhang, C.X.; Qu, X.X.; Xing, B.L.; Huang, G.X.; Xu, B.; Shi, C.L.; Kang, W.W.; Yu, J.; Hong, S.W. Humic acid resin-based amorphous porous carbon as high rate and cycle performance anode for sodium-ion batteries. Electrochim. Acta 2021, 372, 137850. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Prasher, S.; Elsayed, E.; Dhiman, J.; Mawof, A.; Patel, R. Biochar applied to soil under wastewater irrigation remained environmentally viable for the second season of potato cultivation. J. Environ. Manag. 2020, 254, 109822. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Lian, F.; Liu, Z.Q.; Zhu, L.Y.; Song, Z.G. Biochars derived from various crop straws: Characterization and Cd (II) removal potential. Ecotoxicol. Environ. Saf. 2014, 106, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.J.; Ma, J.J. The ecological effects of humic acid fertilizer on the spring wheat under cadmium stress. Appl. Mech. Mater. 2013, 295–298, 1204–1208. [Google Scholar] [CrossRef]
- Ratie, G.; Chrastny, V.; Guinoiseau, D.; Marsac, R.; Vankova, Z.; Komarek, M. Cadmium isotope fractionation during complexation with humic acid. Environ. Sci. Technol. 2021, 55, 7430–7444. [Google Scholar] [CrossRef]
- Luo, Q.H.; Bai, B.; Xie, Y.H.; Yao, D.P.; Zhang, D.M.; Chen, Z.; Zhuang, W.; Deng, Q.Y.; Xiao, Y.H.; Wu, J. Effects of Cd uptake, translocation and redistribution in different hybrid rice varieties on grain Cd concentration. Ecotoxicol. Environ. Saf. 2022, 240, 113683. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Fau-Du, J.; Du, J.; Fau-Liu, J.; Liu, J.; Fau-Yang, S.; Yang, S.; Fau-Pu, X.; Pu, X.; et al. Elemental content in brown rice by inductively coupled plasma atomic emission spectroscopy reveals the evolution of Asian cultivated rice. J. Integr. Plant Biol. 2009, 51, 466–475. [Google Scholar] [CrossRef]
- Celik, H.; Katkat, A.V.; Asik, B.B.; Turan, M.A. Effect of foliar-applied humic acid to dry weight and mineral nutrient uptake of maize under calcareous soil conditions. Commun. Soil Sci. Plant Anal. 2011, 42, 29–38. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.X.; Mitani-Ueno, N.; Yokosho, K.; Ma, J.F. Preferential delivery of zinc to developing tissues in rice is mediated by P-Type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, H.; Gu, J.F.; Zeng, P.; Liao, B.H.; Xie, Y.H.; Ji, X.H. Combined amendment improves soil health and brown rice quality in paddy soils moderately and highly co-contaminated with Cd and As. Environ. Pollut. 2022, 295, 118590. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Buoso, S.; Giacomino, A.; La Gioia, C.; Mentasti, E. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere 2011, 82, 169–178. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Cesco, S.; Varanini, Z.; Pinton, R. Humic substances contribute to plant iron nutrition acting as chelators and biostimulants. Front. Plant Sci. 2019, 10, 00675. [Google Scholar] [CrossRef] [PubMed]
- Nakandalage, N.; Seneweera, S. Chapter 12—Micronutrients use efficiency of crop-plants under changing climate. In Plant Micronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Phan Tran, L.-S., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 209–224. [Google Scholar]
- Bracci, E.L.; Keogh, J.B.; Milte, R.; Murphy, K.J. A comparison of dietary quality and nutritional adequacy of popular energy-restricted diets against the Australian Guide to Healthy Eating and the Mediterranean Diet. Br. J. Nutr. 2022, 128, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chang, J.D.; Chen, R.J.; Li, H.B.; Lu, H.F.; Tao, L.X.; Xiong, J. Comparison on cellular mechanisms of iron and cadmium accumulation in rice: Prospects for cultivating Fe-rich but Cd-free rice. Rice 2016, 9, 39. [Google Scholar] [CrossRef]
- Kailasam, S.; Peiter, E. A path toward concurrent biofortification and cadmium mitigation in plant-based foods. New Phytol. 2021, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Giasuddin, A.S.M.; Jhuma, K.A.; Hossain, M.S.; Haq, A.M.M.J.B.J.o.M.S. Considerations for rice (Oryza sativa) fortification with essential micronutrients in public health intervention. Bangladesh J. Med. Sci. 2020, 19, 189–193. [Google Scholar] [CrossRef]
- Calderon, F.J.; Reeves, J.B.; Collins, H.P.; Paul, E.A. Chemical differences in soil organic matter fractions determined by diffuse-reflectance mid-infrared spectroscopy. Soil Sci. Soc. Am. J. 2011, 75, 568–579. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, X.; Shi, Y.; Song, X.; Qin, Z.; Tang, J. FTIR characteristics of rhizosphere soil of multi-generation continuous Eucalyptus plantation in South Subtropical Region. Ecol. Environ. Sci. 2022, 31, 688–694. (In Chinese) [Google Scholar]
- Ma, F.; Du, C.W.; Zhou, J.M.; Shen, Y.Z. Investigation of soil properties using different techniques of mid-infrared spectroscopy. Eur. J. Soil Sci. 2019, 70, 96–106. [Google Scholar] [CrossRef]
- Cui, L.Q.; Pan, G.X.; Li, L.Q.; Bian, R.J.; Liu, X.Y.; Yan, J.L.; Quan, G.X.; Ding, C.; Chen, T.M.; Liu, Y.; et al. Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: A five-year field experiment. Ecol. Eng. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Janik, L.J.; Skjemstad, J.O.; Shepherd, K.D.; Spouncer, L.R. The prediction of soil carbon fractions using mid-infrared-partial least square analysis. Aust. J. Soil Res. 2007, 45, 73–81. [Google Scholar] [CrossRef]
- Hall, S.J.; Berhe, A.A.; Thompson, A. Order from disorder: Do soil organic matter composition and turnover co-vary with iron phase crystallinity? Biogeochemistry 2018, 140, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.Y.; Zhu, Z.L.; Li, H.H.; Feng, C.L.; An, S.S. Comparison and application of different fourier transform infrared spectroscopy to soil spectral characteristics analysis. Res. Soil Water Conserv. 2022, 29, 121–128. (In Chinese) [Google Scholar] [CrossRef]
- Srivastava, M.; Mishra, A.K. Comparative responses of diazotrophic abundance and community structure to the chemical composition of paddy soil. Environ. Sci. Pollut. R. 2018, 25, 399–412. [Google Scholar] [CrossRef]
- Li, T.; Zhao, S.W.; Li, X.X.; M, S. Characters of soil organic matter functional groups in the fields planted with alfalfa (Medi-cago sativa) for different years in hilly regions of South Ningxia, Northwest China. Chin. J. Appl. Environ. Biol. 2012, 23, 3266–3272. (In Chinese) [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Liu, J.; Li, D.; Liu, F. Characteristics and influencing factors of soil organic carbon functional groups in coastal wetlands with different. Chin. J. Appl. Ecol. 2022, 28, 276–282. (In Chinese) [Google Scholar] [CrossRef]
- Wu, W.X.; Yang, M.; Feng, Q.B.; McGrouther, K.; Wang, H.L.; Lu, H.H.; Chen, Y.X. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenerg. 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Nkoumbou, C.; Villieras, F.; Barres, O.; Bihannic, I.; Pelletier, M.; Razafitianamaharavo, A.; Metang, V.; Ngoune, C.Y.; Njopwouo, D.; Yvon, J. Physicochemical properties of talc ore from Pout-Kelle and Memel deposits (Central Cameroon). Clay Miner. 2008, 43, 317–337. [Google Scholar] [CrossRef]
- Du, C.W.; Zhou, J.M.; Goyne, K.W. Organic and inorganic carbon in paddy soil as evaluated by mid-infrared photoacoustic spectroscopy. PLoS ONE 2012, 7, e43368. [Google Scholar] [CrossRef]
- Wang, S.; Wang, N.; Yao, K.; Fan, Y.C.; Li, W.H.; Han, W.H.; Yin, X.H.; Chen, D.Y. Characterization and interpretation of Cd (II) adsorption by different modified rice straws under contrasting conditions. Sci. Rep. 2019, 9, 17868. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Soil Cd Contaminated Level | Stepwise Multiple Linear Regression a | ANOVA b | All Parameters | No. | ||
|---|---|---|---|---|---|---|
| Equation | R2adj | F | p | p | ||
| S-Cd | Log [CCd in brown rice] = −0.811 − 0.367log [CMK] − 0.278log [VMPn] | 0.969 | 171.9 | 6.7648 × 10−8 *** | <0.01 | (1) |
| M-Cd | Log [CCd in brown rice] = −0.902 − 3.014log [CTDOC] − 0.214log [CFK] | 0.978 | 248 | 1.3467 × 10−8 *** | <0.05 | (2) |
| H-Cd | Log [CCd in brown rice] = 2.532 − 0.664log [VTFstem-flag leaf] − 0.666log [CK in brown rice] –3.09log [CFDOC] | 0.975 | 141.64 | 2.8481 × 10−7 *** | <0.05 | (3) |
| All | Log [CCd in brown rice] = 0.727 − 0.826log [CZn in brown rice] + 0.428log [VTFstem-flag leaf] + 0.458log [Csoil DTPA-Cd] + 0.581log [VTFstem-brown rice] | 0.939 | 136.21 | 3.3602 × 10−19 *** | <0.01 | (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Huang, X.; Li, G.; Zhang, K.; Bai, L.; He, H.; Chen, S.; Dai, J. Effects of Mineral-Based Potassium Humate on Cadmium Accumulation in Rice (Oryza sativa L.) under Three Levels of Cadmium-Contaminated Alkaline Soils. Sustainability 2023, 15, 2836. https://doi.org/10.3390/su15032836
Li S, Huang X, Li G, Zhang K, Bai L, He H, Chen S, Dai J. Effects of Mineral-Based Potassium Humate on Cadmium Accumulation in Rice (Oryza sativa L.) under Three Levels of Cadmium-Contaminated Alkaline Soils. Sustainability. 2023; 15(3):2836. https://doi.org/10.3390/su15032836
Chicago/Turabian StyleLi, Shuangshuang, Xianmin Huang, Guangxian Li, Ke Zhang, Liyong Bai, Huan He, Shibao Chen, and Jiulan Dai. 2023. "Effects of Mineral-Based Potassium Humate on Cadmium Accumulation in Rice (Oryza sativa L.) under Three Levels of Cadmium-Contaminated Alkaline Soils" Sustainability 15, no. 3: 2836. https://doi.org/10.3390/su15032836