Emerging Technologies for the Production of In Vitro Raised Quality Rich Swertia chirayita by Using LED Lights
Abstract
:1. Introduction
2. Material and Methods
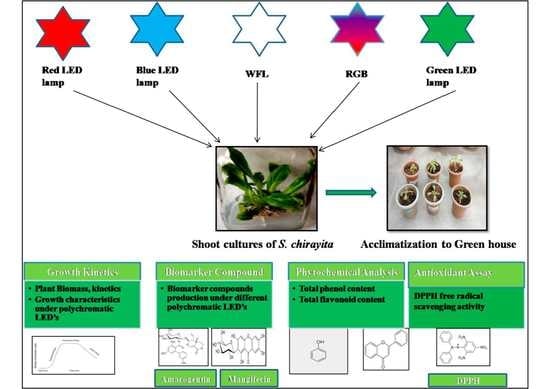
2.1. Selection of Plant Material and Establishment of In Vitro Shoots under LED Lighting
2.2. Light Setup with Growth Conditions
- (a)
- (Control: white fluorescent light at 15 °C ± 1 with 3000 μmol m−2 s −1 intensity (16 h of light and 8 h of dark photoperiod).
- (b)
- Red: 100% red LED at~660 nm wavelength with 20 nm of bandwidth at ½ peak height.
- (c)
- Blue: 100% blue LED at ~460 nm wavelength with 20 nm of bandwidth at ½ peak height.
- (d)
- Green: 100% green LED at ~550 nm wavelength with 20 nm of bandwidth at ½ peak height.
- (e)
- RGB: 40% red, 40% green and 20% blue polychromatic LEDs at 15°C± 1 inside plant tissue culture room.
2.3. Growth Determination of Shoot Cultures
2.4. Phytochemical Screening of Plant Extracts
2.4.1. Preparation of Plant Extract
2.4.2. Quantification of Bioactive Compounds through Reverse-Phase HPLC
2.5. Determination of Total Phenolics and Total Flavonoid Content
2.5.1. Total Phenolic Content
2.5.2. Total Flavonoid Content
2.6. Determination of DPPH Free Radical Scavenging Activity
2.7. Acclimatization and Transplantation of Light Treated Plants of S. chirayita
2.8. Statistical Analysis
3. Result
3.1. Impact of LED Lighting on Growth
3.2. Impact of LED Lighting on Bioactive Compound Production
3.3. Impact of LED Lighting on Phenolic and Flavonoid Content
3.4. Impact of LED Lighting on DPPH Activity
3.5. Acclimatization to Outer Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, C.B. Verbenaceae. In The Flora of British India, 4th ed.; Hooker, J.D., Ed.; L. Reeve and Co.: London, UK, 1885; pp. 560–604. [Google Scholar]
- Bentley, R.; Trimen, H. Medicinal Plants, Being Descriptions, with Original Figures, of the Principal Plants Employed in Medicine, and an Account of the Characters, Properties, and Uses of Their Parts and Products of Medicinal Value; J. & A. Churchill: London, UK, 1880. [Google Scholar]
- Bhatt, A.; Rawal, R.S.; Dhar, U. Ecological features of a critically rare medicinal plant, S. chirayita, in Himalaya. Plant Spec. Biol. 2006, 21, 49–152. [Google Scholar] [CrossRef]
- Joshi, P.; Dhawan, V. S. chirayita—An overview. Curr. Sci. 2005, 89, 635–640. [Google Scholar]
- Phoboo, S.; Pinto, M.D.S.; Barbosa, A.C.L.; Sarkar, D.; Bhowmik, P.C.; Jha, P.K. Phenolic-linked biochemical rationale for the anti-diabetic properties of S. chirayita (Roxb. ex Flem.). Phytother. Res. 2013, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, H.; Goyal, R.K.; Cheema, S.K. Anti-diabetic activity of S.marin is due to an active metabolite, gentianine, that upregulates PPAR-c gene expression in 3T3-L1 cells. Phytother. Res. 2013, 27, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Mandal, S.; Das, A.; Das, S. Amarogentin can reduce hyper proliferation by down regulation of Cox-II and upregulation of apoptosis in mouse skin carcinogenesis model. Cancer Lett. 2006, 244, 252–259. [Google Scholar] [CrossRef]
- Pal, D.; Sur, S.; Mandal, S.; Das, A.; Roy, A.; Das, A. Prevention of liver carcinogenesis by amarogentin through modulation of G1/S cell cycle check point and induction of apoptosis. Carcinogenesis 2012, 33, 2424–2431. [Google Scholar] [CrossRef]
- Pardo-Andreu, G.L.; Paim, B.A.; Castilho, B.A.; Velho, R.F.; Delgado, J.A.; Vercesi, R. Mangifera indica L. extract (Vimang R) and its main poly phenol mangiferin prevent mitochondrial oxidative stress in atherosclerosis-pronehypercholesterolemic mouse. Pharmacol. Res. 2008, 57, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, M.; Nataraj, J.; Essa, M.M.; Memon, M.M.; Manivasagam, M.A. Mangiferin attenuates MPTP induced dopaminergicneurode generation and improves motor impairment, redox balance and Bcl-2/Bax expression in experimental Parkinson’s disease mice. Chem. Biol. Interact. 2013, 206, 239–247. [Google Scholar] [CrossRef]
- Tabassum, S.; Mahmood, S.; Hanif, J.; Hina, M.; Uzair, B. An Overview of Medicinal Importance of S. chirayita. Int. J. Appl. Sci. Technol. 2012, 2, 298–304. [Google Scholar]
- Pradhan, B.K.; Badola, H.K. S. chirayita, a threatened High-value Medicinal Herb: Microhabitats and conservation challenges in Sikkim Himalaya, India. Mt. Res. Dev. 2015, 35, 374–381. [Google Scholar] [CrossRef]
- Sharma, A. Global Medicinal Plants Demand May Touch $5 Trillion by 2050; Indian Express: Chennai, India, 2004. [Google Scholar]
- Joshi, K.; Chavan, P.; Warude, D.; Patwardhan, B. Molecular markers in herbal drug technology. Curr. Sci. 2004, 87, 159–165. [Google Scholar]
- Goraya, G.S.; Jishtu, V.; Rawat, G.S.; Ved, D.K. Wild Medicinal Plants of Himachal Pradesh: An Assessment of Their Conservation Status and Management Prioritisation; Himachal Pradesh Forest Department: Shimla, India, 2013. [Google Scholar]
- Ganesan, M.; Narsingh, V.; Manju, S.; Karuna, S.; Suchitra, B.; Birendra, K.; Laiq, R. Elicitation enhances swerchirin and 1,2,5,6-tetrahydroxyxanthone production in hairy root cultures of S. chirayita (Roxb.) H. Karst. Ind. Crop. Prod. 2022, 177, 114488. [Google Scholar]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus cultures of Rhodiola imbricate Edgew. J. Photochem. Photobiol. 2018, 183, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Darko, É.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. 2014, 369, 20130243. [Google Scholar] [CrossRef] [PubMed]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Schettini, E. Lighting equipment for a crop growing system in microgravity conditions for space mission. Acta Hortic. 2005, 1, 217–224. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Yu, K.W.; Murthy, H.N.; Hahn, E.J.; Paek, K.Y. Ginsenoside production by hairy root cultures of Panax ginseng: Influence of temperature and light quality. Biochem. Eng. J. 2005, 23, 53–56. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ketchum, R.E.B.; Gibson, D.M.; Gallo, L.G. Media optimization for maximum biomass production in cell cultures of pacific yew. Plant Cell Tissue Organ. Cult. 1995, 42, 185–193. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Bekhradnia, A.R. Chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol. 2008, 7, 3188–3192. [Google Scholar]
- Yesmin, M.N.; Uddin, S.N.; Mubassara, S.; Akond, M.A. Antioxidant and Antibacterial Activities of Calotropis procera Linn. Am. J. Agric. Environ. Sci. 2008, 4, 550–553. [Google Scholar]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Halliday, K.J.; Fankhauser, C. Phytochrome-hormonal signalling networks. New Phytol. 2003, 157, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Zhao, B.; Yuan, X. Improved growth of Artemisia annua L. hairy roots and artemisinin production under red light conditions. Biotechnol. Lett. 2001, 23, 1971–1973. [Google Scholar] [CrossRef]
- Yu, W.; Liu, Y.; Song, L.; Jacobs, D.F.; Du, X.; Ying, Y.; Shao, Q.; Wu, J. Effect of Differential Light Quality on Morphology, Photosynthesis, and Antioxidant Enzyme Activity in Camptotheca acuminata Seedlings. J. Plant Growth Regul. 2016, 36, 148–160. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Kivimäenpää, M.; Julkunen-Tiitto, R. New Light for Phytochemicals. Trends Biotechnol. 2018, 36, 7–10. [Google Scholar] [CrossRef]
- Hunter, D.C.; Burritt, D.J. Light quality influences adventitious shoot production from cotyledon explants of lettuce (Lactuca sativa L.). In Vitr. Cell. Dev. Biol. Plant. 2004, 40, 215–220. [Google Scholar] [CrossRef]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red- and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ. Cult. 2008, 92, 147–153. [Google Scholar] [CrossRef]
- Tariq, U.; Ali, M.; Abbasi, B.H. Morphogenic and biochemical variations under different spectral lights in callus cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 130, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Influence of Culture Medium Composition and Light Conditions on the Accumulation of Bioactive Compounds in Shoot Cultures of Scutellaria lateriflora L. (American Skullcap) Grown In Vitro. Appl. Biochem. Biotechnol. 2017, 183, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, R.C.N.; Branquinho, N.A.A.; Hara, A.C.B.A.M.; Costa, A.C.; Silva, F.G.; Pimenta, L.P.; Silva, M.L.A.; Cunha, W.R.; Pauletti, P.M.; Januario, A.H. Impact of light quality on flavonoid production and growth of Hyptis marrubioides seedlings cultivated in vitro. Braz. J. Pharmacogn. 2017, 27, 466–470. [Google Scholar] [CrossRef]
- Lefsrud, M.L.; Kopsell, D.A.; Sams, C.E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, X.; Zhao, B.; Wang, Y. Light intensity and spectral quality influencing the callus growth of Cistanche deserticola and biosynthesis of phenylethanoid glycosides. Plant Sci. 2003, 165, 657–661. [Google Scholar] [CrossRef]
- Zhao, D.; Xing, J.; Li, M.; Lu, D.; Zhao, Q. Optimization of growth and jaceosidin production in callus and cell suspension cultures of Saussurea medusa. Plant Cell Tissue Organ. Cult. 2001, 67, 227–234. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nishida, K.; Noguchi, M.; Tamaki, E. Some factors affecting the anthocyanin formation by populus cells in suspension culture. Agric. Biol. Chem. 1973, 37, 561–567. [Google Scholar] [CrossRef]
- Kumar, V.; Chauhan, R.; Sood, H. In Vitro Production and Efficient Quantification of Major Phyto pharmaceuticalsin an Endangered Medicinal Herb, S. chirata. Int. J. Biotechnol. Bioeng. Res. 2013, 4, 495–506. [Google Scholar]
- Yousefzadi, M.; Sharifi, M.; Behmanesh, M.; Ghasempour, A.; Moyano, E.; Palazon, J. The effect of light on gene expression and podophyllotoxin biosynthesis in Linum album cell culture. Plant Physiol. Biochem. 2012, 56, 41–46. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C.; Saran, M. Flavonoid antioxidants: Rate constants for reactions with oxygen radicals. Methods Enzymol. 1994, 234, 420–429. [Google Scholar]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1250–1318. [Google Scholar]
- Grace, S.C. Phenolics as Antioxidants. In Antioxidants and Reactive Oxygen Species in Plants; Smirnoff, N., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2005; pp. 141–168. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, 81, 268S–276S. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, J.L.; Chen, L.; Zhang, M.S.; Wang, X.Q.; Pang, Y.J.; Yang, Y.H. Effect of light on gene expression and shikonin formation in cultured Onosma paniculatum cells. Plant Cell Tissue Organ. Cult. 2006, 84, 39–46. [Google Scholar] [CrossRef]
- Yáñez, J.A.; Remsberg, C.M.; Takemoto, J.K.; Vega-Villa, K.R.; Andrews, P.K.; Sayre, C.L.; Martinez, S.E.; Davies, N.M. Polyphenols and Flavonoids: An Overview. In Flavonoid Pharmacokinetics: Methods of Analysis, Preclinical and Clinical Pharmacokinetics, Safety, and Toxicology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–69. [Google Scholar]
- Taulavuori, K.; Hyöky, V.; Oksanen, J.; Taulavuori, E.; Julkunen-Tiitto, R. Species specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
- Ahmad, N.; Rab, A.; Ahmad, N. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J. Photochem. Photobiol. B Biol. 2016, 154, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.D.; Karmakar, A. Machine vision based evaluation of impact of light emitting diodes (LEDs) on shoot regeneration and the effect of spectral quality on phenolic content and antioxidant capacity in S. chirata. J. Photochem. Photobiol. B Biol. 2017, 174, 162–172. [Google Scholar] [CrossRef]
- Consentino, L.; Lambert, S.; Martino, C.; Jourdan, N.; Bouchet, P.E.; Witczak, J.; Castello, P.; El-Esawi, M.; Corbineau, F.; d’Harlingue, A.; et al. Blue-light dependent reactive oxygen species formation by Arabidopsis cryptochrome may define a novel evolutionarily conserved signaling mechanism. New Phytol. 2015, 206, 1450–1462. [Google Scholar] [CrossRef]
- Castillon, A.; Shen, H.; Huq, E. Phytochrome Interacting Factors: Central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007, 12, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, H. The importance of applied light quality on the production of lignans and phenolic acids in Schisandra chinensis (Turcz.) Baill. cultures in vitro. Plant Cell Tissue Organ. Cult. 2016, 127, 115–121. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Van Staden, J. A Review of Swertia chirayita (Gentianaceae) as a Traditional Medicinal Plant. Front. Pharmacol. 2016, 12, 308. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, B.; He, J.; Han, L.; Zhan, Y.; Wang, Y. In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J. Ethnopharmacol. 2011, 136, 309–315. [Google Scholar] [CrossRef]







| LED Lamp | Shoot Length (cm) | Root Length (cm) | No. of Shoots/Explants | No. of Roots/ Shoot |
|---|---|---|---|---|
| Red LED | 6.13 ± 0.91 a | 3.09 ± 1.33 a | 5.51 ± 0.82 a | 1.94 ± 1.18 a |
| Blue LED | 4.14 ± 0.61 b | 2.71 ± 1.34 ba | 4.91 ± 0.90 a | 1.70 ± 0.83 a |
| RGB LED | 3.70 ± 0.35 cb | 1.18 ± 0.65 ba | 2.61 ± 0.25 b | 1.42 ± 0.72 a |
| Green LED WFL (Control) | 2.61 ± 0.37 cb 3.96 ± 0.33 cb | 0.00 ± 0.00 b 2.11 ± 1.12 ba | 2.19 ± 0.37 b 3.91 ± 0.93 a | 0.00 ± 0.00 a 1.59 ± 0.54 a |
| Antioxidant Activity | |
|---|---|
| Light Quality | %RSA |
| Blue | 50.40 ± 0.15 a |
| Red | 43.08 ± 0.06 b |
| WFL | 39.02 ± 0.11 c |
| RGB | 35.77 ± 0.05 c |
| Green | 28.45 ± 0.19 d |
| Standard | |
| BHT | 67.47 ± 0.05 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, R.; Sood, H. Emerging Technologies for the Production of In Vitro Raised Quality Rich Swertia chirayita by Using LED Lights. Sustainability 2023, 15, 1714. https://doi.org/10.3390/su15021714
Gupta R, Sood H. Emerging Technologies for the Production of In Vitro Raised Quality Rich Swertia chirayita by Using LED Lights. Sustainability. 2023; 15(2):1714. https://doi.org/10.3390/su15021714
Chicago/Turabian StyleGupta, Rolika, and Hemant Sood. 2023. "Emerging Technologies for the Production of In Vitro Raised Quality Rich Swertia chirayita by Using LED Lights" Sustainability 15, no. 2: 1714. https://doi.org/10.3390/su15021714