Bio-Based Polymeric Flocculants and Adsorbents for Wastewater Treatment
Abstract
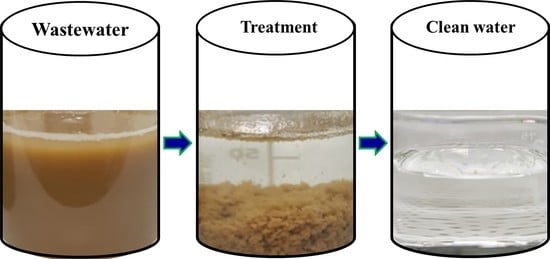
:1. Introduction
2. Methods of Water Purifications
3. Flocculation

Flocculants
| Sl. No. | Flocculent | Pollutant | Results (%) | Refs. |
|---|---|---|---|---|
| 1. | AP-g-PAM | Iron ore Kaolin Coal Silica Paper mill wastewater Municipal wastewater | 89.3%, pH 6.5 86.8%, pH 7.6 90.5%, pH 7.5 43.4%, pH 6.5 70.9%, pH 6.5 88.3%, pH 6.8 | [100,101] |
| 2 | AP-g-PDMA | Iron ore Kaolin Coal Silica Paper mill wastewater Municipal wastewater | 93.5%, pH 6.5 93.3%, pH 7.6 90.9%, pH 7.5 85.8%, pH 6.5 72.5%, pH 6.5 90%, pH 6.8 | [100] |
| 3 | AP-g-PAA | Mining industries’ wastewater | 87.31% | [102] |
| 4 | AP-g-PAA | Paper mill effluent Mine process water Textile wastewater | 82.1% 81.8% 98.0%, pH 8.2 | [103] |
| 5 | AP-g-poly (AM-co-ATMAC) | Kaolin | 5.4 NTU | [104] |
| 6 | Dxt-g-PAM | Kaolin suspension | Settling velocity 0.0370 cm/s | [105] |
| 7 | HES-g-PAM | Iron ore Kaolin Coal Silica Paper mill wastewater Municipal wastewater | 91.8%, pH 6.5 87.2%, pH 7.6 91.3%, pH 7.5 51.5%, pH 6.5 71.8%, pH 6.5 88.9%, pH 6.8 | [100,106] |
| 8 | HES-g-PDMA | Iron ore Kaolin Coal Silica Paper mill wastewater Municipal wastewater | 95.5%, pH 6.5 93.8%, pH 7.6 92.17%, pH 7.5 87.4%, pH 6.5 74.0%, pH 6.5 90.5%, pH 6.8 | [100,106] |
| 9 | HES-g-Poly (DMA-co-AA) | Iron ore Kaolin Coal Silica Paper mill wastewater Municipal wastewater | 89.5%, pH 6.5 94.5%, pH 7.6 90.1%, pH 7.5 51.0%, pH 6.5 76.4%, pH 6.5 77.7%, pH 6.8 | [100] |
| 10 | St-g-PAM | Iron ore Kaolin Coal Silica Paper mill wastewater Municipal wastewater | 86.4%, pH 6.5 83.9%, pH 7.6 88.2%, pH 7.5 42.2%, pH 6.5 69.6%, pH 6.5 79.3%, pH 6.8 | [90,100] |
| 11 | St-g-PDMA | Iron ore Kaolin Coal Silica Paper mill wastewater Municipal wastewater | 92.42%, pH 6.5 85.9%, pH 7.6 89.1%, pH 7.5 83.0%, pH 6.5 70.2%, pH 6.5 88.2%, pH 6.8 | [100] |
| 12 | St-g-poly(AM-co-2-methacryloyloxy ethyl trimethyl ammonium chloride) | Blast furnace effluent | 19.7 NTU | [107] |
| 13 | St-g-poly (2-methacryloyloxyethyl) trimethyl ammonium chloride) | Kaolin suspensions | 25 NTU, pH 4.0 | [108] |
| 14 | St-g-poly(2-methacryloyloxyethyl trimethyl ammonium chloride) | Kaolin Escherichia coli suspensions Kaolin and Escherichia coli suspensions | 98.7%, pH 11.0 95.5%, pH 11.0 98.7%, pH 11.0 | [109] |
| 15 | St-g-poly(AM-co-sodium xanthate) | Kaolin and CuSO4 | ~100%, pH 5.0 | [110] |
| 16 | St-g-poly(AM-co-AA) | Kaolin suspensions | ~100% transparent | [111] |
| 17 | Barly-g-PMMA | Coal fine Iron ore Kaolin Municipal wastewater | 86.0% 75.0% 82.0% 67.61%, pH 7.2 | [112] |
| 18 | CMC-g-p(DADMAC) | Kaolin suspension Coal suspension Iron ore | 80.9% 83.6% 79.5% | [113] |
| 19 | Moringa gum-based | Coal River water Kaolin MWCNTs | 90% 91% 92% 98% | [114] |
| 20 | Chitosan-g-acrylamidopropyltrimethylammonium chloride | Kaolin | 90% | [115] |
| 21 | Kraft lignin-based polymers | Kaolin | 62.91% | [116] |
| 22 | Carboxymethylcellulose-AM-4-vinylpyridine | Kaolin Bentonite | 75% 89% | [117] |
| 23 | Starch-Based Flocculants | Kaolin Hematite | 97.7%, pH 11 98.6%, pH 7 | [118] |



4. Adsorption
4.1. Adsorbents
4.2. Biopolymer-Based Adsorbents
5. Commercial Applications
6. Regeneration of Polymeric Adsorbents
7. Biodegradation of Polymeric Materials
8. Limitations, Challenges, and Opportunities
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Acrylic acid | ||
| AM | Acrylamide | NTU | Nephelometric turbidity unit |
| AML | Amylose | OD | Optical density |
| AN | Acrylonitrile | PAA | Poly(acrylic acid) |
| AP | Amylopectin | PAM | Polyacrylamide |
| ATMAC | (3-acrylamidopropyl) trimethylammonium chloride | PDMA | Poly(N,N-dimethyl acrylamide) |
| CR | Congo red | PEG | Polyethylene glycol |
| DADMAC | Dimethyl diallyl ammonium chloride | PMETAC | Poly (2-methacryloyloxy ethyl trimethyl ammonium chloride) |
| Dextrin | Dt | ppb | Parts per billion |
| DMA | N,N-dimethyl acrylamide | ppm | Parts per million |
| DNS | 2,4-dinitrosalicylic acid | PPS | Potassium peroxydisulfate |
| -g- | Grafting | PVA | Polyvinyl alcohol |
| HES | Hydroxyethyl starch | St | Starch |
| MB | Methylene Blue | UV | Ultraviolet |
| MF | Microfiltration | UV–VIS | Ultraviolet–Visible |
| MG | Malachite Green | ||
| MMA | Methyl methacrylate |
References
- Hussain, D.; Khan, S.A.; Alam Khan, T.; Alharthi, S.S. Efficient liquid phase confiscation of nile blue using a novel hybrid nanocomposite synthesized from guar gum-polyacrylamide and erbium oxide. Sci. Rep. 2022, 12, 14656. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.S.; Ali, N.; Hamid, R.; Ganie, S.A. Genotoxic effect of two commonly used food dyes metanil yellow and carmoisine using Allium cepa L. as indicator. Toxicol. Rep. 2020, 7, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Kaur, J.; Bhat, S.A.; Singh, N.; Bhatti, S.S.; Kaur, V.; Katnoria, J.K. Assessment of the Heavy Metal Contamination of Roadside Soils Alongside Buddha Nullah, Ludhiana, (Punjab) India. Int. J. Environ. Res. Public Health 2022, 19, 1596. [Google Scholar] [CrossRef]
- Pasdaran, A.; Azarpira, N.; Heidari, R.; Nourinejad, S.; Zare, M.; Hamedi, A. Effects of some cosmetic dyes and pigments on the proliferation of human foreskin fibroblasts and cellular oxidative stress; potential cytotoxicity of chlorophyllin and indigo carmine on fibroblasts. J. Cosmet. Dermatol. 2022, 21, 3979–3985. [Google Scholar] [CrossRef]
- Emenike, P.C.; Neris, J.B.; Tenebe, I.T.; Nnaji, C.C.; Jarvis, P. Estimation of some trace metal pollutants in River Atuwara southwestern Nigeria and spatio-temporal human health risks assessment. Chemosphere 2020, 239, 124770. [Google Scholar] [CrossRef]
- Cao, X.; Lu, Y.; Wang, C.; Zhang, M.; Yuan, J.; Zhang, A.; Song, S.; Baninla, Y.; Khan, K.; Wang, Y. Hydrogeochemistry and quality of surface water and groundwater in the drinking water source area of an urbanizing region. Ecotoxicol. Environ. Saf. 2019, 186, 109628. [Google Scholar] [CrossRef]
- Hossain, S.; Anik, A.H.; Khan, R.; Ahmed, F.T.; Siddique, M.A.B.; Khan, A.H.A.N.; Saha, N.; Idris, A.M.; Alam, M. Public Health Vulnerability Due to the Exposure of Dissolved Metal(oid)s in Tap Water from a Mega City (Dhaka, Bangladesh): Source and Quality Appraisals. Expo. Heal. 2022, 14, 713–732. [Google Scholar] [CrossRef]
- Muhammad, S.; Usman, Q.A. Heavy metal contamination in water of Indus River and its tributaries, Northern Pakistan: Evaluation for potential risk and source apportionment. Toxin Rev. 2022, 41, 380–388. [Google Scholar] [CrossRef]
- Rizk, R.; Juzsakova, T.; Ben Ali, M.; Rawash, M.A.; Domokos, E.; Hedfi, A.; Almalki, M.; Boufahja, F.; Shafik, H.M.; Rédey, Á. Comprehensive environmental assessment of heavy metal contamination of surface water, sediments and Nile Tilapia in Lake Nasser, Egypt. J. King Saud Univ. Sci. 2022, 34, 101748. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ahmed, Z.; Seefat, S.M.; Alam, R.; Islam, A.R.M.T.; Choudhury, T.R.; Begum, B.A.; Idris, A.M. Assessment of heavy metal contamination in sediment at the newly established tannery industrial Estate in Bangladesh: A case study. Environ. Chem. Ecotoxicol. 2022, 4, 1–12. [Google Scholar] [CrossRef]
- Küçüksümbül, A.; Akar, A.T.; Tarcan, G. Source, degree and potential health risk of metal(loid)s contamination on the water and soil in the Söke Basin, Western Anatolia, Turkey. Environ. Monit. Assess. 2021, 194, 6. [Google Scholar] [CrossRef]
- Kang, C.-W.; Kolya, H. Green Synthesis of Ag-Au Bimetallic Nanocomposites Using Waste Tea Leaves Extract for Degradation Congo Red and 4-Nitrophenol. Sustainability 2021, 13, 3318. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Biogenic Synthesis of Silver-Iron Oxide Nanoparticles Using Kulekhara Leaves Extract for Removing Crystal Violet and Malachite Green Dyes from Water. Sustainability 2022, 14, 15800. [Google Scholar] [CrossRef]
- Kolya, H.; Maiti, P.; Pandey, A.; Tripathy, T. Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. J. Anal. Sci. Technol. 2015, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose hydrogels: Green and sustainable soft biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Lustenberger, S.; Castro-Muñoz, R. Advanced biomaterials and alternatives tailored as membranes for water treatment and the latest innovative European water remediation projects: A review. Case Stud. Chem. Environ. Eng. 2022, 5, 100205. [Google Scholar] [CrossRef]
- Zaman, A.; Ali, M.S.; Orasugh, J.T.; Banerjee, P.; Chattopadhyay, D. Biopolymer-Based Nanocomposites for Removal of Hazardous Dyes from Water Bodies BT—Innovations in Environmental Biotechnology; Arora, S., Kumar, A., Ogita, S., Yau, Y.-Y., Eds.; Springer Nature: Singapore, 2022; pp. 759–783. ISBN 9789811644450. [Google Scholar]
- Alias, N.H.; Abdullah, N.; Othman, N.H.; Marpani, F.; Zainol, M.M.; Shayuti, M.S.M. Sustainability Challenges and Future Perspectives of Biopolymer BT—Biopolymers: Recent Updates, Challenges and Opportunities; Nadda, A.K., Sharma, S., Bhat, R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 373–389. ISBN 9783030983925. [Google Scholar]
- Kour, G.; Majhi, P.K.; Bharti, A.; Kothari, R.; Jain, A.; Singh, A.; Tyagi, V.V.; Pathania, D. Biopolymer-Based Nanocomposites and Water Treatment: A Global Outlook. In Biorenewable Nanocomposite Materials, Vol. 2: Desalination and Wastewater Remediation; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1411, pp. 2–25. ISBN 9780841297807. [Google Scholar]
- Patel, M.; Patel, J. Improving Sustainable Environment of Biopolymers Using Nanotechnology. In Sustainable Nanotechnology: Strategies, Products, and Applications; Wiley: Hoboken, NJ, USA, 2022; pp. 71–87. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [Green Version]
- Gough, C.R.; Callaway, K.; Spencer, E.; Leisy, K.; Jiang, G.; Yang, S.; Hu, X. Biopolymer-Based Filtration Materials. ACS Omega 2021, 6, 11804–11812. [Google Scholar] [CrossRef]
- Mamba, F.B.; Mbuli, B.S.; Ramontja, J. Recent Advances in Biopolymeric Membranes towards the Removal of Emerging Organic Pollutants from Water. Membranes 2021, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Picos-Corrales, L.A.; Morales-Burgos, A.M.; Ruelas-Leyva, J.P.; Crini, G.; García-Armenta, E.; Jimenez-Lam, S.A.; Ayón-Reyna, L.E.; Rocha-Alonzo, F.; Calderón-Zamora, L.; Osuna-Martínez, U.; et al. Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection. Polymers 2023, 15, 526. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Polymer Systems for Controlled Release of Macromolecules, Immobilized Enzyme Medical Bioreactors, and Tissue Engineering. In Advances in Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 1994; Volume 19, pp. 1–50. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Preparation, investigation of metal ion removal and flocculation performances of grafted hydroxyethyl starch. Int. J. Biol. Macromol. 2013, 62, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Hestekin, J.A.; Brushaber, P.; Cullen, L.; Bachas, L.G.; Sikdar, S.K. Novel poly-glutamic acid functionalized microfiltration membranes for sorption of heavy metals at high capacity. J. Membr. Sci. 1998, 141, 121–135. [Google Scholar] [CrossRef]
- Bajaj, I.; Singhal, R. Poly (glutamic acid)—An emerging biopolymer of commercial interest. Bioresour. Technol. 2011, 102, 5551–5561. [Google Scholar] [CrossRef]
- Lee, C.S.; Chong, M.F.; Robinson, J.; Binner, E. Optimisation of extraction and sludge dewatering efficiencies of bio-flocculants extracted from Abelmoschus esculentus (okra). J. Environ. Manag. 2015, 157, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Vandermeulen, G.W.M.; Boarino, A.; Klok, H.-A. Biodegradation of water-soluble and water-dispersible polymers for agricultural, consumer, and industrial applications—Challenges and opportunities for sustainable materials solutions. J. Polym. Sci. 2022, 60, 1797–1813. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Chapter 4—Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Elsevier: Oxford, UK, 2014; pp. 67–89. ISBN 9780123969835. [Google Scholar]
- Phalle, S.P.; Choudhari, P.B.; Choudhari, S.P.; Bhagwat, D.A.; Kadam, A.M.; Gaikwad, V.L. Microwave-assisted grafting of acrylamide on a natural xylan gum for controlled drug delivery. Polym. Bull. 2023, 1–18. [Google Scholar] [CrossRef]
- Kong, W.; Li, Q.; Liu, J.; Li, X.; Zhao, L.; Su, Y.; Yue, Q.; Gao, B. Adsorption behavior and mechanism of heavy metal ions by chicken feather protein-based semi-interpenetrating polymer networks super absorbent resin. RSC Adv. 2016, 6, 83234–83243. [Google Scholar] [CrossRef]
- Rajabi, M.; Keihankhadiv, S.; Suhas; Tyagi, I.; Karri, R.R.; Chaudhary, M.; Mubarak, N.M.; Chaudhary, S.; Kumar, P.; Singh, P. Comparison and interpretation of isotherm models for the adsorption of dyes, proteins, antibiotics, pesticides and heavy metal ions on different nanomaterials and non-nano materials—A comprehensive review. J. Nanostructure Chem. 2023, 13, 43–65. [Google Scholar] [CrossRef]
- Liu, J.; Su, D.; Yao, J.; Huang, Y.; Shao, Z.; Chen, X. Soy protein-based polyethylenimine hydrogel and its high selectivity for copper ion removal in wastewater treatment. J. Mater. Chem. A 2017, 5, 4163–4171. [Google Scholar] [CrossRef]
- Ekrayem, N.A.; Alhwaige, A.A.; Elhrari, W.; Amer, M. Removal of lead (II) ions from water using chitosan/polyester crosslinked spheres derived from chitosan and glycerol-based polyester. J. Environ. Chem. Eng. 2021, 9, 106628. [Google Scholar] [CrossRef]
- Lin, S.H.; Lin, C.M. Treatment of textile waste effluents by ozonation and chemical coagulation. Water Res. 1993, 27, 1743–1748. [Google Scholar] [CrossRef]
- El-Gohary, F.; Tawfik, A. Decolorization and COD reduction of disperse and reactive dyes wastewater using chemical-coagulation followed by sequential batch reactor (SBR) process. Desalination 2009, 249, 1159–1164. [Google Scholar] [CrossRef]
- Leentvaar, J.; Rebhun, M. Effect of magnesium and calcium precipitation on coagulation-flocculation with lime. Water Res. 1982, 16, 655–662. [Google Scholar] [CrossRef]
- Semerjian, L.; Ayoub, G. High-pH–magnesium coagulation–flocculation in wastewater treatment. Adv. Environ. Res. 2003, 7, 389–403. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: New York, NY, USA, 1984; ISBN 0471866067. [Google Scholar]
- Cooney, D.O. Adsorption Design for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 1998; ISBN 1566703336. [Google Scholar]
- Blöcher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.K.; Matis, K.A. Hybrid flotation—Membrane filtration process for the removal of heavy metal ions from wastewater. Water Res. 2003, 37, 4018–4026. [Google Scholar] [CrossRef]
- Kolya, H.; Singh, V.K.; Kang, C.-W. Polymeric Membranes and Hybrid Techniques for Water Purification Applications BT—Polymer-Based Advanced Functional Materials for Energy and Environmental Applications; Subramani, N.K., Nataraj, S.K., Patel, C., Shivanna, S., Eds.; Springer: Singapore, 2022; pp. 75–91. ISBN 9789811687556. [Google Scholar]
- Mavros, P.; Daniilidou, A.C.; Lazaridis, N.K.; Stergiou, L. Colour removal from aqueous solutions. Part I. Flotation. Environ. Technol. 1994, 15, 601–616. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Lazaridis, N.K.; Grohmann, A. Toxic metals removal from waste waters by upflow filtration with floating filter medium. I. The case of zinc. Sep. Sci. Technol. 2002, 37, 403–416. [Google Scholar] [CrossRef]
- Al-Kdasi, A.; Idris, A.; Saed, K.; Guan, C.T. Treatment of textile wastewater by advanced oxidation processes– A review. Glob. Nest J. 2004, 6, 222–230. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Mo, K.B. Membrane-based solvent extraction for selective removal and recovery of metals. J. Membr. Sci. 1984, 21, 5–19. [Google Scholar] [CrossRef]
- Yang, C.; Qian, Y.; Zhang, L.; Feng, J. Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem. Eng. J. 2006, 117, 179–185. [Google Scholar] [CrossRef]
- Tran, D.; Weidhaas, J. Ion exchange for effective separation of 3-nitro-1,2,4-triazol-5-one (NTO) from wastewater. J. Hazard. Mater. 2022, 436, 129215. [Google Scholar] [CrossRef] [PubMed]
- Edgar, M.; Boyer, T.H. Nitrate adsorption and desorption during biological ion exchange. Sep. Purif. Technol. 2022, 285, 120363. [Google Scholar] [CrossRef]
- Yulistyorini, A.; Sunaryo, K.; Mujiyono; Camargo-Valero, M.A. Hybrid Anaerobic Baffled Reactor and Upflow Anaerobic Filter for Domestic Wastewater Purification BT—Environmental Degradation: Challenges and Strategies for Mitigation; Singh, V.P., Yadav, S., Yadav, K.K., Yadava, R.N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 149–164. ISBN 9783030955427. [Google Scholar]
- Kuroda, K.; Narihiro, T.; Shinshima, F.; Yoshida, M.; Yamaguchi, H.; Kurashita, H.; Nakahara, N.; Nobu, M.K.; Noguchi, T.Q.; Yamauchi, M.; et al. High-rate cotreatment of purified terephthalate and dimethyl terephthalate manufacturing wastewater by a mesophilic upflow anaerobic sludge blanket reactor and the microbial ecology relevant to aromatic compound degradation. Water Res. 2022, 219, 118581. [Google Scholar] [CrossRef] [PubMed]
- Bora, A.; Mohanrasu, K.; Swetha, T.A.; Ananthi, V.; Kumar, P.; Govarthanan, M.; Arun, A. Chapter 21—Microbes incorporated nanomaterials for water purification. In Handbook of Microbial Nanotechnology; Hussain, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 439–459. ISBN 9780128234266. [Google Scholar]
- Zhao, Z.; Cheng, M.; Li, Y.; Song, X.; Wang, Y.; Zhang, Y. A Novel Constructed Wetland Combined with Microbial Desalination Cells and its Application. Microb. Ecol. 2021, 83, 340–352. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Bohari, M.H.; Sicilia, A.; Avignone-Rossa, C.; Bussemaker, M.; Saroj, D.; Lee, J. Tertiary treatment of real abattoir wastewater using combined acoustic cavitation and ozonation. Ultrason. Sonochemistry 2020, 64, 104986. [Google Scholar] [CrossRef]
- Gatidou, G.; Samanides, C.G.; Fountoulakis, M.S.; Vyrides, I. Microbial electrolysis cell coupled with anaerobic granular sludge: A novel technology for real bilge water treatment. Chemosphere 2022, 296, 133988. [Google Scholar] [CrossRef]
- Heebner, A.; Abbassi, B. Electrolysis catalyzed ozonation for advanced wastewater treatment. J. Water Process. Eng. 2022, 46, 102638. [Google Scholar] [CrossRef]
- Kamali, M.; Aminabhavi, T.M.; Tarelho, L.A.; Hellemans, R.; Cuypers, J.; Capela, I.; Costa, M.E.V.; Dewil, R.; Appels, L. Acclimatized activated sludge for enhanced phenolic wastewater treatment using pinewood biochar. Chem. Eng. J. 2022, 427, 131708. [Google Scholar] [CrossRef]
- Lin, R.; Li, Y.; Yong, T.; Cao, W.; Wu, J.; Shen, Y. Synergistic effects of oxidation, coagulation and adsorption in the integrated fenton-based process for wastewater treatment: A review. J. Environ. Manag. 2022, 306, 114460. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Rathilal, S.; Tetteh, E.K. Coagulation Treatment of Wastewater: Kinetics and Natural Coagulant Evaluation. Molecules 2021, 26, 698. [Google Scholar] [CrossRef]
- Lee, H.-K.; Chang, S.; Park, W.; Kim, T.-J.; Park, S.; Jeon, H. Effective treatment of uranium-contaminated soil-washing effluent using precipitation/flocculation process for water reuse and solid waste disposal. J. Water Process. Eng. 2022, 48, 102890. [Google Scholar] [CrossRef]
- Oliveira, C.; Rubio, J. A short overview of the formation of aerated flocs and their applications in solid/liquid separation by flotation. Miner. Eng. 2012, 39, 124–132. [Google Scholar] [CrossRef]
- Zhao, J.; Li, B.; Wang, A.; Ge, W.; Li, W. Floc formation and growth mechanism during magnesium hydroxide and polyacrylamide coagulation process for reactive orange removal. Environ. Technol. 2022, 43, 424–430. [Google Scholar] [CrossRef]
- Wu, Y.; Tran, Q.C.; Zhang, X.; Lu, Y.; Xu, J.; Zhang, H. Experimental investigation on the treatment of different typical marine dredged sludges by flocculant and vacuum preloading. Arab. J. Geosci. 2022, 15, 642. [Google Scholar] [CrossRef]
- Na, S.-H.; Kim, M.-J.; Kim, J.-T.; Jeong, S.; Lee, S.; Chung, J.; Kim, E.-J. Microplastic removal in conventional drinking water treatment processes: Performance, mechanism, and potential risk. Water Res. 2021, 202, 117417. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, M.-K. Chapter 1—Intermolecular Force. In Interface Science and Composites; Park, S.-J., Seo, M.-K., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 18, pp. 1–57. ISBN 1573-4285. [Google Scholar]
- Cheng, Y.; Shi, J.; Zhang, Q.; Fang, C.; Chen, J.; Li, F. Recent Progresses in Adsorption Mechanism, Architectures, Electrode Materials and Applications for Advanced Electrosorption System: A Review. Polymers 2022, 14, 2985. [Google Scholar] [CrossRef]
- Bedzyk, M.J.; Bommarito, G.M.; Caffrey, M.; Penner, T.L. Diffuse-Double Layer at a Membrane-Aqueous Interface Measured with X-Ray Standing Waves. Science 1990, 248, 52–56. [Google Scholar] [CrossRef]
- Grahame, D.C. Diffuse Double Layer Theory for Electrolytes of Unsymmetrical Valence Types. J. Chem. Phys. 1953, 21, 1054–1060. [Google Scholar] [CrossRef]
- Ninham, B.W. On progress in forces since the DLVO theory. Adv. Colloid Interface Sci. 1999, 83, 1–17. [Google Scholar] [CrossRef]
- Amuda, O.S.; Alade, A. Coagulation/flocculation process in the treatment of abattoir wastewater. Desalination 2006, 196, 22–31. [Google Scholar] [CrossRef]
- Amuda, O.S.; Amoo, I.A. Coagulation/flocculation process and sludge conditioning in beverage industrial wastewater treatment. J. Hazard. Mater. 2007, 141, 778–783. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Wong, S.S.; Teng, T.T.; Zuhairi, A. Improvement of alum and PACl coagulation by polyacrylamides (PAMs) for the treatment of pulp and paper mill wastewater. Chem. Eng. J. 2008, 137, 510–517. [Google Scholar] [CrossRef]
- Chong, M.F.; Lee, K.P.; Chieng, H.J.; Ramli, I.I.S.B. Removal of boron from ceramic industry wastewater by adsorption–flocculation mechanism using palm oil mill boiler (POMB) bottom ash and polymer. Water Res. 2009, 43, 3326–3334. [Google Scholar] [CrossRef]
- Martin, M.A.; González, I.; Berrios, M.; Siles, J.A.; Martin, A. Optimization of coagulation–flocculation process for wastewater derived from sauce manufacturing using factorial design of experiments. Chem. Eng. J. 2011, 172, 771–782. [Google Scholar] [CrossRef]
- Sher, F.; Malik, A.; Liu, H. Industrial polymer effluent treatment by chemical coagulation and flocculation. J. Environ. Chem. Eng. 2013, 1, 684–689. [Google Scholar] [CrossRef]
- Irfan, M.; Butt, T.; Imtiaz, N.; Abbas, N.; Khan, R.A.; Shafique, A. The removal of COD, TSS and colour of black liquor by coagulation–flocculation process at optimized pH, settling and dosing rate. Arab. J. Chem. 2017, 10, S2307–S2318. [Google Scholar] [CrossRef] [Green Version]
- Sarika, R.; Kalogerakis, N.; Mantzavinos, D. Treatment of olive mill effluents: Part II. Complete removal of solids by direct flocculation with poly-electrolytes. Environ. Int. 2005, 31, 297–304. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Rishel, K.L.; Sibrell, P.L. Screening and evaluation of polymers as flocculation aids for the treatment of aquacultural effluents. Aquac. Eng. 2005, 33, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.S.; Teng, T.T.; Ahmad, A.L.; Zuhairi, A.; Najafpour, G. Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J. Hazard. Mater. 2006, 135, 378–388. [Google Scholar] [CrossRef]
- Kang, Q.; Gao, B.; Yue, Q.; Zhou, W.; Shen, D. Residual color profiles of reactive dyes mixture during a chemical flocculation process. Colloids Surfaces A: Physicochem. Eng. Asp. 2007, 299, 45–53. [Google Scholar] [CrossRef]
- Yue, Q.Y.; Gao, B.Y.; Wang, Y.; Zhang, H.; Sun, X.; Wang, S.G.; Gu, R.R. Synthesis of polyamine flocculants and their potential use in treating dye wastewater. J. Hazard. Mater. 2008, 152, 221–227. [Google Scholar] [CrossRef]
- Razali, M.A.A.; Ahmad, Z.; Ahmad, M.S.B.; Ariffin, A. Treatment of pulp and paper mill wastewater with various molecular weight of polyDADMAC induced flocculation. Chem. Eng. J. 2011, 166, 529–535. [Google Scholar] [CrossRef]
- Rubio, J. The flocculation properties of poly (ethylene oxide). Colloids Surfaces 1981, 3, 79–95. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Sun, Y.; Ren, J.; Zheng, X.; Sun, Q.; Jiang, S.; Ding, W. Synthesis of novel chitosan-based flocculants with amphiphilic structure and its application in sludge dewatering: Role of hydrophobic groups. J. Clean. Prod. 2020, 249, 119350. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Grafted polysaccharides based on acrylamide and N,N-dimethylacrylamide: Preparation and investi-gation of their flocculation performances. J. Appl. Polym. Sci. 2013, 127, 2786–2795. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M.; Kasirian, N. Development of effective nano-biosorbent based on poly m-phenylenediamine grafted dextrin for removal of Pb (II) and methylene blue from water. Carbohydr. Polym. 2018, 201, 539–548. [Google Scholar] [CrossRef]
- Saya, L.; Malik, V.; Singh, A.; Singh, S.; Gambhir, G.; Singh, W.R.; Chandra, R.; Hooda, S. Guar gum based nanocomposites: Role in water purification through efficient removal of dyes and metal ions. Carbohydr. Polym. 2021, 261, 117851. [Google Scholar] [CrossRef]
- Maji, B.; Maiti, S. Chemical modification of xanthan gum through graft copolymerization: Tailored properties and potential applications in drug delivery and wastewater treatment. Carbohydr. Polym. 2021, 251, 117095. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int. J. Biol. Macromol. 2020, 143, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, M.; Maqsood, H.; Uroos, M.; Iqbal, A. A Comparative Study of Different Methods for Cellulose Extraction from Lignocellulosic Wastes and Conversion into Carboxymethyl Cellulose. Chemistryselect 2022, 7, e202201533. [Google Scholar] [CrossRef]
- Du, Q.; Wang, Y.; Li, A.; Yang, H. Scale-inhibition and flocculation dual-functionality of poly(acrylic acid) grafted starch. J. Environ. Manag. 2018, 210, 273–279. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Tang, X.; Jiang, J.; He, Q.; Xiong, Z.; Zheng, H. Biopolymer-based flocculants: A review of recent technologies. Environ. Sci. Pollut. Res. Int. 2021, 28, 46934–46963. [Google Scholar] [CrossRef]
- Kolya, H.; Sasmal, D.; Tripathy, T. Novel Biodegradable Flocculating Agents Based on Grafted Starch Family for the Industrial Effluent Treatment. J. Polym. Environ. 2017, 25, 408–418. [Google Scholar] [CrossRef]
- Sasmal, D.; Singh, R.P.; Tripathy, T. Synthesis and flocculation characteristics of a novel biodegradable flocculating agent amylopectin-g-poly(acrylamide-co-N-methylacrylamide). Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 575–584. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Mandre, N.R.; Panda, A.B.; Pal, S. Amylopectin grafted with poly (acrylic acid): Development and application of a high performance flocculant. Carbohydr. Polym. 2013, 95, 753–759. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Ghorai, S.; Patra, A.S.; Mishra, B.K.; Mandre, N.R.; Pal, S. Modified amylopectin based flocculant for the treatment of synthetic effluent and industrial wastewaters. Int. J. Biol. Macromol. 2015, 72, 356–363. [Google Scholar] [CrossRef]
- Kumar, K.; Adhikary, P.; Karmakar, N.C.; Gupta, S.; Singh, R.P.; Krishnamoorthi, S. Synthesis, characterization and application of novel cationic and amphoteric flocculants based on amylopectin. Carbohydr. Polym. 2015, 127, 275–281. [Google Scholar] [CrossRef]
- Pal, S.; Nasim, T.; Patra, A.; Ghosh, S.; Panda, A.B. Microwave assisted synthesis of polyacrylamide grafted dextrin (Dxt-g-PAM): Development and application of a novel polymeric flocculant. Int. J. Biol. Macromol. 2010, 47, 623–631. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Polyacrylamide and Poly N, N-Dimethylacrylamide Grafted Hydroxyethyl Starch: Efficient Biode-gradable Flocculants. J. Colloid Sci. Biotechnol. 2014, 3, 150–159. [Google Scholar] [CrossRef]
- Das, S.; Patra, P.; Singha, K.; Biswas, P.; Sarkar, S.; Pal, S. Graft copolymeric flocculant using functionalized starch towards treatment of blast furnace effluent. Int. J. Biol. Macromol. 2019, 125, 35–40. [Google Scholar] [CrossRef]
- Wang, J.-P.; Yuan, S.-J.; Wang, Y.; Yu, H.-Q. Synthesis, characterization and application of a novel starch-based flocculant with high flocculation and dewatering properties. Water Res. 2013, 47, 2643–2648. [Google Scholar] [CrossRef]
- Huang, M.; Liu, Z.; Li, A.; Yang, H. Dual functionality of a graft starch flocculant: Flocculation and antibacterial performance. J. Environ. Manag. 2017, 196, 63–71. [Google Scholar] [CrossRef]
- Chang, Q.; Hao, X.; Duan, L. Synthesis of crosslinked starch-graft-polyacrylamide-co-sodium xanthate and its performances in wastewater treatment. J. Hazard. Mater. 2008, 159, 548–553. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, H.; Yuan, B.; Huang, M.; Yang, H.; Li, A.; Bai, J.; Cheng, R. Synthesis of amphoteric starch-based grafting flocculants for flocculation of both positively and negatively charged colloidal contaminants from water. Chem. Eng. J. 2014, 244, 209–217. [Google Scholar] [CrossRef]
- Dey, K.P.; Mishra, S.; Sen, G. Synthesis and characterization of polymethylmethacrylate grafted barley for treatment of industrial and municipal wastewater. J. Water Process. Eng. 2017, 18, 113–125. [Google Scholar] [CrossRef]
- Mehta, A.; Aryan, A.; Pandey, J.P.; Sen, G. Synthesis of a Novel Water-Soluble Graft Copolymer for Mineral Ore Beneficiation and for River Water Treatment towards Drinking Water Augmentation. Chemistryselect 2022, 7, e202103289. [Google Scholar] [CrossRef]
- Kumari, N.; Mishra, S. Synthesis, characterization and flocculation efficiency of grafted Moringa gum based derivatives. Carbohydr. Polym. 2022, 281, 119079. [Google Scholar] [CrossRef] [PubMed]
- Suresha, P.; Badiger, M.V. Cationic chitosan graft flocculants: Synthesis, characterization and applications in kaolin separation. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Hasan, A.; Fatehi, P. Flocculation of kaolin particles with cationic lignin polymers. Sci. Rep. 2019, 9, 2672. [Google Scholar] [CrossRef] [PubMed]
- Belaid, A.; Bouras, B.; Hocine, T.; Tennouga, L. Flocculation of Clay Suspensions Using Copolymers Based on Acrylamide and Biopolymer. Phys. Chem. Res. 2023, 11, 221–230. [Google Scholar]
- Li, H.; Cai, T.; Yuan, B.; Li, R.; Yang, H.; Li, A. Flocculation of Both Kaolin and Hematite Suspensions Using the Starch-Based Flocculants and Their Floc Properties. Ind. Eng. Chem. Res. 2015, 54, 59–67. [Google Scholar] [CrossRef]
- Jyothi, A.N. Starch Graft Copolymers: Novel Applications in Industry. Compos. Interfaces 2010, 17, 165–174. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Hydroxyethyl Starch-g-Poly-(N,N-dimethylacrylamide-co-acrylic acid): An efficient dye removing agent. Eur. Polym. J. 2013, 49, 4265–4275. [Google Scholar] [CrossRef]
- Guan, G.; Gao, T.; Lou, T.; Wang, X. Lignocellulose–acrylamide–carboxymethyl cellulose copolymer as a cost-effective anionic flocculant. Iran. Polym. J. 2022, 31, 587–594. [Google Scholar] [CrossRef]
- Zhou, P.; Ru, X.; Yang, W.; Dai, Z.; Ofori, M.A.; Chen, J.; Hou, J.; Zhong, Z.; Jin, H. Study on preparation of cationic flocculants by grafting binary monomer on cellulose substrate by γ-ray co-irradiation. J. Environ. Chem. Eng. 2022, 10, 107138. [Google Scholar] [CrossRef]
- Han, Z.; Huo, J.; Zhang, X.; Ngo, H.H.; Guo, W.; Du, Q.; Zhang, Y.; Li, C.; Zhang, D. Characterization and flocculation performance of a newly green flocculant derived from natural bagasse cellulose. Chemosphere 2022, 301, 134615. [Google Scholar] [CrossRef]
- Hu, P.; Shen, S.; Yang, H. Evaluation of hydrophobically associating cationic starch-based flocculants in sludge dewatering. Sci. Rep. 2021, 11, 11819. [Google Scholar] [CrossRef]
- Tripathy, T.; De, B.R. Flocculation: A new way to treat the waste water. J. Phys. Sci. 2006, 10, 93–127. [Google Scholar]
- Lü, T.; Luo, C.; Qi, D.; Zhang, D.; Zhao, H. Efficient treatment of emulsified oily wastewater by using amphipathic chitosan-based flocculant. React. Funct. Polym. 2019, 139, 133–141. [Google Scholar] [CrossRef]
- Mühle, K. Floc Stability in Laminar and Turbulent Flow. In Surfactant Science Series; Marcel Dekker AG: New York, NY, USA, 1993; p. 355. [Google Scholar]
- Nie, Y.; Wang, Z.; Wang, W.; Zhou, Z.; Kong, Y.; Ma, J. Bio-flocculation of Microcystis aeruginosa by using fungal pellets of Aspergillus oryzae: Performance and mechanism. J. Hazard. Mater. 2022, 439, 129606. [Google Scholar] [CrossRef]
- Ma, K.; Zhao, L.; Jiang, Z.; Huang, Y.; Sun, X. Adsorption characteristics of Cr (III) onto starch-graft-poly(acrylic acid)/organo-modifed zeolite 4A composite: A novel path to the adsorption mechanisms. Polym. Compos. 2018, 39, 1223–1233. [Google Scholar] [CrossRef]
- Korhonen, M.H.J.; Rojas, O.J.; Laine, J. Effect of charge balance and dosage of polyelectrolyte complexes on the shear resistance of mineral floc strength and reversibility. J. Colloid Interface Sci. 2015, 448, 73–78. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment; IWA Publishing: London, UK, 2006; ISBN 1843391066. [Google Scholar]
- Singh, R.P.; Tripathy, T.; Karmakar, G.P.; Rath, S.K.; Karmakar, N.C.; Pandey, S.R.; Kannan, K.; Jain, S.K.; Lan, N.T. Novel biodegradable flocculants based on polysaccharides. Curr. Sci. 2000, 78, 798–803. [Google Scholar]
- Pal, S.; Das, R.; Ghorai, S. Hydroxypropyl Methyl Cellulose Grafted with Poly (Acrylamide): Application as Novel Polymeric Material as Flocculant as well as Adsorbent. In Cellulose-Based Graft Copolymers; CRC Press: Boca Raton, FL, USA, 2015; pp. 320–353. [Google Scholar]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent Achievements in Polymer Bio-Based Flocculants for Water Treat-ment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef]
- Lapointe, M.; Jahandideh, H.; Farner, J.M.; Tufenkji, N. Super-bridging fibrous materials for water treatment. NPJ Clean Water 2022, 5, 11. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Kurusu, R.S.; Lapointe, M.; Tufenkji, N. Sustainable iron-grafted cellulose fibers enable coagulant recycling and improve contaminant removal in water treatment. Chem. Eng. J. 2022, 430, 132927. [Google Scholar] [CrossRef]
- Ngah, W.W.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Kolya, H.; Tripathy, T. Metal complexation studies of amylopectin-graft -poly[(N,N -dimethylacrylamide)-co -(acrylic acid)]: A biodegradable synthetic graft copolymer. Polym. Int. 2015, 64, 1336–1351. [Google Scholar] [CrossRef]
- Jana, S.; Ray, J.; Mondal, B.; Pradhan, S.S.; Tripathy, T. pH responsive adsorption/desorption studies of organic dyes from their aqueous solutions by katira gum-cl-poly(acrylic acid-co-N-vinyl imidazole) hydrogel. Colloids Surfaces A: Physicochem. Eng. Asp. 2018, 553, 472–486. [Google Scholar] [CrossRef]
- Sasmal, D.; Maity, J.; Kolya, H.; Tripathy, T. Study of congo red dye removal from its aqueous solution using sulfated acrylamide and N, N- dimethyl acrylamide grafted amylopectin. J. Water Process. Eng. 2017, 18, 7–19. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Lu, L.; Pan, Z.; Hao, N.; Peng, W. A novel acrylamide-free flocculant and its application for sludge dewatering. Water Res. 2014, 57, 304–312. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Sadeghi, U.; Maleki, A.; Hayati, B.; Najafi, F. Synthesis of cationic polymeric adsorbent and dye removal isotherm, kinetic and thermodynamic. J. Ind. Eng. Chem. 2014, 20, 2745–2753. [Google Scholar] [CrossRef]
- Yue, Q.-Y.; Li, Q.; Gao, B.-Y.; Yuan, A.-J.; Wang, Y. Formation and characteristics of cationic-polymer/bentonite complexes as adsorbents for dyes. Appl. Clay Sci. 2007, 35, 268–275. [Google Scholar] [CrossRef]
- Dhodapkar, R.; Rao, N.N.; Pande, S.P.; Nandy, T.; Devotta, S. Adsorption of cationic dyes on Jalshakti®, super absorbent polymer and photocatalytic regeneration of the adsorbent. React. Funct. Polym. 2007, 67, 540–548. [Google Scholar] [CrossRef]
- Tekin, N.; Demirbaş, O.; Alkan, M. Adsorption of cationic polyacrylamide onto kaolinite. Microporous Mesoporous Mater. 2005, 85, 340–350. [Google Scholar] [CrossRef]
- Tanaka, H. Copolymerization of cationic monomers with acrylamide in an aqueous solution. J. Polym. Sci. Part A Polym. Chem. 1986, 24, 29–36. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, A.; Zhang, Y.; Li, M.; Li, K.; Zhao, Y.; Xing, W. Novel polyamidoamine dendrimer-functionalized palygorskite adsorbents with high adsorption capacity for Pb2+ and reactive dyes. Appl. Clay Sci. 2015, 107, 220–229. [Google Scholar] [CrossRef]
- Kislenko, V.N.; Oliynyk, L.P. Complex formation of polyethyleneimine with copper(II), nickel(II), and cobalt(II) ions. J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 914–922. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Ning, J.; Gao, M.; Jiang, W.; Zhou, Z.; Li, G. Preparation and characterization of a novel polyethyleneimine cation-modified persimmon tannin bioadsorbent for anionic dye adsorption. J. Environ. Manag. 2018, 217, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Briones, O.X.; Tapia, R.A.; Campodónico, P.R.; Urzúa, M.; Leiva, A.; Contreras, R.; González-Navarrete, J. Synthesis and characterization of poly (ionic liquid) derivatives of N-alkyl quaternized poly(4-vinylpyridine). React. Funct. Polym. 2018, 124, 64–71. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, X.; Zhang, X.; Huang, L.; Liu, Z.; Sun, G. Highly efficient removal of trace metal ions by using poly(acrylic acid) hydrogel adsorbent. Mater. Des. 2019, 181, 107934. [Google Scholar] [CrossRef]
- Rahchamani, J.; Mousavi, H.Z.; Behzad, M. Adsorption of methyl violet from aqueous solution by polyacrylamide as an adsorbent: Isotherm and kinetic studies. Desalination 2011, 267, 256–260. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.; Prabakaran, E.; Pillay, K. Carbohydrate biopolymers, lignin based adsorbents for removal of heavy metals (Cd2+, Pb2+, Zn2+) from wastewater, regeneration and reuse for spent adsorbents including latent fingerprint detection: A review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Pinjari, D.V.; Sonawane, S.H.; Bhanvase, B.A.; Sheikh, J.; Sillanpää, M. Cellulose-based nanomaterials for water and wastewater treatments: A review. J. Environ. Chem. Eng. 2021, 9, 106626. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Advances in the application of chitosan-based metal organic frameworks as adsorbents for environmental remediation. Carbohydr. Polym. 2022, 283, 119153. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.K.; Kabir, S.F.; Rahman, F.B.A.; Sakib, M.N.; Efty, S.S.; Rahman, M.M. Cu(II) removal from wastewater using chitosan-based adsorbents: A review. J. Environ. Chem. Eng. 2022, 10, 108048. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Yoon, D.H.; Joo, J.; Cheong, I.W. Graphene oxide-embedded chitosan/gelatin hydrogel particles for the adsorptions of multiple heavy metal ions. J. Mater. Sci. 2020, 55, 9354–9363. [Google Scholar] [CrossRef]
- Sheoran, K.; Kaur, H.; Siwal, S.S.; Saini, A.K.; Vo, D.-V.N.; Thakur, V.K. Recent advances of carbon-based nanomaterials (CBNMs) for wastewater treatment: Synthesis and application. Chemosphere 2022, 299, 134364. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Du, J.; Qi, F.; Donne, S.W.; Islam, M.M. Mesoporous Biopolymer Architecture Enhanced the Adsorption and Selectivity of Aqueous Heavy-Metal Ions. ACS Omega 2021, 6, 15316–15331. [Google Scholar] [CrossRef]
- Liu, S.; Wei, W.; Wu, S.; Zhang, F. Preparation of hierarchical porous activated carbons from different industrial lignin for highly efficient adsorption performance. J. Porous Mater. 2020, 27, 1523–1533. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, X.; Xiong, F.; Ding, J.; Qing, Y.; Wu, Y. Preparation of lignin-based porous carbon as an efficient absorbent for the removal of methylene blue. Ind. Crop. Prod. 2021, 171, 113980. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, J.; Kuang, Y.; Cheng, Z.; Wu, Q.; Xie, J.; Wang, B.; Gao, W.; Zeng, J.; Li, J.; et al. Lignin-derived sulfonated porous carbon from cornstalk for efficient and selective removal of cationic dyes. Ind. Crop. Prod. 2021, 159, 113071. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Sun, X.; Bai, L.; Han, C.; Zhang, P. The potential of biochar and lignin-based adsorbents for wastewater treatment: Comparison, mechanism, and application—A review. Ind. Crop. Prod. 2021, 166, 113473. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, L.; Niu, N. Enhanced adsorption for malachite green by functionalized lignin magnetic composites: Optimization, performance and adsorption mechanism. J. Mol. Struct. 2022, 1260, 132842. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, J.; Yu, W.; Kuang, Y.; Wang, B.; Ying, G.; Li, J.; Cheng, Z.; Li, J.; Chen, K. Simultaneous production of clean water and organic dye from dyeing wastewater by reusable lignin-derived porous carbon. Ind. Crop. Prod. 2022, 187, 115314. [Google Scholar] [CrossRef]
- Sasmal, D.; Maity, J.; Kolya, H.; Tripathy, T. Selective adsorption of Pb (II) ions by amylopectin-g-poly (acrylamide-co-acrylic acid): A bio-degradable graft copolymer. Int. J. Biol. Macromol. 2017, 97, 585–597. [Google Scholar] [CrossRef]
- Sasmal, D.; Kolya, H.; Tripathy, T. Amylopectin-g-poly(methylacrylate-co-sodium acrylate): An efficient Cd(II) binder. Int. J. Biol. Macromol. 2016, 91, 934–945. [Google Scholar] [CrossRef]
- Chai, K.; Lu, K.; Xu, Z.; Tong, Z.; Ji, H. Rapid and selective recovery of acetophenone from petrochemical effluents by crosslinked starch polymer. J. Hazard. Mater. 2018, 348, 20–28. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Synthesis of Guar gum/bentonite a novel bionanocomposite: Isotherms, kinetics and thermodynamic studies for the removal of Pb (II) and crystal violet dye. J. Mol. Liq. 2018, 249, 805–814. [Google Scholar] [CrossRef]
- Zhou, L.S.; Zhou, H.; Yang, X. Preparation and performance of a novel starch-based inorganic/organic composite coagulant for textile wastewater treatment. Sep. Purif. Technol. 2019, 210, 93–99. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, L.; Yang, X. Optimization of preparing a high yield and high cationic degree starch graft copolymer as environmentally friendly flocculant: Through response surface methodology. Int. J. Biol. Macromol. 2018, 118, 1431–1437. [Google Scholar] [CrossRef]
- Bahrami, M.; Amiri, M.J.; Bagheri, F. Optimization of the lead removal from aqueous solution using two starch based adsorbents: Design of experiments using response surface methodology (RSM). J. Environ. Chem. Eng. 2018, 7, 102793. [Google Scholar] [CrossRef]
- Abdel-Halim, E. Preparation of starch/poly(N,N-Diethylaminoethyl methacrylate) hydrogel and its use in dye removal from aqueous solutions. React. Funct. Polym. 2013, 73, 1531–1536. [Google Scholar] [CrossRef]
- Wei, H.; Ren, J.; Li, A.; Yang, H. Sludge dewaterability of a starch-based flocculant and its combined usage with ferric chloride. Chem. Eng. J. 2018, 349, 737–747. [Google Scholar] [CrossRef]
- Mahmoodi-Babolan, N.; Nematollahzadeh, A.; Heydari, A.; Merikhy, A. Bioinspired catecholamine/starch composites as superadsorbent for the environmental remediation. Int. J. Biol. Macromol. 2019, 125, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Peng, S.; Li, X.; Yang, L.; Soares, J.B.P.; Li, G. Preparation of amphoteric starch-based flocculants by reactive extrusion for removing useless solids from water-based drilling fluids. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 558, 343–350. [Google Scholar] [CrossRef]
- Ibrahim, B.; Fakhre, N. Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater. Int. J. Biol. Macromol. 2019, 123, 70–80. [Google Scholar] [CrossRef]
- Kolya, H.; Roy, A.; Tripathy, T. Starch-g-Poly-(N, N-dimethyl acrylamide-co-acrylic acid): An efficient Cr (VI) ion binder. Int. J. Biol. Macromol. 2015, 72, 560–568. [Google Scholar] [CrossRef]
- Kolya, H.; Das, S.; Tripathy, T. Synthesis of Starch-g-Poly-(N-methylacrylamide-co-acrylic acid) and its application for the removal of mercury (II) from aqueous solution by adsorption. Eur. Polym. J. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Mondal, S.K.; Wu, C.; Nwadire, F.C.; Rownaghi, A.; Kumar, A.; Adewuyi, Y.; Okoronkwo, M.U. Examining the Effect of a Chitosan Biopolymer on Alkali-Activated Inorganic Material for Aqueous Pb(II) and Zn(II) Sorption. Langmuir 2022, 38, 903–913. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Chen, D.-Q. An investigation of adsorption of lead(II) and copper(II) ions by water-insoluble starch graft copolymers. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 205, 231–236. [Google Scholar] [CrossRef]
- Xun, J.; Lou, T.; Xing, J.; Zhang, W.; Xu, Q.; Peng, J.; Wang, X. Synthesis of a starch-acrylic acid-chitosan copolymer as flocculant for dye removal. J. Appl. Polym. Sci. 2019, 136, 47437. [Google Scholar] [CrossRef]
- Ying, C.; Gongwei, T.; Yuning, L.; Qi, Z.; Jing, L.; Ji, L. Ammonium persulfate-initiated polymerization of cationic starch-grafted-cationic polyacrylamide flocculant for the enhanced flocculation of oil sludge suspension. J. Dispers. Sci. Technol. 2018, 40, 1246–1255. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S.; Ahmari, A. Dextran-graft-poly(hydroxyethyl methacrylate) biosorbents for removal of dyes and metal cations. Mater. Res. Express 2019, 6, 055324. [Google Scholar] [CrossRef]
- Wang, X.-M.; Zhang, C.-N.; Dai, W.-Y.; Xu, Q. Study on the adsorption of crystal violet by starch-g-poly (acrylic acid). In Materials Engineering and Environmental Science; World Scientific: Singapore, 2015; pp. 21–29. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Zheng, G. Synthesis of cross-linking cationic starch and its adsorption properties for reactive dyes. IOP Conf. Series: Earth Environ. Sci. 2019, 227, 062034. [Google Scholar] [CrossRef]
- Lou, T.; Wang, X.; Song, G.; Cui, G. Synthesis and flocculation performance of a chitosan-acrylamide-fulvic acid ternary copolymer. Carbohydr. Polym. 2017, 170, 182–189. [Google Scholar] [CrossRef]
- Fan, X.-M.; Yu, H.-Y.; Wang, D.-C.; Mao, Z.-H.; Yao, J.; Tam, K.M.C. Facile and Green Synthesis of Carboxylated Cellulose Nanocrystals as Efficient Adsorbents in Wastewater Treatments. ACS Sustain. Chem. Eng. 2019, 7, 18067–18075. [Google Scholar] [CrossRef]
- Hussain, G.; Haydar, S. Textile Effluent Treatment Using Natural Coagulant Opuntia strictain Comparison with Alum. CLEAN –Soil, Air, Water 2021, 49, 2000342. [Google Scholar] [CrossRef]
- Du, J.; Yang, X.; Xiong, H.; Dong, Z.; Wang, Z.; Chen, Z.; Zhao, L. Ultrahigh Adsorption Capacity of Acrylic Acid-Grafted Xanthan Gum Hydrogels for Rhodamine B from Aqueous Solution. J. Chem. Eng. Data 2021, 66, 1264–1272. [Google Scholar] [CrossRef]
- Elbedwehy, A.M.; Atta, A.M. Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal. Polymers 2020, 12, 338. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Huang, J.; He, B.; Zhang, F.; Li, H. Peach gum for efficient removal of methylene blue and methyl violet dyes from aqueous solution. Carbohydr. Polym. 2014, 101, 574–581. [Google Scholar] [CrossRef]
- Jeon, C.; Höll, W.H. Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res. 2003, 37, 4770–4780. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Han, Y.; Zhou, Z. Biosorption of Pb2+ and Zn2+ by Ca-alginate immobilized and free extracellular polysaccharides produced by Leuconostoc citreum B-2. Int. J. Biol. Macromol. 2021, 193, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Atassi, Y.; Tally, M. Synthesis and swelling behavior of metal-chelating superabsorbent hydrogels based on sodium alginate-g-poly(AMPS-co-AA-co-AM) obtained under microwave irradiation. Polym. Bull. 2017, 74, 4453–4481. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Preparation of poly(acrylic acid)/starch hydrogel and its application for cadmium ion removal from aqueous solutions. React. Funct. Polym. 2014, 75, 1–8. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Staelens, J.-N.; LoIacono, S.; Martel, B.; Chanet, G.; Crini, G. Simultaneous removal of Cd, Co, Cu, Mn, Ni, and Zn from synthetic solutions on a hemp-based felt. III. Real discharge waters. J. Appl. Polym. Sci. 2020, 137, 48823. [Google Scholar] [CrossRef]
- Moradi, M.; Sabzevari, M.H.; Marahel, F.; Shameli, A. Response surface methodology for the high efficiency removal of lead and zinc from effluents using natural sepiolite particles on the corn silk. Int. J. Environ. Anal. Chem. 2022, 1–18. [Google Scholar] [CrossRef]
- Li, R.; Zhang, T.; Zhong, H.; Song, W.; Zhou, Y.; Yin, X. Bioadsorbents from algae residues for heavy metal ions adsorption: Chemical modification, adsorption behaviour and mechanism. Environ. Technol. 2021, 42, 3132–3143. [Google Scholar] [CrossRef]
- Kumar, A.; Patra, C.; Rajendran, H.K.; Narayanasamy, S. Activated carbon-chitosan based adsorbent for the efficient removal of the emerging contaminant diclofenac: Synthesis, characterization and phytotoxicity studies. Chemosphere 2022, 307, 135806. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Liu, K.; Gibril, M.E.; Kong, F.; Wang, S. Lignin-based superhydrophobic melamine resin sponges and their application in oil/water separation. Ind. Crop. Prod. 2021, 170, 113798. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, J.; Wang, B.; Xie, J.; Ying, G.; Li, J.; Cheng, Z.; Li, J.; Chen, K. Highly efficient and rapid purification of organic dye wastewater using lignin-derived hierarchical porous carbon. J. Colloid Interface Sci. 2022, 625, 158–168. [Google Scholar] [CrossRef]
- Salim, M.H.; Kassab, Z.; Ablouh, E.-H.; Sehaqui, H.; Aboulkas, A.; Bouhfid, R.; Qaiss, A.E.K.; El Achaby, M. Manufacturing of macroporous cellulose monolith from green macroalgae and its application for wastewater treatment. Int. J. Biol. Macromol. 2022, 200, 182–192. [Google Scholar] [CrossRef]
- He, Y.; Dietrich, A.M.; Jin, Q.; Lin, T.; Yu, D.; Huang, H. Cellulose adsorbent produced from the processing waste of brewer’s spent grain for efficient removal of Mn and Pb from contaminated water. Food Bioprod. Process. 2022, 135, 227–237. [Google Scholar] [CrossRef]
- Si, R.; Chen, Y.; Wang, D.; Yu, D.; Ding, Q.; Li, R.; Wu, C. Nanoarchitectonics for High Adsorption Capacity Carboxymethyl Cellulose Nanofibrils-Based Adsorbents for Efficient Cu2+ Removal. Nanomaterials 2022, 12, 160. [Google Scholar] [CrossRef]
- Bisiriyu, I.O.; Meijboom, R. Adsorption of Cu(II) ions from aqueous solution using pyridine-2,6-dicarboxylic acid crosslinked chitosan as a green biopolymer adsorbent. Int. J. Biol. Macromol. 2020, 165, 2484–2493. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total. Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Omorogie, M.O.; Babalola, J.O.; Unuabonah, E.I. Regeneration strategies for spent solid matrices used in adsorption of organic pollutants from surface water: A critical review. Desalination Water Treat. 2014, 57, 518–544. [Google Scholar] [CrossRef]
- Arabkhani, P.; Javadian, H.; Asfaram, A.; Hosseini, S.N. A reusable mesoporous adsorbent for efficient treatment of hazardous triphenylmethane dye wastewater: RSM-CCD optimization and rapid microwave-assisted regeneration. Sci. Rep. 2021, 11, 2275. [Google Scholar] [CrossRef]
- McQuillan, R.V.; Stevens, G.W.; Mumford, K.A. The electrochemical regeneration of granular activated carbons: A review. J. Hazard. Mater. 2018, 355, 34–49. [Google Scholar] [CrossRef]
- Gautam, R.; Bassi, A.S.; Yanful, E.K. A review of biodegradation of synthetic plastic and foams. Appl. Biochem. Biotechnol. 2007, 141, 85–108. [Google Scholar] [CrossRef]
- Kyrikou, I.; Briassoulis, D. Biodegradation of Agricultural Plastic Films: A Critical Review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Biodegradable flocculants based on polyacrylamide and poly(N,N-dimethylacrylamide) grafted amylopectin. Int. J. Biol. Macromol. 2014, 70, 26–36. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ramin, S. Fabrication of starch-graft-poly(acrylamide)/graphene oxide/hydroxyapatite nanocomposite hydrogel adsorbent for removal of malachite green dye from aqueous solution. Int. J. Biol. Macromol. 2018, 106, 101–115. [Google Scholar] [CrossRef] [PubMed]






| Water Purification Method | Meaning | Advantages | Limitations |
|---|---|---|---|
| Boiling water | Boiled water in a clean and covered pot. |
|
|
| Sedimentation | Heavy particles settle down at the bottom. |
|
|
| Decantation | Separation of immiscible liquid–liquid and liquid–solid mixtures. |
|
|
| Chlorination | Mixing chlorine in the water. |
|
|
| Filtration | Contaminated water passes through the sands and activated carbon cartridge. |
|
|
| Coagulation | Inorganic chemicals are applied to remove suspended particles. For electro coagulation, there is no need for chemicals. |
|
|
| Flocculation | Adding organic chemicals or polymers to separate colloidal particles or suspended particles. |
|
|
| Adsorption | Adding adsorbents to the contaminated water and attaching contaminates to the surface of the adsorbent. |
|
|
| Membrane filtration | Physically separates contaminates using pressure difference between the two sides of the membrane. Ultrafiltration, nanofiltration, and reverse osmosis are different membrane filtration processes. |
|
|
| Froth floatation | Selectively separates hydrophobic contaminates from hydrophilic contaminates. |
|
|
| Advanced oxidation techniques | Separation of organic and inorganic contaminates from water using •OH radicals. |
|
|
| Solvent extraction | Separation of aromatic compounds from water, transforming solubility from one solvent to another solvent. |
|
|
| Ion exchange | Ionic pollutants separation by exchange with other ions passing through resin matrix. |
|
|
| Anaerobic treatment | Transform organic matters to biogas in the absence of oxygen. |
|
|
| Microbial treatment | Removing pollutants from water using bacteria. |
|
|
| Acoustic cavitation | Separation of contaminates using high-power ultrasounds. |
|
|
| Electrolysis | A sacrificial metal anode and cathode generate electrically active coagulants and tiny bubbles of hydrogen and oxygen in the water. |
|
|
| Catalytic process | Adding catalyst improved the rate of removal of contaminates’ selectively. It includes a catalytic oxidation and reduction process. |
|
|
| Activated sludge treatment | Used microorganisms to treat wastewater. |
|
|
| Disinfection | It is the final step of the water treatment. Adding disinfectant chemicals, ultraviolet radiation treatment, or ultrafiltration |
|
|
| Sl. No. | Commercial Polymeric Flocculants | Charge | Type of Effluent Used | Optimum Results (TSS %) | Ref. |
|---|---|---|---|---|---|
| 1. | Polyacrylamide | Anionic | Slaughterhouse | 94.0 | [75] |
| 2. | Polyacrylamide | Non-ionic | Beverage and food units | 97.0 | [76] |
| 3. | Organopol 5415 | Cationic | Paper mills | 99.5 | [77] |
| 4. | Chemfloc 430A | Anionic | Pulp industries | 99.5 | [77] |
| 5. | KP 1200B | Cationic | Ceramic (Boron) | 80.0 | [78] |
| 6. | AP 825C | Anionic | Ceramic plants (Boron) | 40.0 | [78] |
| 7. | Actipol A-401 | Anionic | Sauce manufacturing units | 72.0 | [79] |
| 8. | Magnafloc 155 | Anionic | Polymer effluents | 91.0 | [80] |
| 9. | Polyacrylamide | Amphoteric | Paper mills | 95.0 | [81] |
| 10. | Flocan 23 | Anionic | Olive mills | ~100 | [82] |
| 11. | Magnafloc LT 7991 Magnafloc LT 7992 Magnafloc LT 7995 Magnafloc LT 22S Magnafloc E 38 | Cationic | Aquaculture | 89.0 84.0 84.0 91.0 45.0 | [83] |
| 12. | Hyperfloc CE 854 Hyperfloc CE 834 Hyperfloc CE 1950 | Cationic | Aquaculture | 98.0 87.0 94.0 | [83] |
| 13. | Organopol 5415 | Anionic | Pulp and paper mills | 97.0 | [84] |
| 14. | Polydiallyldimethylammonium chloride | Cationic | Textile dye | 90.0 | [85] |
| 15. | Polyamine | Cationic | Dye effluents | 96.0 | [86] |
| 16. | Polydiallyldimethylammonium chloride | Cationic | Wastewater of paper mills | ~100 | [87] |
| 17. | Poly etheleneoxide | Non-ionic | Tale, graphite, chalcopyrite, and covellite dispersions | 98.0, 99.5, 82.0 and 93.0 | [88] |
| Name | Properties | Chemical Structures | Ref. |
|---|---|---|---|
| Starch | Sources: seeds and stem crops, wheat, potato, corn, sago, sweet potato, arrowroot, cassava, and rice. Chain of D-glucose-α-glycosidic linkages mixtures with amylose [(1-4)-α-D-glucan)] and amylopectin [(1-4)-α-D-glucan and (1-6)-α-D linkages]. |  | [90] |
| Hydroxyethyl starch | It is chemically modified starch and dextrin. Primarily used in the medical specialty pharmaceutical field and industrial effluent treatment. |  | [90] |
| Dextrin and cyclodextrin | Potato, corn, wheat, rice, and tapioca all contain cyclic oligosaccharides. |  | [91] |
| Guar gum | Plant’s polysaccharide; linear chain of mannose and galactose. |  | [92] |
| Xanthan gum | Natural polysaccharides: Chain β-(1→4)-linked- d-glucopyranose glucan and side chain β-(1→3)-α-linked d-mannopyranose-(1→2)-β- d-glucuronic acid-(1→4) β-d- mannopyranose. |  | [93] |
| Locust bean gum | Source is the seeds of carob trees: linkage of (1-4)-β-D mannose and the side chain of (1-6)-α-D galactose. |  | [94] |
| Chitosan | Source is the shells of shrimps, fish scales, crabs, and lobsters. Linkage of β-1,4-D-glucosamine. |  | [95] |
| Cellulose | Main source of cellulose is wood, cotton, sugarcane bagasse, grass, and banana peel. Covalently linked β-D-anhydroglucopyranose units. |  | [96] |
| Sl. No. | Commercial Polymeric Adsorbents | Charge | Type of Effluent Used | Maximum Adsorption Capacity (mg/g)/Removal (%) | Ref. |
|---|---|---|---|---|---|
| 1. | Poly(quaternary ammonium salt) | Cationic | Acid Blue 25 and Acid Red 18 dye removal from single and binary systems | 2000 and 1667 | [144] |
| 2. | Epicholorohydrin-dimethylamine polyamine | Cationic | Bentonite clay | 94.9% at 323 K | [145] |
| 3. | Jalshakti® | Anionic | Cationic dye such as Safranine T, Methylene Blue, Malachite Green, Brilliant Green, Rhodamine B, Crystal Violet, and Basic Fuchsine | 181.8, 172.4, 34.2, 17.6, 15.4, 12.9, and 11.7 | [146] |
| 4. | Polyacrylamide | Cationic | Kaolinite | 46.9% at 298 K | [147] |
| 5. | 2-acrylamide-2-methylpropanedimethyl ammonium chloride | Cationic | Activated sludge | Not available | [148] |
| 6. | Polyamidoamine | Cationic | Pb (II) ions | 694.4 at 293 K | [149] |
| 7. | Poly (ethyleneimine) | Cationic | Cu (II), Ni (II) and Co (II) ions | Not available | [150] |
| 8. | Poly (ethyleneimine) | Cationic | Methyl orange | 275.74 at 323 K | [151] |
| 9. | Poly (4-vinyl pyridine) | Cationic | Cr (VI) ions | 72.2% at 298 K | [152] |
| 10. | Poly acryl acid | Anionic | Cu (II) removal | 303 at 298 K | [153] |
| 11. | Polyacrylamide | Non-ionic | Methylviolet | 1136 at 298 K | [154] |
| Sl. No. | Adsorbent | Pollutant | Results % or Qmax mg/g | Ref. |
|---|---|---|---|---|
| 1. | AP-g-poly(DMA-co-AA) | Cu (II), Ni (II), and Zn (II) | 11.23 mg/g, 8.02 mg/g, 7.77 mg/g | [139] |
| 2. | AP-g-poly(AM-co-AA) | Pb (II) | 58.8 mg/g | [169] |
| 3. | AP-g-Poly(NMA-co-AA) | MG | 99.1% | [101] |
| 4. | AP-g-poly(MA-co-sodium acrylate) | Cd(II) | 5.742 mg/g | [170] |
| 5. | AP-g-AM (sulphated) AP-g-PDMA (sulphated) AP (sulphated) | CR CR CR | 66.97 mg/g 51.41 mg/g 37.18 mg/g | [141] |
| 6. | AP-g-poly (AM-co-ATMAC) | MB Kaolin | 97.74%, pH 7 5.4 NTU | [104] |
| 7. | Cross-linked starch polymer | Acetophenone 1-phenylethanol | 1.29 mg/g 0.70 mg/g | [171] |
| 8. | Guar gum/bentonite | Pb Crystal Violet | 72.5 mg/g 48.40 mg/g | [172] |
| 9. | HES-g-Poly (DMA-co-AA) | MG | 93.53%, pH 5.5 | [120] |
| 10. | Polyaluminum-FeCl3-St-g-poly (AM-co-Dimethyl diallyl ammonium chloride) | Dye blue KN-R Green KE-4B Textile wastewater | 89.7%, ~100% | [173] |
| 11. | St-g-poly(AM-co-dimethyl diallyl ammonium chloride) | Disperse Yellow E-3G Dye blue KN-R Reactive Green KE-4B Disperse blue 2BLN | 96.0% 89.8% 96.0% 96.0% | [174] |
| 12. | St-phosphate | Pb (II) | 99.99%, pH 5.5 | [175] |
| 13. | St-g-poly(N,N-Diethylaminoethyl methacrylate) hydrogel | Direct Red 81 | 95.65%, pH 1.0 | [176] |
| 14. | St-g-3-chloro-2-hydroxypropyl trimethylammonium chloride | Sludge dewatering S-EPS LB-EPS TB-EPS | 76.40% 50.90% 48.30% | [177] |
| 15. | FeCl3-St-3-chloro-2-hydroxypropyl trimethylammonium chloride | Sludge dewatering of extracellular polymeric substances (EPS) Soluble EPS Loosely bound EPS Tightly bound EPS | 93.5% 55.4% 53.9% | [177] |
| 16. | St-g-poly(AM-co-AA) | MB | 2276 mg/g | [178] |
| 17. | St-g-DADMAC | Kaolinite and minerals from drilling fluids | 98% | [179] |
| 18. | Cross-linked dibenzo-18-crown-6-St | Cd (II) Zn (II) Ni (II) Cu (II) | 45 mg/g 42 mg/g 50 mg/g 84 mg/g | [180] |
| 19. | St-g-poly(DMA-co-AA) | Cr (VI) | 6.70 mg/g | [181] |
| 20. | St-g-poly(NMA-co-AA) | Hg (II) | 11.0 mg/g | [182] |
| 21. | Chitosan on alkali-activated inorganic material | Zn (II) and Cu (II) | 98–100% | [183] |
| 22. | St-g-poly(2-methacryloyloxyethyl trimethyl ammonium chloride) | Kaolin Escherichia coli suspensions Kaolin and Escherichia coli suspensions | 98.7%, pH 11.0 95.5%, pH 11.0 98.7%, pH 11.0 | [109] |
| 23. | St-g-dimethylaminoethyl methacrylate | Cu (II) Pb (II) | 2.12 mg/g 2.09 mg/g | [184] |
| 24. | St-g-poly(AM-co-sodium xanthate) | Kaolin and CuSO4 | ~100%, pH 5.0 | [110] |
| 25. | St-g-(AA–chitosan) | Acid Blue 113 | 99.7% pH 4–10.0 | [185] |
| 26. | St-g-poly (AM-co- DMDAAC) | Oil―sludge suspension | 93.6% transmittance, pH 6.0 | [186] |
| 27. | Dxt-g-poly(hydroxyethyl methacrylate) | Methylene Blue Red rose Bengal Fe (III) Cu (II) | 91.6% 88.6% 92.4% 81.9% | [187] |
| 28. | St-g-poly(AM-co-AA) | Kaolin suspensions | ~100% transparent | [111] |
| 29. | St-g-PAA | Crystal Violet | 0.8 mg/g, pH 12.0 | [188] |
| 30. | St-g- 3-chloro-2-hydroxypropyltrimethyl ammonium chloride | Red 195 Golden yellow SNE | 101.73 mg/g 120.14 mg/g | [189] |
| 31. | Chitosan-g-AM-fulvic acid | Acid blue 113 MO Reactive Black 5 | 97.0% 91.6% 38.2% | [190] |
| 32. | Carboxylated cellulose nanocrystals | MB Cu (II) | 95.6% 82.3% | [191] |
| 33. | Opuntia stricta | TSS Color | 80% pH 10.6 77.8%, pH 10.6 | [192] |
| 34. | AA-g- xanthan gum hydrogel | Rhodamine B | 2777.77 mg/g, 323 K | [193] |
| 35. | Arabic gum-g-PAA/PAM | MB | 2300 mg/g, pH 7.0 | [194] |
| 36. | Peach gum | MB and MV | 98%, pH 6–10 | [195] |
| 37. | Modified chitosan beads | Hg (II) | 2.3 mmol/g, pH 7 | [196] |
| 38. | Leuconostoc citreum B-2 extracellular polysaccharides | Pb (II) Zn (II) | 269.54 mg/g, pH 5.5, 298 K 49.88 mg/g, pH 5.5, 318 K | [197] |
| 39. | Alginate-hydrogel | Pb (II) Cd (II) Ni (II) Cu (II) | 534.25 mg/g 258.6 mg/g 187.0 mg/g 224.5 mg/g | [198] |
| 40. | Polyacrylic acid/starch graft copolymer | Cd (II) | 588 mg/g | [199] |
| 41. | Modified HEMP | Cd (II), Co (III), Ni (II), Cu (II), Mn (II), and Zn (II) | 80–100% | [200] |
| 42. | Corn silk/sepiolite | Pb (II) Zn (II) | 93.13%, pH 5.5 89.04%, pH 5.5 | [201] |
| 43. | Algae residue CCLR | Cu (II), Pb (II), Cd (II), and Mn (II) | 78.1, 108.8, 87.3, 57.8 mg/g | [202] |
| 44. | Activated carbon–chitosan beads | Diclofenac | 99.29 mg/g | [203] |
| 45. | Lignin-based superhydrophobic melamine resin sponges | Oil/water | 98.6% | [204] |
| 46. | Lignin-derived hierarchical porous carbon | Organic dye | 1980.63 mg/g | [205] |
| 47. | Macroporous cellulose | Methylene Blue | 454 mg/g | [206] |
| 48. | Oxidized cellulose | Pb (II) and Mn (II) | 272.5 and 52.9 mg/g | [207] |
| 49. | Carboxymethyl Cellulose Nanofibrils | Cu (II) | 380.03 ± 23 mg/g | [208] |
| 50 | Pyridine-2,6-dicarboxylic acid cross-linked chitosan | Cu (II) | 2186 mg/g, pH 7.5 | [209] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolya, H.; Kang, C.-W. Bio-Based Polymeric Flocculants and Adsorbents for Wastewater Treatment. Sustainability 2023, 15, 9844. https://doi.org/10.3390/su15129844
Kolya H, Kang C-W. Bio-Based Polymeric Flocculants and Adsorbents for Wastewater Treatment. Sustainability. 2023; 15(12):9844. https://doi.org/10.3390/su15129844
Chicago/Turabian StyleKolya, Haradhan, and Chun-Won Kang. 2023. "Bio-Based Polymeric Flocculants and Adsorbents for Wastewater Treatment" Sustainability 15, no. 12: 9844. https://doi.org/10.3390/su15129844