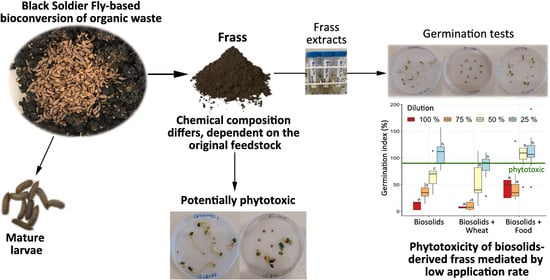
Analysis of Chemical and Phytotoxic Properties of Frass Derived from Black Soldier Fly-Based Bioconversion of Biosolids
Abstract
:1. Introduction
2. Material and Methods
2.1. Origin of BSFL Feedstock and Frass
2.2. Chemical Analysis of BSFL Feedstocks and Frass
2.3. Seed Germination Assay with Extracts from BSFL Feedstock and Frass
2.4. Statistical Analysis
3. Results
3.1. Analysis of the Chemical Composition of BSFL Frass and Feedstocks
3.2. Phytotoxicity of BSFL Frass and Feedstocks against Lettuce and Radish
3.3. Chemical Parameters Related to Germination Success and Seedling Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018; p. 38. [Google Scholar]
- Mateo-Sagasta, J.; Raschid-Sally, L.; Thebo, A. Global wastewater and sludge production, treatment and use. In Wastewater; Springer: Berlin/Heidelberg, Germany, 2015; pp. 15–38. [Google Scholar]
- Paramashivam, D.; Dickinson, N.M.; Clough, T.J.; Horswell, J.; Robinson, B.H. Potential environmental benefits from blending biosolids with other organic amendments before application to land. J. Environ. Qual. 2017, 46, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenat, A.; Zurbrugg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and middle-income settings. Rev. Environ. Sci. Bio/Technol. 2017, 16, 81–130. [Google Scholar] [CrossRef] [Green Version]
- Lopes, I.G.; Yong, J.W.; Lalander, C. Frass derived from black soldier fly larvae treatment of biodegradable wastes. A critical review and future perspectives. Waste Manag. 2022, 142, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Nordberg, A.; Vinneras, B. A comparison in product-value potential in four treatment strategies for food waste and faeces—Assessing composting, fly larvae composting and anaerobic digestion. Gcb Bioenergy 2018, 10, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Smetana, S.; Spykman, R.; Heinz, V. Environmental aspects of insect mass production. J. Insects Food Feed 2021, 7, 553–571. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Yuwono, N.W.; Nisa, K.; Mategeko, B.; Smetana, S.; Saki, M.; Nawaz, A.; et al. Black soldier fly larvae (BSFL) and their affinity for organic waste processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef]
- Ndegwa, P.; Thompson, S.; Das, K. Effects of stocking density and feeding rate on vermicomposting of biosolids. Bioresour. Technol. 2000, 71, 5–12. [Google Scholar] [CrossRef]
- Ermolaev, E.; Lalander, C.; Vinnerås, B. Greenhouse gas emissions from small-scale fly larvae composting with Hermetia illucens. Waste Manag. 2019, 96, 65–74. [Google Scholar] [CrossRef]
- Mertenat, A.; Diener, S.; Zurbrugg, C. Black Soldier Fly biowaste treatment—Assessment of global warming potential. Waste Manag. 2019, 84, 173–181. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Chen, J.; Nowar, E.E.; Li, Z.; Hu, R.; Tomberlin, J.K.; Yu, Z.; Li, Q.; Wang, S. Reducing greenhouse gas emissions and enhancing carbon and nitrogen conversion in food wastes by the black soldier fly. J. Environ. Manag. 2020, 260, 110066. [Google Scholar] [CrossRef]
- Kaczor, M.; Bulak, P.; Proc-Pietrycha, K.; Kirichenko-Babko, M.; Bieganowski, A. The variety of applications of Hermetia illucens in industrial and agricultural areas—Review. Biology 2022, 12, 25. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrugg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in Black Soldier Fly larval treatment: A review. Waste Manag. 2018, 82, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Gebremikael, M.T.; Wickeren, N.V.; Hosseini, P.S.; De Neve, S. The impacts of Black Soldier Fly frass on nitrogen availability, microbial activities, C sequestration, and plant growth. Front. Sustain. Food Syst. 2022, 6, 795950. [Google Scholar] [CrossRef]
- Bohm, K.; Hatley, G.A.; Robinson, B.H.; Gutierrez-Gines, M.J. Black Soldier Fly-based bioconversion of biosolids creates high-value products with low heavy metal concentrations. Resour. Conserv. Recycl. 2022, 180, 106149. [Google Scholar] [CrossRef]
- Mohajerani, A.; Ukwatta, A.; Jeffrey-Bailey, T.; Swaney, M.; Ahmed, M.; Rodwell, G.; Bartolo, S.; Eshtiaghi, N.; Setunge, S. A proposal for recycling the world’s unused stockpiles of treated wastewater sludge (biosolids) in fired-clay bricks. Buildings 2019, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for Chemical Analysis of Soils; New Zealand Soil Bureau Scientific Report 10A; New Zealand Soil Bureau: Wellington, New Zealand, 1981; p. 102. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA: Madison, WI, USA, 1996; Volume 5, pp. 1123–1184. [Google Scholar]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- NZS 4454:2005; Composts, Soil Conditioners and Mulches. Standards New Zealand Te Mana Tautikanga o Aotearoa: Wellington, New Zealand, 2005. Available online: https://www.standards.govt.nz/shop/nzs-44542005/ (accessed on 1 April 2023).
- Armas, I.; Pogrebnyak, N.; Raskin, I. A rapid and efficient in vitro regeneration system for lettuce (Lactuca sativa L.). Plant Methods 2017, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests, R Package Version 0.7.2; 2023. Available online: https://cran.r-project.org/web/packages/rstatix/ (accessed on 1 April 2023).
- Conigrave, J. Corx: Create and Format Correlation Matrices, R Package Version 1.0.6.1; 2020. Available online: https://cran.r-project.org/web/packages/corx/ (accessed on 1 April 2023).
- Kassambara, A. ggcorrplot: Visualization of a Correlation Matrix Using ‘ggplot2’, R Package Version 0.1.3; 2019. Available online: https://cran.r-project.org/web/packages/ggcorrplot (accessed on 1 April 2023).
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Di Salvatore, M.; Carafa, A.M.; Carratù, G. Assessment of heavy metals phytotoxicity using seed germination and root elongation tests: A comparison of two growth substrates. Chemosphere 2008, 73, 1461–1464. [Google Scholar] [CrossRef]
- Emino, E.R.; Warman, P.R. Biological assay for compost quality. Compost Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V.; Guida, M. Compost from organic solid waste: Quality assessment and European regulations for its sustainable use. Resour. Conserv. Recycl. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Thompson, W.; Leege, P.; Millner, P.; Watson, M.E. (Eds.) TMECC. Test Methods for the Examination of Composts and Composting; The US Composting Council, US Government Printing Office: Washington, DC, USA, 2002. [Google Scholar]
- Liu, T.; Awasthi, M.K.; Awasthi, S.K.; Duan, Y.; Zhang, Z. Effects of black soldier fly larvae (Diptera: Stratiomyidae) on food waste and sewage sludge composting. J. Environ. Manag. 2020, 256, 109967. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.; Popa, R. Enhanced ammonia content in compost leachate processed by Black Soldier Fly larvae. Appl. Biochem. Biotechnol. 2012, 166, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Klammsteiner, T.; Turan, V.; Juarez, M.F.D.; Oberegger, S.; Insam, H. Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization. Agronomy 2020, 10, 1578. [Google Scholar] [CrossRef]
- Setti, L.; Francia, E.; Pulvirenti, A.; Gigliano, S.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Bortolini, S.; Maistrello, L.; Ronga, D. Use of black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae processing residue in peat-based growing media. Waste Manag. 2019, 95, 278–288. [Google Scholar] [CrossRef]
- Liu, T.; Kumar, A.M.; Chen, H.Y.; Duan, Y.M.; Awasthi, S.K.; Zhang, Z.Q. Performance of black soldier fly larvae (Diptera: Stratiomyidae) for manure composting and production of cleaner compost. J. Environ. Manag. 2019, 251, 109593. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y.; Hodgkiss, I.J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]
- Zubillaga, M.S.; Lavado, R.S. Phytotoxicity of biosolids compost at different degrees of maturity compared to biosolids and animal manures. Compost Sci. Util. 2006, 14, 267–270. [Google Scholar] [CrossRef]
- Carter, J.M.; Brown, E.M.; Grace, J.P.; Salem, A.K.; Irish, E.E.; Bowden, N.B. Improved growth of pea, lettuce, and radish plants using the slow release of hydrogen sulfide from GYY-4137. PLoS ONE 2018, 13, e0208732. [Google Scholar] [CrossRef] [Green Version]
- Hase, T.; Kawamura, K. Evaluating compost maturity with a newly proposed index based on a germination test using Komatsuna (Brassica rapa var. peruviridis) seeds. J. Mater. Cycles Waste Manag. 2012, 14, 220–227. [Google Scholar] [CrossRef]
- de Carvalho, M.J.A.; Mirth, C.K. Food intake and food choice are altered by the developmental transition at critical weight in Drosophila melanogaster. Anim. Behav. 2017, 126, 195–208. [Google Scholar] [CrossRef]
- Ramírez, W.A.; Domene, X.; Andrés, P.; Alcañiz, J.M. Phytotoxic effects of sewage sludge extracts on the germination of three plant species. Ecotoxicology 2008, 17, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Lopes, I.G.; de Souza, L.F.; da Cruz, M.C.P.; Vidotti, R.M. Composting as a strategy to recycle aquatic animal waste: Case study of a research centre in Sao Paulo State, Brazil. Waste Manag. Res. 2019, 37, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Monedero, M.A.; Roig, A.; Paredes, C.; Bernal, M.P. Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Kirchmann, H.; Johnston, A.E.J.; Bergstrom, L.F. Possibilities for reducing nitrate leaching from agricultural land. Ambio 2002, 31, 404–408. [Google Scholar] [CrossRef]
- Camut, L.; Gallova, B.; Jilli, L.; Sirlin-Josserand, M.; Carrera, E.; Sakvarelidze-Achard, L.; Ruffel, S.; Krouk, G.; Thomas, S.G.; Hedden, P.; et al. Nitrate signaling promotes plant growth by upregulating gibberellin biosynthesis and destabilization of DELLA proteins. Curr. Biol. 2021, 31, 4971–4982.e4974. [Google Scholar] [CrossRef]
- Lin, D.; Huang, Y.; Zhao, J.; Wu, Z.; Liu, S.; Qin, W.; Wu, D.; Chen, H.; Zhang, Q. Evaluation of seed nitrate assimilation and stimulation of phenolic-linked antioxidant on pentose phosphate pathway and nitrate reduction in three feed-plant species. BMC Plant Biol. 2020, 20, 267. [Google Scholar] [CrossRef]
- Sarpong, D.; Oduro-Kwarteng, S.; Gyasi, S.F.; Buamah, R.; Donkor, E.; Awuah, E.; Baah, M.K. Biodegradation by composting of municipal organic solid waste into organic fertilizer using the Black Soldier Fly (Hermetia illucens) (Diptera: Stratiomyidae) larvae. Int. J. Recycl. Org. Waste Agric. 2019, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Wangu, M.M.; Dubois, T.; Ekesi, S.; et al. Low-cost technology for recycling agro-industrial waste into nutrient-rich organic fertilizer using black soldier fly. Waste Manag. 2021, 119, 183–194. [Google Scholar] [CrossRef]
- Takeda, A.; Tsukada, H.; Takaku, Y.; Hisamatsu, S.i.; Inaba, J.; Nanzyo, M. Extractability of major and trace elements from agricultural soils using chemical extraction methods: Application for phytoavailability assessment. Soil Sci. Plant Nutr. 2006, 52, 406–417. [Google Scholar] [CrossRef]
- Saalidong, B.M.; Aram, S.A.; Otu, S.; Lartey, P.O. Examining the dynamics of the relationship between water pH and other water quality parameters in ground and surface water systems. PLoS ONE 2022, 17, e0262117. [Google Scholar] [CrossRef] [PubMed]
- Chiam, Z.Y.; Lee, J.T.E.; Tan, J.K.N.; Song, S.; Arora, S.; Tong, Y.W.; Tan, H.T.W. Evaluating the potential of okara-derived black soldier fly larval frass as a soil amendment. J. Environ. Manag. 2021, 286, 112163. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kawasaki, T.; Hirayasu, H.; Matsumoto, Y.; Fujitani, Y. Evaluation of fertilizer value of residues obtained after processing household organic waste with Black Soldier Fly larvae (Hermetia illucens). Sustainability 2020, 12, 4920. [Google Scholar] [CrossRef]
- Gutierrez-Gines, M.J.; Lehto, N.J.; Madejon, E.; Robinson, B.H. The effect of contrasting biosolids application strategies on soil quality. Plant Soil 2023. [Google Scholar] [CrossRef]
- Song, S.; Ee, A.W.L.; Tan, J.K.N.; Cheong, J.C.; Chiam, Z.; Arora, S.; Lam, W.N.; Tan, H.T.W. Upcycling food waste using Black Soldier Fly larvae: Effects of further composting on frass quality, fertilising effect and its global warming potential. J. Clean. Prod. 2021, 288, 125664. [Google Scholar] [CrossRef]
- Chen, B.Q.; Liu, E.K.; Tian, Q.Z.; Yan, C.R.; Zhang, Y.Q. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.M.; Klose, S.; Tabatabai, M.A. Soil microbial biomass carbon and nitrogen as affected by cropping systems. Biol. Fertility Soils 2000, 31, 200–210. [Google Scholar] [CrossRef]
- Rummel, P.S.; Beule, L.; Hemkemeyer, M.; Schwalb, S.A.; Wichern, F. Black Soldier Fly diet impacts soil greenhouse gas emissions from frass applied as fertilizer. Front. Sustain. Food Syst. 2021, 5, 709993. [Google Scholar] [CrossRef]




| Parameter | n | Statistic | df | p-Value |
|---|---|---|---|---|
| Total C (%) | 30 | 19.5 | 1 | 1.0 × 10−5 **** |
| Total N (%) | 30 | 2.29 | 1 | 0.13 |
| C/N ratio | 30 | 0.795 | 1 | 0.373 |
| Total P (g/kg) | 30 | 10.1 | 1 | 0.0015 ** |
| NO3−-N (mg/kg) | 30 | 0.876 | 1 | 0.349 |
| NH4+-N (mg/kg) | 30 | 10.9 | 1 | 0.00097 *** |
| Organic matter (g/kg) | 30 | 13.5 | 1 | 0.00024 *** |
| EC (mS/cm) | 30 | 4.56 | 1 | 0.0327 * |
| pH | 30 | 21.8 | 1 | 3.1 × 10−6 **** |
| Trace Element | Feedstock | Frass | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | Food | Biosolids 1 | Biosolids 1 + Wheat | Biosolids 1 + Food | Wheat | Food | Biosolids 1 | Biosolids 1 + Wheat | Biosolids 1 + Food | Origin 1 | Treatment | O × T 2 | |
| Al | 0.14 ± 0.13 d | 0.60 ± 0.07 d | 15.1 ± 3.13 cd | 12.60 ± 1.13 cd | 47.80 ± 9.24 ab | 0.32 ± 0.24 d | 0.37 ± 0.08 d | 2.14 ± 0.97 d | 4.07 ± 1.05 d | 2.75 ± 0.18 d | <0.001 | <0.001 | <0.001 |
| Cr | 0.010 ± 0.002 d | 0.020 ± 0.002 d | 0.31 ± 0.02 bc | 0.32 ± 0.02 b | 0.63 ± 0.12 a | 0.01 ± 0.00 d | 0.01 ± 0.00 d | 0.04 ± 0.01 d | 0.13 ± 0.01 bd | 0.040 ± 0.004 d | <0.001 | <0.001 | <0.001 |
| Mn | 6.10 ± 1.04 bd | 25.00 ± 1.13 a | 5.40 ± 0.35 bd | 11.80 ± 1.13 b | 10.90 ± 1.34 b | 7.07 ± 2.12 bd | 0.36 ± 0.04 d | 2.28 ± 0.41 cd | 7.87 ± 0.56 bc | 1.65 ± 0.03 cd | <0.001 | <0.001 | <0.001 |
| Fe | 2.0 ± 0.2 b | 0.75 ± 0.15 b | 60.30 ± 7.92 a | 57.20 ± 3.55 a | 57.7 ± 11.5 a | 5.51 ± 0.86 b | 0.21 ± 0.03 b | 6.03 ± 1.64 b | 11.20 ± 0.59 b | 4.34 ± 0.11 b | <0.001 | <0.001 | <0.001 |
| Co | 0.04 ± 0.002 ef | 0.020 ± 0.002 f | 0.31 ± 0.02 a | 0.23 ± 0.01 ab | 0.24 ± 0.06 ab | 0.08 ± 0.01 df | 0.01 ± 0.00 f | 0.13 ± 0.02 cde | 0.21 ± 0.01 bc | 0.100 ± 0.004 df | 0.001 | <0.001 | <0.001 |
| Ni | 0.69 ± 0.06 cd | 1.13 ± 0.08 bc | 2.17 ± 0.18 a | 1.60 ± 0.10 ab | 2.05 ± 0.33 a | 0.24 ± 0.02 d | 0.91 ± 0.08 c | 0.22 ± 0.04 d | 0.49 ± 0.03 cd | 0.20 ± 0.02 d | <0.001 | <0.001 | <0.001 |
| Cu | 0.98 ± 0.06 f | 0.77 ± 0.03 f | 35.6 ± 2.0 a | 29.40 ± 1.24 b | 15.10 ± 1.23 c | 1.54 ± 0.16 ef | 0.24 ± 0.03 f | 3.69 ± 0.53 df | 6.70 ± 0.33 d | 1.68 ± 0.18 ef | <0.001 | <0.001 | <0.001 |
| Zn | 1.31 ± 0.23 c | 5.41 ± 0.11 c | 74.10 ± 6.06 a | 35.20 ± 0.66 b | 51.2 ± 10.1 b | 1.37 ± 0.06 c | 0.31 ± 0.02 c | 2.83 ± 0.58 c | 3.24 ± 0.32 c | 1.52 ± 0.08 c | <0.001 | <0.001 | <0.001 |
| As | 0.030 ± 0.001 d | 0.01 ± 0.00 d | 1.02 ± 0.07 a | 0.73 ± 0.06 bc | 0.86 ± 0.11 ac | 0.07 ± 0.01 d | 0.010 ± 0.001 d | 0.99 ± 0.04 a | 0.97 ± 0.05 ab | 0.68 ± 0.06 c | 0.535 | <0.001 | 0.01 |
| Cd | 0.009 ± 0.000 b | 0.009 ± 0.000 b | 0.030 ± 0.001 a | 0.030 ± 0.001 a | 0.03 ± 0.01 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.010 ± 0.001 b | 0.01 ± 0.00 b | <0.001 | <0.001 | <0.001 |
| Pb | 0.02 ± 0.01 d | 0.020 ± 0.004 d | 0.12 ± 0.03 ab | 0.097 ± 0.004 bc | 0.18 ± 0.03 a | 0.01 ± 0.00 d | 0.01 ± 0.00 d | 0.02 ± 0.01 d | 0.04 ± 0.01 cd | 0.020 ± 0.003 d | <0.001 | <0.001 | <0.001 |
| Plant Species | Treatment | n1 | n2 | Statistic | df | p-Value |
|---|---|---|---|---|---|---|
| Lettuce | Wheat | 3 | 3 | 5.52 | 2.97 | 0.012 * |
| Lettuce | Food | 3 | 3 | −5.5 | 2.12 | 0.028 * |
| Lettuce | Biosolids | 3 | 3 | −0.552 | 2.24 | 0.631 |
| Lettuce | Biosolids + wheat | 3 | 3 | 5.24 | 2.07 | 0.032 * |
| Lettuce | Biosolids + food | 3 | 3 | −2.31 | 2.44 | 0.124 |
| Radish | Wheat | 3 | 3 | 2.43 | 3.99 | 0.072 |
| Radish | Food | 3 | 3 | −5.43 | 3.99 | 0.006 ** |
| Radish | Biosolids | 3 | 3 | 4.66 | 2.26 | 0.034 * |
| Radish | Biosolids + wheat | 3 | 3 | 2.98 | 3.54 | 0.048 * |
| Radish | Biosolids + food | 3 | 3 | −2.35 | 2.54 | 0.116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohm, K.; Hatley, G.A.; Robinson, B.H.; Gutiérrez-Ginés, M.J. Analysis of Chemical and Phytotoxic Properties of Frass Derived from Black Soldier Fly-Based Bioconversion of Biosolids. Sustainability 2023, 15, 11526. https://doi.org/10.3390/su151511526
Bohm K, Hatley GA, Robinson BH, Gutiérrez-Ginés MJ. Analysis of Chemical and Phytotoxic Properties of Frass Derived from Black Soldier Fly-Based Bioconversion of Biosolids. Sustainability. 2023; 15(15):11526. https://doi.org/10.3390/su151511526
Chicago/Turabian StyleBohm, Kristin, Gregory A. Hatley, Brett H. Robinson, and María J. Gutiérrez-Ginés. 2023. "Analysis of Chemical and Phytotoxic Properties of Frass Derived from Black Soldier Fly-Based Bioconversion of Biosolids" Sustainability 15, no. 15: 11526. https://doi.org/10.3390/su151511526