Soil–Plant–Microbe Interactions Determine Soil Biological Fertility by Altering Rhizospheric Nutrient Cycling and Biocrust Formation
Abstract
:1. Introduction
- How does the rhizosphere foster plant growth, development, and nutrient cycling through soil microorganisms (e.g., bacteria, fungi)?
- How can soil microorganisms regulate macronutrient cycling and facilitate biocrust formation?
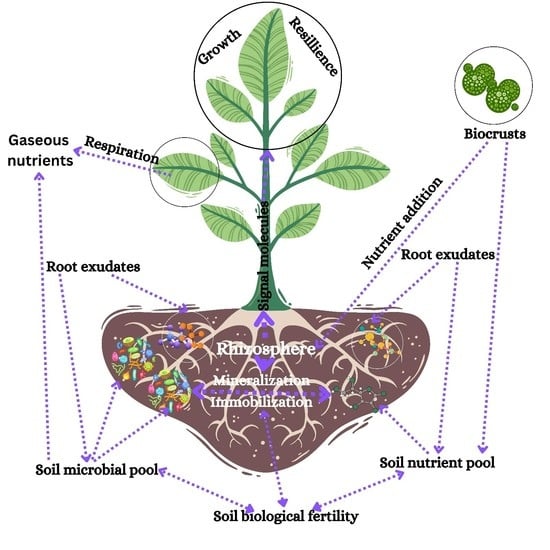
2. Rhizosphere: The Substrate for Soil–Plant–Microbial Interactions
2.1. Root Exudates
2.2. Rhizobacteria
- (1)
- Increasing the interaction between available nutrients in the soil and the plants;
- (2)
- Preventing pathogen growth/activity to protect plants;
- (3)
- Directly stimulating plant growth, e.g., by producing phytohormones.
2.3. Fungi: Special Emphasis on Arbuscular Mycorrhizal Fungi
2.4. Nematodes, Rhizospheric Microorgansims and Their Inter- and Intra-Interactions
3. Nutrient Cycling and Soil Microbial Community under Different Agronomic Managements
3.1. Carbon and Nitrogen Cycling
3.2. Phosphorus and Potassium Cycling
3.2.1. Phosphorus Cycling
3.2.2. K Cycling
4. Biological Soil Crusts (BSCs): An In-Depth Overview of Classification and Types
- (1)
- Cyanobacteria-dominated crust: smooth, composed of filamentous cyanobacteria, 1.5 mm thick bio-rich zone;
- (2)
- Short moss–lichen crust: ≥50% mosses-lichen cover, some cyanobacteria, 11 mm thick bio-rich zone;
- (3)
- Tall moss–lichen pinnacle crust: ≥50% mosses-lichen cover, some cyanobacteria, 22 mm thick bio-rich zone.
- (1)
- Physical crusts (rain/desiccation)—bacteria, fungi and radicle thrive before cyanobacteria and algae;
- (2)
- Cyanobacterial/algal crusts—dominated by cyanobacteria (e.g., Microcoleus and Scytonema sp.);
- (3)
- Lichen crust—composed of lichens, but also various saprotrophic fungi;
- (4)
- Bryophyte crusts—dominated by bryophytes (e.g., Microcoleus sp.), developing in the presence of large amounts of organic matter deposited by wind and precipitation;
- (5)
- Mixed biotic crusts—a complex structure of communities: bryophytes, lichens, algae, cyanobacteria, and associated decomposing microorganisms (humicolous, lignicolous fungi).
4.1. Role of Soil Bio-Crusts in Physicochemical Properties of Soils and Nutrient Cycling
- BSCs can alter the physicochemical properties of soil [190,202,203] (Figure 2). Primarily, they stabilize the soil surface and protect against erosive forces [191]. Seitz et al. [204] showed that bryophyte-dominated BSCs strongly reduce soil erosion in forest environments (southeastern China), being more effective than abiotic soil surface cover. Additionally, Bowker et al. [193] showed that extracellular polysaccharides from cyanobacteria increase soil stability and reduce soil erosion. BSCs can affect water availability, stability, and soil fertility in semi-arid areas [205,206]. More silt or clay is typically present in crust-covered soils, which improves plant macronutrient assimilation and increases the soil’s fertility [190]. BSCs under dry (desert) conditions can facilitate the accumulation, morphology, and ecosystem services of silt and increase water availability (bind surface water) [192,205]. Miralles-Mellado, Cantón, and Solé-Benet [207] showed that BSCs also prevent soil desertification by influencing the detachment and transport of soil particles. Studies in cool deserts have shown that BSCs can increase the nutrient content (i.e., N, K, Ca, Mg, P, Fe, Mn) of soil [208], and modify soil pH [209].
- BSCs from arid ecosystems affect N cycling through nitrification and N fixation [211]. Among the organisms that form BSCs are cyanobacteria and cyanolichens (e.g., Collema, Microcoleus, Nostoc), which fix nitrogen that is then released into the soil environment. Up to 70% of the nitrogen bound by these microorganisms ends up in the soil. Studies have shown that the presence of BSCs significantly increases soil N content [208,212]. Elbert et al. [212] estimate nitrogen uptake by cryptogamic covers at about 49 Tg per year, suggesting that nitrogen fixation by covers may be crucial for carbon sequestration by plants. In addition, the polysaccharide and total carbon content of soil increases due to the presence of BSCs [213,214]. This is because crust-forming organisms secrete extracellular carbon within a short time of its acquisition. In the case of cyanobacteria, this can be as high as 50% of the acquired carbon. An increase in soil carbon is associated with an increase in heterotrophic microorganisms, and a faster rate of decomposition. Globally, cryptogamic communities—which form crusts—are estimated to take up about 3.9 Pg of carbon per year, equivalent to about 7% of net primary production by terrestrial vegetation [212].
- Studies have shown that communities of organisms in BSCs play a key role in the biogeochemical cycling of P, especially by converting stable P into labile, readily available P [215,216]. Beraldi-Campesi et al. [208] showed that bio-crusts have higher total P concentrations than adjacent soils. Baumann et al. [217] showed that especially P-containing mineral concentrations decreased, and organic P concentrations increased in BSCs compared to volumetric soil in a temperate forest in Germany. Cyanobacteria (e.g., Anabaea, Anacystis, Lyngbya, Nostoc), but also fungi, lichens and some bacteria can chelate metals [218,219]. These increases availability of Cu, Zn, Ni, Fe etc. to plants [208]. Chelation of metals by microorganisms is particularly important in soils with a pH higher than 7 (e.g., deserts), where some elements form insoluble oxides/hydroxides [220]. In addition, BSCs can take up significant amounts of available metallic nutrients (Cu, Fe, K, Mg, Mn, Na, and Zn) and play an important role in conserving and protecting these nutrients in dry soils from leaching and erosion [221].
- Apart from these, BSCs has the potential to impact plant growth and development. For example, BSCs in a temperate pine barren ecosystem impacted germination and other early stages of the development of plant species including Lespedeza capitata, Lupinus perennis [225]. The development of BSCs also increases the survival of some vascular plant species [226], leading to greater ecosystem diversity. In postglacial areas, BSCs have been shown to colonize the soil first and successionally facilitate piedmont [227]. At the same time, Song, Li and Hui [228] suggest that BSCs can act as natural regulators of vegetation patterns and thus promote the stability and sustainability of ecosystems. A high cover of BSCs can also protect soils from the negative consequences of climate change as evidenced in a dry ecosystem of Spain [229].
4.2. Establishing BSCs on Agricultural Land Warrants More Research
5. Conclusions and Perspectives
- The interaction between soil, microorganisms, and plants makes the rhizosphere an ideal space for microorganism growth. This is mostly related to the quantity and type of root secretions and their predominant components that attract microorganisms and influence nutrient mobility.
- Rhizospheric microorganisms exhibit a wide array of positive effects through atmospheric nitrogen fixation, decomposition of organic matter, resilience against plant pathogens, immobilization/mineralization of nutrients, production of phytohormones (ABA, IAA, et al.), osmoprotectants, exopolysaccharides, etc., and by reducing the harmful chemical footprint (e.g., salinity) in the environment.
- The introduction of PGPR in agricultural soils may reduce the chemical fertilizer-associated costs of farmers because of their high efficiency in nutrient cycling. Additionally, they can make crop plants more resilient to biotic/abiotic stresses, thus documenting their responses under elevated CO2 conditions can facilitate future research on climate change mitigation.
- A quantitative estimation of the composition of root exudates is necessary since it maintains synergistic/antagonistic associations among plant, soil, and soil-dwelling microorganisms.
- The spatiotemporal dynamics of KSB distribution and their role in K cycling in soils should be included in future research.
- Knowledge about the diversity and functionality of the organisms forming BSCs is diverse, and although this topic has been of interest to researchers for a long time, still not all the relationships occurring in BSCs are known. There is still a lack of information on the biological conservation of BSCs species, the possibilities for soil remediation with BSCs. and the impact of climate change on the structure and function of bio-crusts. It is worth noting the lack of broad studies from other than desert/semi-desert/arid areas and the problem of biodiversity assessment due to the use of different methods by researchers. Future research should be based on accurate, molecular methods using DNA analysis. It is estimated that crusts occupy about 12% of the terrestrial land surface, so researchers should try to analyze BSCs from more ecosystems, to assess whether they also affect the soil environment in systems other than forests and deserts.
- Research on the rhizosphere tends to focus on bacteria and fungi, while other organisms are often neglected. Despite the nature of the threat posed by nematodes to plant crops, the topic of their interactions with microorganisms and plants is not fully understood. The network of these interactions is very complex and requires in-depth analyses. Understanding these interactions could make it possible to control nematodes and thus help protect plants against parasites.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Intensive Agriculture and the Soil Carbon Pool. J. Crop Improv. 2013, 27, 735–751. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Q.; Wang, C.; Sardans, J.; Vancov, T.; Fang, Y.; Wu, L.; Huang, X.; Gargallo-Garriga, A.; Peñuelas, J.; Wang, W. Effect of soil degradation on the carbon concentration and retention of nitrogen and phosphorus across Chinese rice paddy fields. CATENA 2022, 209, 105810. [Google Scholar] [CrossRef]
- Hunke, P.; Mueller, E.N.; Schröder, B.; Zeilhofer, P. The Brazilian Cerrado: Assessment of water and soil degradation in catchments under intensive agricultural use. Ecohydrology 2015, 8, 1154–1180. [Google Scholar] [CrossRef]
- Pylro, V.S.; Fulthorpe, R.; Roesch, L.F.W. Editorial: Microbe-Mediated Processes in Soils. Front. Environ. Sci. 2020, 8, 124. [Google Scholar] [CrossRef]
- Saccá, M.L.; Barra Caracciolo, A.; Di Lenola, M.; Grenni, P. Ecosystem services provided by soil microorganisms. In Soil Biological Communities and Ecosystem Resilience; Springer International Publishing: Cham, Switzerland, 2017; pp. 9–24. [Google Scholar]
- Abbott, L.K.; Murphy, D.V. Soil Biological Fertility: A Key to Sustainable Land Use in Agriculture; Springer: Heidelberg, The Netherlands, 2007; ISBN 9781402066184. [Google Scholar]
- Adedeji, A.A.; Häggblom, M.M.; Babalola, O.O. Sustainable agriculture in Africa: Plant growth-promoting rhizobacteria (PGPR) to the rescue. Sci. African 2020, 9, e00492. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; Enshasy, H. El Plant growth promoting rhizobacteria (Pgpr) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Nazari, M.; Smith, D.L. A PGPR-Produced Bacteriocin for Sustainable Agriculture: A Review of Thuricin 17 Characteristics and Applications. Front. Plant Sci. 2020, 11, 916. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [Green Version]
- Barra Caracciolo, A.; Terenzi, V. Rhizosphere microbial communities and heavy metals. Microorganisms 2021, 9, 1462. [Google Scholar] [CrossRef]
- Fan, K.; Cardona, C.; Li, Y.; Shi, Y.; Xiang, X.; Shen, C.; Wang, H.; Gilbert, J.A.; Chu, H. Rhizosphere-associated bacterial network structure and spatial distribution differ significantly from bulk soil in wheat crop fields. Soil Biol. Biochem. 2017, 113, 275–284. [Google Scholar] [CrossRef]
- Finzi, A.C.; Abramoff, R.Z.; Spiller, K.S.; Brzostek, E.R.; Darby, B.A.; Kramer, M.A.; Phillips, R.P. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob. Change Biol. 2015, 21, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Peng, F.; Dang, Z.; Jiang, X.; Zhang, J.; Zhang, Y.; Shu, H. Influence of rhizosphere ventilation on soil nutrient status, root architecture and the growth of young peach trees. Soil Sci. Plant Nutr. 2015, 61, 775–787. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Yang, J.; Brookes, P.C.; Gunina, A. Rhizosphere priming regulates soil organic carbon and nitrogen mineralization: The significance of abiotic mechanisms. Geoderma 2021, 385, 114877. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, L.; Zhang, L.; Liu, C.; Han, M. Effects of cultivation ages and modes on microbial diversity in the rhizosphere soil of panax ginseng. J. Ginseng Res. 2016, 40, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar]
- Hoo, H.; Hashidoko, Y.; Islam, M.T.; Tahara, S. Requirement of a relatively high threshold level of Mg2+ for cell growth of a rhizoplane bacterium, Sphingomonas yanoikuyae EC-S. Appl. Environ. Microbiol. 2004, 70, 5214–5221. [Google Scholar] [CrossRef] [Green Version]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.; Anli, M.; Meddich, A.; Oufdou, K. Use of Rhizobacteria and Mycorrhizae Consortium in the Open Field as a Strategy for Improving Crop Nutrition, Productivity and Soil Fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef] [Green Version]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al. Biofertilizers as Strategies to Improve Photosynthetic Apparatus, Growth, and Drought Stress Tolerance in the Date Palm. Front. Plant Sci. 2020, 11, 1560. [Google Scholar] [CrossRef]
- Atieno, M.; Herrmann, L.; Nguyen, H.T.; Phan, H.T.; Nguyen, N.K.; Srean, P.; Than, M.M.; Zhiyong, R.; Tittabutr, P.; Shutsrirung, A.; et al. Assessment of biofertilizer use for sustainable agriculture in the Great Mekong Region. J. Environ. Manag. 2020, 275, 111300. [Google Scholar] [CrossRef]
- Hawkes, C.V. Nitrogen Cycling Mediated by Biological Soil Crusts And Arbuscular Mycorrhizal Fungi. Ecology 2003, 84, 1553–1562. [Google Scholar] [CrossRef]
- Strauss, S.L.; Day, T.A.; Garcia-Pichel, F. Nitrogen cycling in desert biological soil crusts across biogeographic regions in the Southwestern United States. Biogeochemistry 2012, 108, 171–182. [Google Scholar] [CrossRef]
- Heindel, R.C.; Governali, F.C.; Spickard, A.M.; Virginia, R.A. The Role of Biological Soil Crusts in Nitrogen Cycling and Soil Stabilization in Kangerlussuaq, West Greenland. Ecosystems 2019, 22, 243–256. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, H.; Zuo, X.; Drake, S.; Zhao, X. Biological soil crust development and its topsoil properties in the process of dune stabilization, Inner Mongolia, China. Environ. Geol. 2008, 54, 653–662. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Ha-tran, D.M.; Nguyen, T.T.M.; Hung, S.H.; Huang, E.; Huang, C.C. Roles of plant growth-promoting rhizobacteria (Pgpr) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight into the Role of PGPR in Sustainable Agriculture and Environment. Front. Sustain. Food Syst. 2021, 5, 183. [Google Scholar] [CrossRef]
- Gall, C.; Ohan, J.; Glaser, K.; Karsten, U.; Schloter, M.; Scholten, T.; Schulz, S.; Seitz, S.; Kurth, J.K. Biocrusts: Overlooked hotspots of managed soils in mesic environments. J. Plant Nutr. Soil Sci. 2022, 185, 745–751. [Google Scholar] [CrossRef]
- Faist, A.M.; Antoninka, A.J.; Barger, N.N.; Bowker, M.A.; Chaudhary, V.B.; Havrilla, C.A.; Huber-Sannwald, E.; Reed, S.C.; Weber, B. Broader Impacts for Ecologists: Biological Soil Crust as a Model System for Education. Front. Microbiol. 2021, 11, 3284. [Google Scholar] [CrossRef]
- Kennedy, A.C.; de Luna, L.Z. Rhizosphere. In Encyclopedia of Soils in the Environment; Elsevier Inc.: Amsterdam, The Netherlands, 2004; Volume 4, pp. 399–406. ISBN 9780080547954. [Google Scholar]
- Hinsinger, P.; Gobran, G.R.; Gregory, P.J.; Wenzel, W.W. Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol. 2005, 168, 293–303. [Google Scholar] [CrossRef]
- Turner, T.R.; Ramakrishnan, K.; Walshaw, J.; Heavens, D.; Alston, M.; Swarbreck, D.; Osbourn, A.; Grant, A.; Poole, P.S. Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. ISME J. 2013, 7, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cañizares, C.; Jorrín, B.; Poole, P.S.; Tkacz, A. Understanding the holobiont: The interdependence of plants and their microbiome. Curr. Opin. Microbiol. 2017, 38, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Elhady, A.; Topalović, O.; Heuer, H. Plants Specifically Modulate the Microbiome of Root-Lesion Nematodes in the Rhizosphere, Affecting Their Fitness. Microorganisms 2021, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nihorimbere, V.; Ongena, M.; Smargiassi, M.; Thonart, P. Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol. Agron. Soc. Environ. 2011, 15, 327–337. [Google Scholar]
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current Perspectives on Plant Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root Exudates: Mechanistic Insight of Plant Growth Promoting Rhizobacteria for Sustainable Crop Production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef]
- Pérez-Jaramillo, J.E.; Mendes, R.; Raaijmakers, J.M. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol. Biol. 2016, 90, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Masurkar, P.; Pandey, S.K.; Kumar, S. Rhizobacteria–plant interaction, alleviation of abiotic stresses. In Microorganisms for Sustainability; Springer: Singapore, 2019; Volume 12, pp. 345–353. [Google Scholar]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3874–3883. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Singh, P.; Sharma, A.; Guo, D.J.; Upadhyay, S.K.; Song, Q.Q.; Verma, K.K.; Li, D.P.; Malviya, M.K.; Song, X.P.; et al. Unraveling Nitrogen Fixing Potential of Endophytic Diazotrophs of Different Saccharum Species for Sustainable Sugarcane Growth. Int. J. Mol. Sci. 2022, 23, 6242. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, L.; Liu, W.; Zhang, Y.; Zhou, Q.; Wu, Z.; He, F. Effects of root exudates on rhizosphere bacteria and nutrient removal in pond-ditch circulation systems (PDCSs) for rural wastewater treatment. Sci. Total Environ. 2021, 785, 147282. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Badri, D.V.; Kidd, B.N.; Vivanco, J.M.; Schenk, P.M. Linking Jasmonic acid signaling, root exudates, and rhizosphere microbiomes. Mol. Plant-Microbe Interact. 2015, 28, 1049–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrodi, O.V.; McWilliams, J.R.; Peter, J.O.; Berim, A.; Hassan, K.A.; Elbourne, L.D.H.; LeTourneau, M.K.; Gang, D.R.; Paulsen, I.T.; Weller, D.M.; et al. Root Exudates Alter the Expression of Diverse Metabolic, Transport, Regulatory, and Stress Response Genes in Rhizosphere Pseudomonas. Front. Microbiol. 2021, 12, 698. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Déziel, E.; Schweizer, H.P.; Rahme, L.G.; Fall, R.; Vivanco, J.M. Pseudomonas aeruginosa-Plant Root Interactions. Pathogenicity, Biofilm Formation, and Root Exudation. Plant Physiol. 2004, 134, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Lanoue, A.; Burlat, V.; Henkes, G.J.; Koch, I.; Schurr, U.; Röse, U.S.R. De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol. 2010, 185, 577–588. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Bowya, T.; Balachandar, D. Rhizosphere engineering through exogenous growth-regulating small molecules improves the colonizing efficiency of a plant growth-promoting rhizobacterium in rice. 3 Biotech 2020, 10, 277. [Google Scholar] [CrossRef]
- Chamam, A.; Sanguin, H.; Bellvert, F.; Meiffren, G.; Comte, G.; Wisniewski-Dyé, F.; Bertrand, C.; Prigent-Combaret, C. Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum-Oryza sativa association. Phytochemistry 2013, 87, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- White, L.J.; Ge, X.; Brözel, V.S.; Subramanian, S. Root isoflavonoids and hairy root transformation influence key bacterial taxa in the soybean rhizosphere. Environ. Microbiol. 2017, 19, 1391–1406. [Google Scholar] [CrossRef]
- Szoboszlay, M.; White-Monsant, A.; Moe, L.A. The effect of root exudate 7,40-Dihydroxyflavone and naringenin on soil bacterial community structure. PLoS ONE 2016, 11, e0146555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.C.; Sim, H.J.; Kim, S.G.; Ryu, C.M. Root-mediated signal transmission of systemic acquired resistance against above-ground and below-ground pathogens. Ann. Bot. 2016, 118, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Glinwood, R.; Pettersson, J.; Ahmed, E.; Ninkovic, V.; Birkett, M.; Pickett, J. Change in acceptability of barley plants to aphids after exposure to allelochemicals from couch-grass (Elytrigia repens). J. Chem. Ecol. 2003, 29, 261–274. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Dippold, M.A.; Kuzyakov, Y.; Razavi, B.S. Spatial pattern of enzyme activities depends on root exudate composition. Soil Biol. Biochem. 2019, 133, 83–93. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef]
- Sipilä, T.P.; Yrjälä, K.; Alakukku, L.; Palojärvi, A. Cross-site soil microbial communities under tillage regimes: Fungistasis and microbial biomarkers. Appl. Environ. Microbiol. 2012, 78, 8191–8201. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Ladha, J.K.; Gupta, R.K.; Bhushan, L.; Rao, A.N.; Sivaprasad, B.; Singh, P.P. Evaluation of mulching, intercropping with Sesbania and herbicide use for weed management in dry-seeded rice (Oryza sativa L.). Crop Prot. 2007, 26, 518–524. [Google Scholar] [CrossRef]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant–microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef]
- Long, H.H.; Schmidt, D.D.; Baldwin, I.T. Native Bacterial Endophytes Promote Host Growth in a Species-Specific Manner; Phytohormone Manipulations Do Not Result in Common Growth Responses. PLoS ONE 2008, 3, e2702. [Google Scholar] [CrossRef] [Green Version]
- Dimkpa, C.O.; Merten, D.; Svatoš, A.; Büchel, G.; Kothe, E. Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol. Biochem. 2009, 41, 154–162. [Google Scholar] [CrossRef]
- Sikora, R.A.; Schäfer, K.; Dababat, A.A. Modes of action associated with microbially induced in planta suppression of plant-parasitic nematodes. In Proceedings of the Australasian Plant Pathology; Springer: Berlin/Heidelberg, Germany, 2007; Volume 36, pp. 124–134. [Google Scholar]
- Vaughan, M.M.; Wang, Q.; Webster, F.X.; Kiemle, D.; Hong, Y.J.; Tantillo, D.J.; Coates, R.M.; Wray, A.T.; Askew, W.; O’Donnell, C.; et al. Formation of the Unusual Semivolatile Diterpene Rhizathalene by the Arabidopsis Class I Terpene Synthase TPS08 in the Root Stele Is Involved in Defense against Belowground Herbivory. Plant Cell 2013, 25, 1108–1125. [Google Scholar] [CrossRef] [Green Version]
- Forchetti, G.; Masciarelli, O.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria in sunflower (Helianthus annuus L.): Isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl. Microbiol. Biotechnol. 2007, 76, 1145–1152. [Google Scholar] [CrossRef]
- Perrig, D.; Boiero, M.L.; Masciarelli, O.A.; Penna, C.; Ruiz, O.A.; Cassán, F.D.; Luna, M.V. Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl. Microbiol. Biotechnol. 2007, 75, 1143–1150. [Google Scholar] [CrossRef]
- Park, Y.G.; Mun, B.G.; Kang, S.M.; Hussain, A.; Shahzad, R.; Seo, C.W.; Kim, A.Y.; Lee, S.U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [Green Version]
- Jha, Y.; Subramanian, R. Paddy plants inoculated with PGPR show better growth physiology and nutrient content under saline conditions. Chil. J. Agric. Res. 2013, 73, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Housh, A.B.; Waller, S.; Sopko, S.; Powell, A.; Benoit, M.; Wilder, S.L.; Guthrie, J.; Schueller, M.J.; Ferrieri, R.A. Azospirillum brasilense Bacteria Promotes Mn2+ Uptake in Maize with Benefits to Leaf Photosynthesis. Microorganisms 2022, 10, 1290. [Google Scholar] [CrossRef]
- Rafi, M.M.; Varalakshmi, T.; Charyulu, P.B.B.N. Influence of Azospirillum and PSB inoculation on growth and yield of Foxtail Millet. J. Microbiol. Biotechnol. Res. 2017, 2, 558–565. [Google Scholar]
- Rizvi, A.; Zaidi, A.; Ameen, F.; Ahmed, B.; Alkahtani, M.D.F.; Khan, M.S. Heavy metal induced stress on wheat: Phytotoxicity and microbiological management. RSC Adv. 2020, 10, 38379–38403. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of bacillus spp. In disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Sasirekha, B.; Srividya, S. Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chilli. Agric. Nat. Resour. 2016, 50, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Asante, M.; Ahiabor, B.D.K.; Atakora, W.K. Growth, Nodulation, and Yield Responses of Groundnut (Arachis hypogaea L.) as Influenced by Combined Application of Rhizobium Inoculant and Phosphorus in the Guinea Savanna Zone of Ghana. Int. J. Agron. 2020, 2020, 8691757. [Google Scholar] [CrossRef] [Green Version]
- Ayuso-Calles, M.; García-Estévez, I.; Jiménez-Gómez, A.; Flores-Félix, J.D.; Escribano-Bailón, M.T.; Rivas, R. Rhizobium laguerreae Improves Productivity and Phenolic Compound Content of Lettuce (Lactuca sativa L.) under Saline Stress Conditions. Foods 2020, 9, 1166. [Google Scholar] [CrossRef]
- Khaitov, B.; Kurbonov, A.; Abdiev, A.; Adilov, M. Effect of chickpea in association with Rhizobium to crop productivity and soil fertility. Eurasian J. Soil Sci. 2016, 5, 105. [Google Scholar] [CrossRef]
- Badawi, F.S.F.; Biomy, A.M.M.; Desoky, A.H. Peanut plant growth and yield as influenced by co-inoculation with Bradyrhizobium and some rhizo-microorganisms under sandy loam soil conditions. Ann. Agric. Sci. 2011, 56, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Jha, P.N. The PGPR stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 2017, 8, 1945. [Google Scholar] [CrossRef] [Green Version]
- Akbari, A.; Gharanjik, S.; Koobaz, P.; Sadeghi, A. Plant growth promoting Streptomyces strains are selectively interacting with the wheat cultivars especially in saline conditions. Heliyon 2020, 6, e03445. [Google Scholar] [CrossRef]
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front. Microbiol. 2019, 10, 290. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Jha, P.N. The Multifarious PGPR Serratia marcescens CDP-13 Augments Induced Systemic Resistance and Enhanced Salinity Tolerance of Wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Devi, K.A.; Pandey, P.; Sharma, G.D. Plant Growth-Promoting Endophyte Serratia marcescens AL2-16 Enhances the Growth of Achyranthes aspera L., a Medicinal Plant. HAYATI J. Biosci. 2016, 23, 173–180. [Google Scholar] [CrossRef]
- Bashan, Y.; Levanony, H. Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can. J. Microbiol. 1990, 36, 591–608. [Google Scholar] [CrossRef] [Green Version]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Guseva, K.; Darcy, S.; Simon, E.; Alteio, L.V.; Montesinos-Navarro, A.; Kaiser, C. From diversity to complexity: Microbial networks in soils. Soil Biol. Biochem. 2022, 169, 108604. [Google Scholar] [CrossRef]
- Díaz-Zorita, M.; Fernández-Canigia, M.V. Field performance of a liquid formulation of Azospirillum brasilense on dryland wheat productivity. Eur. J. Soil Biol. 2009, 45, 3–11. [Google Scholar] [CrossRef]
- Youseif, S.; Abd El-Megeed, F.; Saleh, S. Improvement of Faba Bean Yield Using Rhizobium/Agrobacterium Inoculant in Low-Fertility Sandy Soil. Agronomy 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Stürmer, S.L.; Kemmelmeier, K. The Glomeromycota in the Neotropics. Front. Microbiol. 2021, 11, 3200. [Google Scholar] [CrossRef]
- Tedersoo, L.; Smith, M.E. Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol. Rev. 2013, 27, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Islam, M.T.; Kang, S. Secreted metabolite-mediated interactions between rhizosphere bacteria and Trichoderma biocontrol agents. PLoS ONE 2019, 14, e0227228. [Google Scholar] [CrossRef] [Green Version]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant—Fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 1–11. [Google Scholar] [CrossRef]
- Baslam, M.; Qaddoury, A.; Goicoechea, N. Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 2014, 28, 161–172. [Google Scholar] [CrossRef]
- Barea, J.M.; Azcón, R.; Azcón-Aguilar, C. Mycorrhizosphere interactions to improve a sustainable production of legumes. In Microbes for Legume Improvement, Second Edition; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 199–225. ISBN 9783319591742. [Google Scholar]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Boldt, K.; Pörs, Y.; Haupt, B.; Bitterlich, M.; Kühn, C.; Grimm, B.; Franken, P. Photochemical processes, carbon assimilation and RNA accumulation of sucrose transporter genes in tomato arbuscular mycorrhiza. J. Plant Physiol. 2011, 168, 1256–1263. [Google Scholar] [CrossRef]
- Qiao, X.; Bei, S.; Li, C.; Dong, Y.; Li, H.; Christie, P.; Zhang, F.; Zhang, J. Enhancement of faba bean competitive ability by arbuscular mycorrhizal fungi is highly correlated with dynamic nutrient acquisition by competing wheat. Sci. Rep. 2015, 5, 8122. [Google Scholar] [CrossRef] [Green Version]
- Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef]
- Campo, S.; Martín-Cardoso, H.; Olivé, M.; Pla, E.; Catala-Forner, M.; Martínez-Eixarch, M.; San Segundo, B. Effect of Root Colonization by Arbuscular Mycorrhizal Fungi on Growth, Productivity and Blast Resistance in Rice. Rice 2020, 13, 42. [Google Scholar] [CrossRef]
- Aganchich, B.; Wahbi, S.; Yaakoubi, A.; El-Aououad, H.; Bota, J. Effect of arbuscular mycorrhizal fungi inoculation on growth and physiology performance of olive tree under regulated deficit irrigation and partial rootzone drying. S. Afr. J. Bot. 2022, 148, 1–10. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis on Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 00307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soonvald, L.; Loit, K.; Runno-Paurson, E.; Astover, A.; Tedersoo, L. Characterising the effect of crop species and fertilisation treatment on root fungal communities. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhady, A.; Adss, S.; Hallmann, J.; Heuer, H. Rhizosphere Microbiomes Modulated by Pre-crops Assisted Plants in Defense Against Plant-Parasitic Nematodes. Front. Microbiol. 2018, 9, 1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, M.; Westphal, A.; Hallmann, J.; Heuer, H. Specific microbial attachment to root knot nematodes in suppressive soil. Appl. Environ. Microbiol. 2014, 80, 2679–2686. [Google Scholar] [CrossRef] [Green Version]
- Topalović, O.; Bredenbruch, S.; Schleker, A.S.S.; Heuer, H. Microbes Attaching to Endoparasitic Phytonematodes in Soil Trigger Plant Defense Upon Root Penetration by the Nematode. Front. Plant Sci. 2020, 11, 138. [Google Scholar] [CrossRef]
- Ruanpanun, P.; Laatsch, H.; Tangchitsomkid, N.; Lumyong, S. Nematicidal activity of fervenulin isolated from a nematicidal actinomycete, Streptomyces sp. CMU-MH021, on Meloidogyne incognita. World J. Microbiol. Biotechnol. 2011, 27, 1373–1380. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Qi, G.; Yin, R.; Zhang, H.; Li, C.; Zhao, X. Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Olsen, D.P.; Phu, D.; Libby, L.J.M.; Cormier, J.A.; Montez, K.M.; Ryder, E.F.; Politz, S.M. Chemosensory control of surface antigen switching in the nematode Caenorhabditis elegans. Genes Brain Behav. 2007, 6, 240–252. [Google Scholar] [CrossRef]
- Lok, J.B. Signaling in Parasitic Nematodes: Physicochemical Communication Between Host and Parasite and Endogenous Molecular Transduction Pathways Governing Worm Development and Survival. Curr. Clin. Microbiol. Rep. 2016, 3, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Tang, W.; Yang, F.; Meng, J.; Chen, W.; Li, X. Influence of biochar application on potassium-solubilizing Bacillus Mucilaginosus as potential biofertilizer. Prep. Biochem. Biotechnol. 2017, 47, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, X.; Zhang, X.; Ju, W.; Duan, C.; Guo, X.; Wang, Y.; Fang, L. Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Hu, J.; Du, N.; Chen, F. Functional diversity of the microbial community in healthy subjects and periodontitis patients based on sole carbon source utilization. PLoS ONE 2014, 9, e91977. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagerty, S.B.; Allison, S.D.; Schimel, J.P. Evaluating soil microbial carbon use efficiency explicitly as a function of cellular processes: Implications for measurements and models. Biogeochemistry 2018, 140, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Luo, S.; Xu, X.; Gou, Y.; Wang, J. The soil carbon cycle determined by GeoChip 5.0 in sugarcane and soybean intercropping systems with reduced nitrogen input in South China. Appl. Soil Ecol. 2020, 155, 103653. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Sun, L.; Qi, G.; Chen, S.; Zhao, X. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci. Rep. 2017, 7, 343. [Google Scholar] [CrossRef] [Green Version]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: Insights from meta-analysis and theoretical models. Ecol. Lett. 2013, 16, 887–894. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Poeplau, C.; Herrmann, A.; Kätterer, T. Opposing effects of nitrogen and phosphorus on soil microbial metabolism and the implications for soil carbon storage. Soil Biol. Biochem. 2016, 100, 83–91. [Google Scholar] [CrossRef]
- Wu, R.; Cheng, X.; Zhou, W.; Han, H. Microbial regulation of soil carbon properties under nitrogen addition and plant inputs removal. PeerJ 2019, 2019, e7343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.; Li, Z.; Wakelin, S.A.; Yu, W.; Liang, Y. Mineral fertilizer alters cellulolytic community structure and suppresses soil cellobiohydrolase activity in a long-term fertilization experiment. Soil Biol. Biochem. 2012, 55, 70–77. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Townsend, A.R.; Sattin, S.R.; Freeman, K.R.; Fierer, N.; Neff, J.C.; Bowman, W.D.; Schadt, C.W.; Weintraub, M.N.; Schmidt, S.K. The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: Implications for carbon and nitrogen cycling. Environ. Microbiol. 2008, 10, 3093–3105. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Xu, J.; Zheng, Z.; Fa, K. Enhanced atmospheric nitrogen deposition triggered little change in soil microbial diversity and structure in a desert ecosystem. Glob. Ecol. Conserv. 2021, 31, e01879. [Google Scholar] [CrossRef]
- Li, J.; Yang, C.; Zhou, H.; Shao, X. Responses of plant diversity and soil microorganism diversity to water and nitrogen additions in the Qinghai-Tibetan Plateau. Glob. Ecol. Conserv. 2020, 22, e01003. [Google Scholar] [CrossRef]
- Liu, W.; Qiao, C.; Yang, S.; Bai, W.; Liu, L. Microbial carbon use efficiency and priming effect regulate soil carbon storage under nitrogen deposition by slowing soil organic matter decomposition. Geoderma 2018, 332, 37–44. [Google Scholar] [CrossRef]
- Moore, J.A.M.; Anthony, M.A.; Pec, G.J.; Trocha, L.K.; Trzebny, A.; Geyer, K.M.; van Diepen, L.T.A.; Frey, S.D. Fungal community structure and function shifts with atmospheric nitrogen deposition. Glob. Change Biol. 2021, 27, 1349–1364. [Google Scholar] [CrossRef]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Wang, R.; Cai, J.; Meng, Y.; Wang, Z.; Feng, X.; Liu, H.; Turco, R.F.; Jiang, Y. Enhanced carbon acquisition and use efficiency alleviate microbial carbon relative to nitrogen limitation under soil acidification. Ecol. Process. 2021, 10, 32. [Google Scholar] [CrossRef]
- Wang, H.; He, X.; Zhang, Z.; Li, M.; Zhang, Q.; Zhu, H.; Xu, S.; Yang, P. Eight years of manure fertilization favor copiotrophic traits in paddy soil microbiomes. Eur. J. Soil Biol. 2021, 106, 103352. [Google Scholar] [CrossRef]
- Ma, Q.; Wen, Y.; Wang, D.; Sun, X.; Hill, P.W.; Macdonald, A.; Chadwick, D.R.; Wu, L.; Jones, D.L. Farmyard manure applications stimulate soil carbon and nitrogen cycling by boosting microbial biomass rather than changing its community composition. Soil Biol. Biochem. 2020, 144, 107760. [Google Scholar] [CrossRef]
- Elfstrand, S.; Hedlund, K.; Mårtensson, A. Soil enzyme activities, microbial community composition and function after 47 years of continuous green manuring. Appl. Soil Ecol. 2007, 35, 610–621. [Google Scholar] [CrossRef]
- Tian, W.; Wang, L.; Li, Y.; Zhuang, K.; Li, G.; Zhang, J.; Xiao, X.; Xi, Y. Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen. Agric. Ecosyst. Environ. 2015, 213, 219–227. [Google Scholar] [CrossRef]
- Sun, X.; Ye, Y.; Ma, Q.; Guan, Q.; Jones, D.L. Variation in enzyme activities involved in carbon and nitrogen cycling in rhizosphere and bulk soil after organic mulching. Rhizosphere 2021, 19, 100376. [Google Scholar] [CrossRef]
- Wang, J.; Zou, Y.; Di Gioia, D.; Singh, B.K.; Li, Q. Conversion to agroforestry and monoculture plantation is detrimental to the soil carbon and nitrogen cycles and microbial communities of a rainforest. Soil Biol. Biochem. 2020, 147, 107849. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.; Zhou, B.; Chen, X.; Jin, J.; Liu, X.; et al. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 2022, 418, 115846. [Google Scholar] [CrossRef]
- Starke, R.; Mondéjar, R.L.; Human, Z.R.; Navrátilová, D.; Štursová, M.; Větrovský, T.; Olson, H.M.; Orton, D.J.; Callister, S.J.; Lipton, M.S.; et al. Niche differentiation of bacteria and fungi in carbon and nitrogen cycling of different habitats in a temperate coniferous forest: A metaproteomic approach. Soil Biol. Biochem. 2021, 155, 108170. [Google Scholar] [CrossRef]
- Too, C.C.; Ong, K.S.; Yule, C.M.; Keller, A. Putative roles of bacteria in the carbon and nitrogen cycles in a tropical peat swamp forest. Basic Appl. Ecol. 2021, 52, 109–123. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Zhang, Z.; Song, N.; Zhou, H.; Li, Y.; Wang, Y.; Li, C.; Hale, L. Alteration of desert soil microbial community structure in response to agricultural reclamation and abandonment. CATENA 2021, 207, 105678. [Google Scholar] [CrossRef]
- Nuccio, E.E.; Hodge, A.; Pett-Ridge, J.; Herman, D.J.; Weber, P.K.; Firestone, M.K. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ. Microbiol. 2013, 15, 1870–1881. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; He, J.; Zhu, Y.; Qiao, N.; Ge, Y. Arbuscular mycorrhizal fungi and plant diversity drive restoration of nitrogen-cycling microbial communities. Mol. Ecol. 2021, 30, 4133–4146. [Google Scholar] [CrossRef] [PubMed]
- Legay, N.; Clément, J.C.; Grassein, F.; Lavorel, S.; Lemauviel-Lavenant, S.; Personeni, E.; Poly, F.; Pommier, T.; Robson, T.M.; Mouhamadou, B.; et al. Plant growth drives soil nitrogen cycling and N-related microbial activity through changing root traits. Fungal Ecol. 2020, 44, 100910. [Google Scholar] [CrossRef]
- Qian, K.; Wang, L.; Yin, N. Effects of AMF on soil enzyme activity and carbon sequestration capacity in reclaimed mine soil. Int. J. Min. Sci. Technol. 2012, 22, 553–557. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Ren, T.; Yan, J.; Zhu, D.; Liao, S.; Zhang, Y.; Lu, Z.; Cong, R.; Li, X.; Lu, J. Straw returning mediates soil microbial biomass carbon and phosphorus turnover to enhance soil phosphorus availability in a rice-oilseed rape rotation with different soil phosphorus levels. Agric. Ecosyst. Environ. 2022, 335, 107991. [Google Scholar] [CrossRef]
- Eder, S.; Shi, L.; Jensen, K.; Yamane, K.; Hulett, F.M. A Bacillus subtilis secreted phosphodiesterase/alkaline phosphatase is the product of a Pho regulon gene, phoD. Microbiology 1996, 142, 2041–2047. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.t.; Zeng, Q.w.; Zhang, S.c.; Liao, B.; Shu, W.s.; Li, J.t. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Gong, J.; Li, X.; Zhang, Z.; Zhang, W.; Li, Y.; Song, L.; Zhang, S.; Dong, J.; Baoyin, T.t. Plant–microbial linkages regulate soil organic carbon dynamics under phosphorus application in a typical temperate grassland in northern China. Agric. Ecosyst. Environ. 2022, 335, 108006. [Google Scholar] [CrossRef]
- Zhang, D.; Kuzyakov, Y.; Zhu, H.; Alharbi, H.A.; Li, H.; Rengel, Z. Increased microbial biomass and turnover underpin efficient phosphorus acquisition by Brassica chinensis. Soil Tillage Res. 2022, 223, 105492. [Google Scholar] [CrossRef]
- Luo, G.; Sun, B.; Li, L.; Li, M.; Liu, M.; Zhu, Y.; Guo, S.; Ling, N.; Shen, Q. Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phoD- and phoC-harboring functional microbial populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, M.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Chen, S.; Cao, F.; Li, L.; Yang, X.; et al. Impact of 36 years of nitrogen fertilization on microbial community composition and soil carbon cycling-related enzyme activities in rhizospheres and bulk soils in northeast China. Appl. Soil Ecol. 2019, 136, 148–157. [Google Scholar] [CrossRef]
- Hillel, D. Soil fertility and plant nutrition. In Soil in the Environment; Elsevier: Amsterdam, The Netherlands, 2008; pp. 151–162. [Google Scholar]
- Lalitha, M.; Dhakshinamoorthy, M. Forms of soil potassium-A review. Agric. Rev. 2014, 35, 64. [Google Scholar] [CrossRef]
- Zhang, M.; Riaz, M.; Liu, B.; Xia, H.; El-desouki, Z.; Jiang, C. Two-year study of biochar: Achieving excellent capability of potassium supply via alter clay mineral composition and potassium-dissolving bacteria activity. Sci. Total Environ. 2020, 717, 137286. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lv, L.; Wang, W.; Liu, Y.; Yin, C.; Xu, Q.; Yan, H.; Fu, J.; Liu, X. Differences in Distribution of Potassium-Solubilizing Bacteria in Forest and Plantation Soils in Myanmar. Int. J. Environ. Res. Public Health 2019, 16, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Riaz, M.; Liu, B.; Li, Y.; El-Desouki, Z.; Jiang, C. Over two years study: Peanut biochar promoted potassium availability by mediating the relationship between bacterial community and soil properties. Appl. Soil Ecol. 2022, 176, 104485. [Google Scholar] [CrossRef]
- Xiao, B.; Lian, B.; Shao, W. Do Bacterial Secreted Proteins Play a Role in The Weathering of Potassium-Bearing Rock Powder? Geomicrobiol. J. 2012, 29, 497–505. [Google Scholar] [CrossRef]
- Nutaratat, P.; Monprasit, A.; Srisuk, N. High-yield production of indole-3-acetic acid by Enterobacter sp. DMKU-RP206, a rice phyllosphere bacterium that possesses plant growth-promoting traits. 3 Biotech 2017, 7, 305. [Google Scholar] [CrossRef]
- Melero, S.; Madejón, E.; Herencia, J.F.; Ruiz, J.C. Effect of Implementing Organic Farming on Chemical and Biochemical Properties of an Irrigated Loam Soil. Agron. J. 2008, 100, 136–144. [Google Scholar] [CrossRef]
- Yuan, Z.S.; Liu, F.; Zhang, G.F. Characteristics and biodiversity of endophytic phosphorus- and potassiumsolubilizing bacteria in moso bamboo (Phyllostachys edulis). Acta Biol. Hung. 2015, 66, 449–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Almeida, R.F.; Naves, E.R.; da Mota, R.P. Soil quality: Enzymatic activity of soil β-glucosidase. Glob. J. Agric. Res. Rev. 2015, 3, 146–150. [Google Scholar]
- Singhania, R.R.; Patel, A.K.; Sukumaran, R.K.; Larroche, C.; Pandey, A. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour. Technol. 2013, 127, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Kang, E.; Zhang, K.; Li, Y.; Hao, Y.; Wu, H.; Li, M.; Zhang, X.; Wang, J.; Yan, L.; et al. Plant and Soil Enzyme Activities Regulate CO2 Efflux in Alpine Peatlands After 5 Years of Simulated Extreme Drought. Front. Plant Sci. 2021, 12, 2208. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Shi, Y.; He, N.; Wen, X.; Yu, Q.; Zheng, C.; Sun, X.; Qiu, W. Responses of soil hydrolytic enzymes, ammonia-oxidizing bacteria and archaea to nitrogen applications in a temperate grassland in Inner Mongolia. Sci. Rep. 2016, 6, 32791. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, J.; Wang, J. Clay Minerals Change the Toxic Effect of Cadmium on the Activities of Leucine Aminopeptidase. Adsorpt. Sci. Technol. 2021, 2021, 1024085. [Google Scholar] [CrossRef]
- Poshina, D.N.; Raik, S.V.; Poshin, A.N.; Skorik, Y.A. Accessibility of chitin and chitosan in enzymatic hydrolysis: A review. Polym. Degrad. Stab. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Baldrian, P.; Voříšková, J.; Dobiášová, P.; Merhautová, V.; Lisá, L.; Valášková, V. Production of extracellular enzymes and degradation of biopolymers by saprotrophic microfungi from the upper layers of forest soil. Plant Soil 2011, 338, 111–125. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Alokika; Singh, B. Production, characteristics, and biotechnological applications of microbial xylanases. Appl. Microbiol. Biotechnol. 2019, 103, 8763–8784. [Google Scholar] [CrossRef]
- Bosetto, A.; Justo, P.I.; Zanardi, B.; Venzon, S.S.; Graciano, L.; dos Santos, E.L.; de Cássia Garcia Simão, R. Research Progress Concerning Fungal and Bacterial β-Xylosidases. Appl. Biochem. Biotechnol. 2016, 178, 766–795. [Google Scholar] [CrossRef]
- Greenfield, L.M.; Puissant, J.; Jones, D.L. Synthesis of methods used to assess soil protease activity. Soil Biol. Biochem. 2021, 158, 108277. [Google Scholar] [CrossRef]
- Mekonnen, E.; Kebede, A.; Nigussie, A.; Kebede, G.; Tafesse, M. Isolation and Characterization of Urease-Producing Soil Bacteria. Int. J. Microbiol. 2021, 2021, 8888641. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shi, W. Soil peroxidase regulates organic matter decomposition through improving the accessibility of reducing sugars and amino acids. Biol. Fertil. Soils 2014, 50, 785–794. [Google Scholar] [CrossRef]
- Abou-el-Seoud, I.I.; Abdel-Megeed, A. Impact of rock materials and biofertilizations on P and K availability for maize (Zea Maize) under calcareous soil conditions. Saudi J. Biol. Sci. 2012, 19, 55–63. [Google Scholar] [CrossRef]
- Basak, B.B.; Biswas, D.R. Influence of potassium solubilizing microorganism (Bacillus mucilaginosus) and waste mica on potassium uptake dynamics by sudan grass (Sorghum vulgare Pers.) grown under two Alfisols. Plant Soil 2009, 317, 235–255. [Google Scholar] [CrossRef]
- Liu, D.; Lian, B.; Dong, H. Isolation of Paenibacillus sp. and Assessment of its Potential for Enhancing Mineral Weathering. Geomicrobiol. J. 2012, 29, 413–421. [Google Scholar] [CrossRef]
- Guo, X.Q.; Gu, J.Y.; Yu, Y.J.; Zhang, W.B.; He, L.Y.; Sheng, X.F. Paenibacillus susongensis sp. nov., a mineral-weathering bacterium. Int. J. Syst. Evol. Microbiol. 2014, 64, 3958–3963. [Google Scholar] [CrossRef]
- Sheng, X.F.; Zhao, F.; He, L.Y.; Qiu, G.; Chen, L. Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can. J. Microbiol. 2008, 54, 1064–1068. [Google Scholar] [CrossRef]
- Sarikhani, M.R.; Oustan, S.; Ebrahimi, M.; Aliasgharzad, N. Isolation and identification of potassium-releasing bacteria in soil and assessment of their ability to release potassium for plants. Eur. J. Soil Sci. 2018, 69, 1078–1086. [Google Scholar] [CrossRef]
- Song, M.; Pedruzzi, I.; Peng, Y.; Li, P.; Liu, J.; Yu, J. K-Extraction from Muscovite by the Isolated Fungi. Geomicrobiol. J. 2015, 32, 771–779. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, L.; Peng, Y.; Li, P.; Yu, J. Effects of Mineral Structure and Microenvironment on K Release from Potassium Aluminosilicate Minerals by Cenococcum geophilum fr. Geomicrobiol. J. 2019, 36, 11–18. [Google Scholar] [CrossRef]
- Rodriguez-Caballero, E.; Belnap, J.; Büdel, B.; Crutzen, P.J.; Andreae, M.O.; Pöschl, U.; Weber, B. Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosci. 2018, 11, 185–189. [Google Scholar] [CrossRef]
- Biological Soil Crusts: Structure, Function, and Management; Ecological Studies; Belnap, J.; Lange, O.L. (Eds.) Springer: Berlin/Heidelberg, Germany, 2003; Volume 150, ISBN 978-3-540-43757-4. [Google Scholar]
- Weber, B.; Büdel, B.; Belnap, J. (Eds.) Biological Soil Crusts: An Organizing Principle in Drylands; Ecological Studies; Springer International Publishing: Cham, Switzerland, 2016; Volume 226, ISBN 978-3-319-30212-6. [Google Scholar]
- Williams, A.J.; Buck, B.J.; Beyene, M.A. Biological Soil Crusts in the Mojave Desert, USA: Micromorphology and Pedogenesis. Soil Sci. Soc. Am. J. 2012, 76, 1685–1695. [Google Scholar] [CrossRef]
- Bowker, M.A.; Reed, S.C.; Maestre, F.T.; Eldridge, D.J. Biocrusts: The living skin of the earth. Plant Soil 2018, 429, 1–7. [Google Scholar] [CrossRef]
- Maestre, F.T.; Benito, B.M.; Berdugo, M.; Concostrina-Zubiri, L.; Delgado-Baquerizo, M.; Eldridge, D.J.; Guirado, E.; Gross, N.; Kéfi, S.; Le Bagousse-Pinguet, Y.; et al. Biogeography of global drylands. New Phytol. 2021, 231, 540–558. [Google Scholar] [CrossRef]
- Chen, N.; Yu, K.; Jia, R.; Teng, J.; Zhao, C. Biocrust as one of multiple stable states in global drylands. Sci. Adv. 2020, 6, eaay3763. [Google Scholar] [CrossRef]
- Warren, S.D.; Clair, L.L.; Stark, L.R.; Lewis, L.A.; Pombubpa, N.; Kurbessoian, T.; Stajich, J.E.; Aanderud, Z.T. Reproduction and Dispersal of Biological Soil Crust Organisms. Front. Ecol. Evol. 2019, 7, 344. [Google Scholar] [CrossRef] [Green Version]
- Büdel, B. Microorganisms of biological crusts on soil surfaces. In Microorganisms in Soils: Roles in Genesis and Functions. Soil Biology; Varma, A., Buscot, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 307–323. ISBN 978-3-540-26609-9. [Google Scholar]
- Kidron, G.J.; Vonshak, A.; Abeliovich, A. Recovery rates of microbiotic crusts within a dune ecosystem in the Negev Desert. Geomorphology 2008, 100, 444–452. [Google Scholar] [CrossRef]
- Belnap, J.; Lange, O.L. Lichens and microfungi in biological soil crusts. In The Fungal Community. Its Organization and Role in the Ecosystem, 4th ed.; Dighton, J., White, J.F., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 137–158. ISBN 9781315119496. [Google Scholar]
- Ladrón De Guevara, M.; Maestre, F.T. Ecology and responses to climate change of biocrust-forming mosses in drylands. J. Exp. Bot. 2022, 73, 4380–4395. [Google Scholar] [CrossRef]
- Concostrina-Zubiri, L.; Valencia, E.; Ochoa, V.; Gozalo, B.; Mendoza, B.J.; Maestre, F.T. Biocrust-forming lichens increase soil available phosphorus under simulated climate change. Eur. J. Soil Sci. 2022, 73, e13284. [Google Scholar] [CrossRef]
- Bu, C.; Wu, S.; Zhang, K.; Yang, Y.; Gao, G. Biological soil crusts: An eco-adaptive biological conservative mechanism and implications for ecological restoration. Plant Biosyst. 2015, 149, 364–373. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, M.; Xu, M.X. Characterising the diversity and functionality of the microbial community within biocrusts associated with different vegetation communities and soil habitats. Appl. Soil Ecol. 2022, 175, 104458. [Google Scholar] [CrossRef]
- Seitz, S.; Nebel, M.; Goebes, P.; Käppeler, K.; Schmidt, K.; Shi, X.; Song, Z.; Webber, C.L.; Weber, B.; Scholten, T. Bryophyte-dominated biological soil crusts mitigate soil erosion in an early successional Chinese subtropical forest. Biogeosciences 2017, 14, 5775–5788. [Google Scholar] [CrossRef] [Green Version]
- Chamizo, S.; Cantón, Y.; Miralles, I.; Domingo, F. Biological soil crust development affects physicochemical characteristics of soil surface in semiarid ecosystems. Soil Biol. Biochem. 2012, 49, 96–105. [Google Scholar] [CrossRef]
- Chamizo, S.; Cantón, Y.; Lázaro, R.; Solé-Benet, A.; Domingo, F. Crust Composition and Disturbance Drive Infiltration Through Biological Soil Crusts in Semiarid Ecosystems. Ecosystems 2012, 15, 148–161. [Google Scholar] [CrossRef]
- Miralles-Mellado, I.; Cantón, Y.; Solé-Benet, A. Two-Dimensional Porosity of Crusted Silty Soils: Indicators of Soil Quality in Semiarid Rangelands? Soil Sci. Soc. Am. J. 2011, 75, 1330–1342. [Google Scholar] [CrossRef] [Green Version]
- Beraldi-Campesi, H.; Hartnett, H.E.; Anbar, A.; Gordon, G.W.; Garcia-Pichel, F. Effect of biological soil crusts on soil elemental concentrations: Implications for biogeochemistry and as traceable biosignatures of ancient life on land. Geobiology 2009, 7, 348–359. [Google Scholar] [CrossRef]
- Harper, K.T.; Belnap, J. The influence of biological soil crusts on mineral uptake by associated vascular plants. J. Arid Environ. 2001, 47, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Chamizo, S. Soil Inoculation with Cyanobacteria: Reviewing Its’ Potential for Agriculture Sustainability in Drylands. Agric. Res. Technol. Open Access J. 2018, 18, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Nevins, C.; Strauss, S.L.; Inglett, P. Contrasting effects of agroecosystem biocrusts on seedling growth and nitrogen accumulation in a greenhouse environment. Agrosystems Geosci. Environ. 2022, 5, e20295. [Google Scholar] [CrossRef]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Büdel, B.; Andreae, M.O.; Pöschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Sancho, L.G.; Belnap, J.; Colesie, C.; Raggio, J.; Weber, B. Carbon Budgets of Biological Soil Crusts at Micro-, Meso-, and Global Scales; Springer: Berlin/Heidelberg, Germany, 2016; pp. 287–304. [Google Scholar]
- Nevins, C.J.; Strauss, S.L.; Inglett, P.W. Biological soil crusts enhance moisture and nutrients in the upper rooting zone of sandy soil agroecosystems. J. Plant Nutr. Soil Sci. 2020, 183, 615–626. [Google Scholar] [CrossRef]
- Baumann, K.; Siebers, M.; Kruse, J.; Eckhardt, K.U.; Hu, Y.; Michalik, D.; Siebers, N.; Kar, G.; Karsten, U.; Leinweber, P. Biological soil crusts as key player in biogeochemical P cycling during pedogenesis of sandy substrate. Geoderma 2019, 338, 145–158. [Google Scholar] [CrossRef]
- Qi, J.; Liu, Y.; Wang, Z.; Zhao, L.; Zhang, W.; Wang, Y.; Li, X. Variations in microbial functional potential associated with phosphorus and sulfur cycling in biological soil crusts of different ages at the Tengger Desert, China. Appl. Soil Ecol. 2021, 165, 104022. [Google Scholar] [CrossRef]
- Baumann, K.; Glaser, K.; Mutz, J.E.; Karsten, U.; MacLennan, A.; Hu, Y.; Michalik, D.; Kruse, J.; Eckhardt, K.U.; Schall, P.; et al. Biological soil crusts of temperate forests: Their role in P cycling. Soil Biol. Biochem. 2017, 109, 156–166. [Google Scholar] [CrossRef]
- Gehlot, P.; Vivekanand, V.; Pareek, N. Cyanobacterial and microalgal bioremediation: An efficient and eco-friendly approach toward industrial wastewater treatment and value-addition. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 343–362. [Google Scholar]
- Magan, N.; Gouma, S.; Fragoeiro, S.; Shuaib, M.E.; Bastos, A.C. Bacterial and fungal bioremediation strategies. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 193–212. [Google Scholar]
- Olaniran, A.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Jiménez, E.; Ochoa-Hueso, R.; Plaza, C.; Aceña-Heras, S.; Flagmeier, M.; Elouali, F.Z.; Ochoa, V.; Gozalo, B.; Lázaro, R.; Maestre, F.T. Biocrusts buffer against the accumulation of soil metallic nutrients induced by warming and rainfall reduction. Commun. Biol. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Belnap, J. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol. Process. 2006, 20, 3159–3178. [Google Scholar] [CrossRef]
- Rutherford, W.A.; Painter, T.H.; Ferrenberg, S.; Belnap, J.; Okin, G.S.; Flagg, C.; Reed, S.C. Albedo feedbacks to future climate via climate change impacts on dryland biocrusts. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Couradeau, E.; Karaoz, U.; Lim, H.C.; Nunes da Rocha, U.; Northen, T.; Brodie, E.; Garcia-Pichel, F. Bacteria increase arid-land soil surface temperature through the production of sunscreens. Nat. Commun. 2016, 7, 10373. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.A.; Corbin, J.D. Biological soil crusts inhibit seed germination in a temperate pine barren ecosystem. PLoS ONE 2019, 14, e0212466. [Google Scholar] [CrossRef]
- Thiet, R.K.; Doshas, A.; Smith, S.M. Effects of biocrusts and lichen-moss mats on plant productivity in a US sand dune ecosystem. Plant Soil 2014, 377, 235–244. [Google Scholar] [CrossRef]
- Yoshitake, S.; Uchida, M.; Koizumi, H.; Kanda, H.; Nakatsubo, T. Production of biological soil crusts in the early stage of primary succession on a High Arctic glacier foreland. New Phytol. 2010, 186, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Li, X.; Hui, R. Effect of biological soil crusts on seed germination and growth of an exotic and two native plant species in an arid ecosystem. PLoS ONE 2017, 12, e0185839. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Martínez, P.; Panettieri, M.; García-Palacios, P.; Moreno, E.; Plaza, C.; Maestre, F.T. Biocrusts Modulate Climate Change Effects on Soil Organic Carbon Pools: Insights From a 9-Year Experiment. Ecosystems 2022. [Google Scholar] [CrossRef]
- Evans, R.D.; Lange, O.L. Biological soil crusts and ecosystem nitrogen and carbon dynamics. In Biological Soil Crusts: Structure, Function, and Management. Ecological Studies; Belnap, J., Lange, O.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 263–279. ISBN 978-3-642-56475-8. [Google Scholar]
- Nevins, C.J.; Inglett, P.W.; Strauss, S.L. Biological soil crusts structure the subsurface microbiome in a sandy agroecosystem. Plant Soil 2021, 462, 311–329. [Google Scholar] [CrossRef]
- Nevins, C.J.; Inglett, P.W.; Reardon, C.L.; Strauss, S.L. Seasonality drives microbiome composition and nitrogen cycling in soil below biocrusts. Soil Biol. Biochem. 2022, 166, 108551. [Google Scholar] [CrossRef]
- Zaady, E.; Arbel, S.; Barkai, D.; Sarig, S. Long-term impact of agricultural practices on biological soil crusts and their hydrological processes in a semiarid landscape. J. Arid Environ. 2013, 90, 5–11. [Google Scholar] [CrossRef]
- Ferrenberg, S.; Faist, A.M.; Howell, A.; Reed, S.C. Biocrusts enhance soil fertility and Bromus tectorum growth, and interact with warming to influence germination. Plant Soil 2018, 429, 77–90. [Google Scholar] [CrossRef]
- Peng, X.; Bruns, M.A. Development of a nitrogen-fixing cyanobacterial consortium for surface stabilization of agricultural soils. J. Appl. Phycol. 2019, 31, 1047–1056. [Google Scholar] [CrossRef]
- Peng, X.; Bruns, M.A. Cyanobacterial Soil Surface Consortia Mediate N Cycle Processes in Agroecosystems. Front. Environ. Sci. 2019, 6, 156. [Google Scholar] [CrossRef] [Green Version]
- Sosa-Quintero, J.; Camargo-Ricalde, S.L.; Herrera-Campos, M.; de los Ángeles Herrera-Campos, M.; Godínez-Alvarez, H. Rainfed agriculture and firewood extraction modify differently the taxonomic and functional structure of biocrusts in a tropical semiarid region. Geoderma 2022, 406, 115459. [Google Scholar] [CrossRef]
- Sosa-Quintero, J.; Godínez-Alvarez, H.; Camargo-Ricalde, S.L.; Gutiérrez-Gutiérrez, M.; Huber-Sannwald, E.; Jiménez-Aguilar, A.; Maya-Delgado, Y.; Mendoza-Aguilar, D.; Montaño, N.M.; Pando-Moreno, M.; et al. Biocrusts in Mexican deserts and semideserts: A review of their species composition, ecology, and ecosystem function. J. Arid Environ. 2022, 199, 104712. [Google Scholar] [CrossRef]
- Zaady, E.; Eldridge, D.J.; Bowker, M.A. Effects of local-scale disturbance on biocrusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 429–449. ISBN 978-3-319-30214-0. [Google Scholar]

| Compounds of Root Exudates | Microorganisms | Plant | References |
|---|---|---|---|
| 2,4-diacetylphloroglucinol Organic acids | Pseudomonas spp. Azospirillum spp. Arthobacter spp. Devosia spp. | Wheat | [53] |
| Flavonoids Salicylic acid | Azospirillum spp. Pseudomonas spp. | Rice | [54,55] |
| Azelaic acid | Bacillus spp. | Tomato | [45] |
| Amino acids, proline, exopolysaccharides | Bacillus spp. | Maize | [56] |
| Isoflavonoids | Rhizobium spp. | Soybean | [57] |
| 7,4-Dihydroxyflavone | Acidobacteria | Alfalfa | [58] |
| Bacteria | Implications for the Plant | Plant | References |
|---|---|---|---|
| Azospirillum sp. | Fixation of atmospheric Nitrogen Production of phytohormones (auxins) | - | [75] |
| Showed higher panicle and seed weight | foxtail millet | [76] | |
| Azotobacter sp. | Increased grain yield 16.5–19.42% over control | wheat | [77] |
| Bacillus sp. | Produce many potent antifungal metabolites Production of phytohormones | - | [78] |
| Pseudomonas sp. | Siderophore production Production of phytohormones (e.g., IAA, cytokinins) | - | [79] |
| Pseudomonas putida | Improving growth and development under moisture stress conditions | maize | [80] |
| Bradyrhizobium sp. | Increased yield of haulm yield Increased yield of pod yield (13–40%) | groundnut | [81] |
| Increasing leaf number, shoot weight and the content of certain bioactive compounds | lettuce | [82] | |
| Increase in total nitrogen, carbon and phosphorus in the soil | chickpea | [83] | |
| Accumulation of dry matter, higher yield of pods and straw | peanut | [84] | |
| Stenotrophomonas maltophilia | Higher levels of defence enzymes (including β-1,3 glucanase, peroxidases (PO) and polyphenol oxidases (PPO)) Significantly increased plant growth (increased shoot length/root length (20–39%), fresh weight/dry weight (28–42%)) higher chlorophyll content (24–56%) | wheat | [85] |
| Streptomyces sp. | Higher chlorophyll and carotenoid content Lower APX and SOD activity lower Na+ content | wheat | [86] |
| Growth promotion Inhibiting the growth of virulent strains of B. glumae, as well as a wide range of bacterial and fungal species | rice | [87] | |
| Serratia marcescens | Reduction in plant growth inhibition (15 to 85%) caused by salt stress Increase in the concentration (20 to 75%) of various osmoprotectants (proline, indoleacetic acid) in plants | wheat | [88] |
| Serratia sp. AL2-16 | Increase in shoot length by 95.52%, shoot fresh weight by 602.38%, root fresh weight by 438% and leaf area by 127.2% | Achyranthes aspera L. (prickly chaff flower) | [89] |
| Agronomic Practice(s)/Forest Ecosystem/Desert Ecosystem | Soil Type and Climate | Region | Soil Microbial Activities | Carbon Cycling | Nitrogen Cycling | Reference(s) |
|---|---|---|---|---|---|---|
| Rainforest conversion into rubber-based plantation | Oxisols developed from sandstone and granite; tropical and monsoonal climate. | Baisha, Hainan, China | Relative abundance of Ascomycota and Zygomycota decreased but Basiodiomycota increased. Relative abundance of Actinobacteria and Verrucomicrobia decreased but Acidobacteria and Chloroflexi increased. | Reduced soil C mineralization rate. Microbial biomass C (MBC) increased. | Increased soil available N. Microbial biomass N (MBN) increased. | [142] |
| Manure addition compared to fertilization | Black soil and temperate continental monsoonal climate. | Northeast China | Manure can increase the functioning and abundances of Proteobacteria and Planctomycetes (C and N cycler) but inhibited growth of Verrucomicrobia. | Manure can reduce the abundance of cooC (reductive acetyl-CoA pathway) and coxS (CO oxidation) genes but enhance the abundance of icd genes. | Manures can boost the abundances of nasA, nasD, napA, and napC genes. | [143] |
| Organic mulching | Northern subtropical monsoonal climate. | Nanjing, China | N.E. | SOC, dissolved C and MBC increased. | Total N, soil available N, dissolved N and MBN increased. | [141] |
| Temperate Coniferous Forest | Peat soils, Temperate and harsh climatic conditions. | Central Europe | Dominant abundance of bacteria include Proteobacteria, Acidobacteria and Actinobacteria. Dominant abundance of fungi include Basidiomycota and Ascomycota. | Fungi regulated C cycling through enzyme stoichiometry. | Bacteria regulated N cycling through enzyme stoichiometry. | [144] |
| Peat Swamp Forest | Waterlogged peat soils, tropical moist climate. | Malaysia | Bacterial taxa e.g., Dyella spp., Paraburkholderia spp., Klebsiella spp. were involved in the dilapidation of lignocellulose, carbohydrates, sugar alcohols, organic acids and aromatic compounds. | [145] | ||
| Conversion of desert to croplands (reclamation) | Aeolian silty loam soils. | Northwest China | Increased bacterial (Proteobacteria, Nitrospirae) and archaeal gene (Euryarchaeota, Thaumarchaeota) abundances observed. | Total organic carbon, SOC, dissolved organic carbon, MBC increased. | Total N content and soil available N increased. | [146] |
| Enzymes | Function(s) | Reference(s) |
|---|---|---|
| β-glucosidase | 1. Directly controls the quality and quantity of SOM by regulating SOM decomposition. 2. Split cellobiose into glucose molecules. | [168,169] |
| β-cellobiosidase | 1. Regulate C cycling and ecosystem respiration. | [170] |
| N-acetyl-glucosaminidase | 1. N mineralization in soils. 2. Reducing ecosystem respiration. | [170,171] |
| L-leucine aminopeptidase | 1. Helps soil microbes to acquire N. | [172] |
| Cellobiohydrolase | 1. Facilitates microbial breakdown of cellulose and chitin. 2. Regulates the decomposition of SOC. | [173,174] |
| α-glucosidase | 1. Degrades carbohydrates in soils. | [175] |
| Xylosidase | 1. Regulate C cycle. 2. Produce microbes’ accessible simple sugars from compound carbohydrates. | [176,177] |
| Proteases | 1. Regulates N cycle. 2. Converts protein to oligopeptides and amino acids. | [178] |
| Ureases | 1. Hydrolyzes urea into NH3 and CO2. | [179] |
| Dehydrogenase | 1. Regulates soil microbial metabolism. | [141] |
| Peroxidase | 1. Regulates SOM decomposition. 2. Converts phenolics to form carbohydrates and proteins. | [180] |
| Reference | Agricultural Practices/Sampling Sites | Region | Soil Type | Inference | Impact on Soil Microbial Activities | Impact on Soil Enzymatic Activities |
|---|---|---|---|---|---|---|
| [163] | Peanut shell biochar addition | China | K-deficient acidic soil | Increased soil K content. | Enhanced growth and relative abundance of K-dissolving bacteria e.g., Actinomycetes, Chloroflexi, Proteobacteria, Acidobacteriota, Firmicutes. | Improved functionality of urease, dehydrogenases, and extracellular enzymes. |
| [161] | Peanut shell biochar addition | China | Yellow-brown soil | Improved soil available K. | Relative abundance of Sphingomonas, Gaiella, Elev-16S-1332, Gemmatimonas was improved by 28–377%. | N.E. |
| [181] | Soil fertilization with rock K and K-dissolving bacteria (Bacillus mucilaginosus and B. subtilis) | Egypt | Calcareous soil | Soil available K increased significantly i.e., bacterial inoculation and rock K+ bacterial inoculation increase K+ 6% and 80%, respectively. | N.E. | N.E. |
| [182] | Sudan grass (Sorghum vulgare Pers.) var Sudanensis cultivation using waste mica inoculated with Bacillus mucilaginosus | India | Alfisol | B. mucilaginosus led to around 59% increase in soil available K+ | N.E. | N.E. |
| Soil Microorganisms’ Type | Genus/Species | Mineral | References |
|---|---|---|---|
| Bacteria | Bacillus mucilaginosus (strain K02) | Feldspar | [164] |
| Paenibacillus sp. | Feldspar, mica | [183,184] | |
| Bacillus globisporus | Feldspar, muscovite, biotite | [185] | |
| Pseudomonas sp. (strain S10-3) | Biotite, muscovite | [186] | |
| Plesiomonas, Bacillus mucilaginosus, Bacillus subtillis | Feldspar | [167] | |
| Fungi | Penicillium purpurogenum, Taromyces radicus, Aspergillus fumigatus, Aureobasidium pullulan | Muscovite | [187] |
| Cenococcum geophilum Fr | Nepheline, illite, muscovite, biotite and feldspar | [188] |
| Crust Categories | Occurrence | Characteristics |
|---|---|---|
| Smooth | Hot deserts. | Composed mainly of endedaphic cyanobacteria, algae and fungi. Chemical crusting; |
| Rugose | Temperate regions. | Low surface roughness. Lots of scattered lichen and/or moss clumps. At high humidity, dominated by filamentous algae, which penetrate the soil to ~4 mm. |
| Pinnacled | Areas where the soil freezes in winter. | Dominated by cyanobacteria. Locally up to 40% moss lichen cover. Height to ~15 cm. |
| Rolling | Areas where the soil freezes in winter. High rainfall. | Lichen-moss cover. Height to ~5 cm. |
| BSCs Type | Climate | Examples of Sites |
|---|---|---|
| Smooth | Hyper-arid | Negev, Oman, Israel |
| Arid | Israel, Egypt, Iran, Negev, Clark Mountain, Gurbantunggut desert, Oman | |
| Semi-arid | Canyonlands national park, Yanchi Research Station, Colorado, Niger, Burkina Faso, Utah (USA), National Park (Australia), Chihuahuan desert | |
| No dryland | Gemrany, Hobq Desert, Tenger desert, HIhnerwasser, Neuer Lugteich, Niger | |
| Rugose | Arid | Navajo, Israel, Gurbantunggut Desert, Clark Mountain |
| Semi-arid | Negev, Mongolia, North China, Colorado Plateau, Belichon, Idaho, Mojave Desert, Southen Kalahari, New Mexico, Utah | |
| No dryland | Germany, Hobq Desert, Tenger desert, Iceland, Sweden, Florida, Slovakia | |
| Rolling | Arid | Nizzana, Hidden Valley Area |
| Semi-arid | SE Spain, El Cautivo, Aranjuez, Tengger desert, Soebatsfontein, Kane Creek Road, Gurbantunggut Desert | |
| No dryland | Hochtor, Austria, Germany, Hobq Desert, Tenger desert, Iceland, Germany, Slovakia | |
| Pinnacled | Arid | Gurbantunggut Desert, Clark Mountain |
| Semi-arid | Mongolia, North China, Colorado Plateau, Cannonville, Boulder, Big Water, Utah, Gurbantunggut Desert | |
| No dryland | Dalateqi County, Tenger desert, Germany, Gurbantunggut desert | |
| Hypolithic | No data | Gobabeb, Moerdverloren 208, Baja california |
| Only lichen | Arid | Nizanna |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharyya, S.S.; Furtak, K. Soil–Plant–Microbe Interactions Determine Soil Biological Fertility by Altering Rhizospheric Nutrient Cycling and Biocrust Formation. Sustainability 2023, 15, 625. https://doi.org/10.3390/su15010625
Bhattacharyya SS, Furtak K. Soil–Plant–Microbe Interactions Determine Soil Biological Fertility by Altering Rhizospheric Nutrient Cycling and Biocrust Formation. Sustainability. 2023; 15(1):625. https://doi.org/10.3390/su15010625
Chicago/Turabian StyleBhattacharyya, Siddhartha Shankar, and Karolina Furtak. 2023. "Soil–Plant–Microbe Interactions Determine Soil Biological Fertility by Altering Rhizospheric Nutrient Cycling and Biocrust Formation" Sustainability 15, no. 1: 625. https://doi.org/10.3390/su15010625