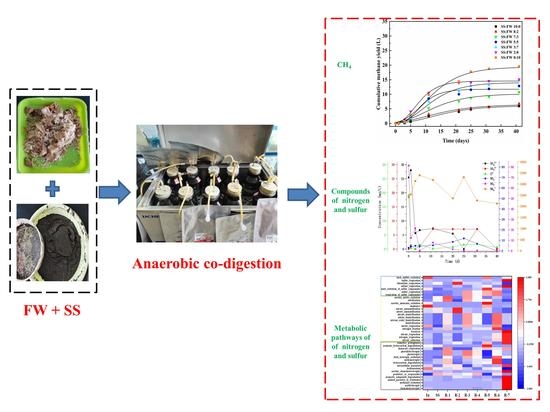
Temporal Variation of Nitrogen and Sulfur Species of Food Waste and Sludge during Anaerobic Co-Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. AcoD Experiments
2.3. Analytical Methods
2.4. Reagent
2.5. Statistical and Kinetic Analysis of Biogas Generation
2.6. Microbial Community Analysis
3. Results
3.1. Cumulative Methane Production
3.2. Kinetic Features
3.3. Stability of AcoD
3.4. Dynamics of Sulfur Chemical Forms
3.5. Dynamics of Nitrogen Chemical Forms
3.6. Microbial Community Analysis
3.6.1. Microbial Community Diversity and Richness
3.6.2. Taxonomic Composition of the Microbial Community
3.6.3. Relationships of the Bacterial Community with N and S Compounds
3.6.4. Functional Prediction of Bacterial Community
3.7. Integrated Dynamic of N and S
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AcoD | Anaerobic co-digestion |
| FW | Food waste |
| SS | Sweage sludge |
| N | Nitrogen |
| S | Sulfur |
| SCOD | Soluble chemical oxygen demand |
| VFAs | Volatile fatty acids |
| CMY | Cumulative methane yield |
| OTUs | Operational taxonomic units |
| RDA | Redundancy analysis |
| FAPROTAX | Functional Annotation of Prokaryotic Taxa |
References
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste–Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Azarmanesh, R.; Zonoozi, M.H.; Ghiasinejad, H. Characterization of food waste and sewage sludge mesophilic anaerobic co-digestion under different mixing ratios of primary sludge, secondary sludge and food waste. Biomass Bioenergy 2020, 139, 105610. [Google Scholar] [CrossRef]
- Zaharioiu, A.; Bucura, F.; Ionete, E.I.; Ionete, R.E.; Ebrasu, D.; Sandru, C.; Marin, F.; Oancea, S.; Niculescu, V.; Miricioiu, M.G.; et al. Thermochemical Decomposition of Sewage Sludge-An Eco-Friendly Solution for a Sustainable Energy Future by Using Wastes. Rev. Chim. 2020, 71, 171–181. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Zaharioiu, A.; Oancea, S.; Bucura, F.; Raboaca, M.S.; Filote, C.; Ionete, R.E. Violeta Carolina Niculescu, Marius Constantinescu.Sewage Sludge Derived Materials for CO2 Adsorption. Appl. Sci. 2021, 11, 7139. [Google Scholar] [CrossRef]
- Bardi, M.J.; Aminirad, H. Synergistic effects of co-trace elements on anaerobic co-digestion of food waste and sewage sludge at high organic load. Environ. Sci. Pollut. Res. 2020, 27, 18129–18144. [Google Scholar] [CrossRef]
- Sheng, K.C.; Chen, X.; Pan, J.M.; Klossa, R.; Wei, Y.; Ying, Y.B. Effect of ammonia and nitrate on biogas production from food waste via anaerobic digestion. Biosyst. Eng. 2013, 116, 205–212. [Google Scholar] [CrossRef]
- Fisgativa, H.; Tremier, A.; Dabert, P. Characterizing the variability of food waste quality: A need for efficient valorisation through anaerobic digestion. Waste Manag. 2016, 50, 264–274. [Google Scholar] [CrossRef]
- Dewil, R.; Baeyens, J.; Roels, J.; Steene, B.V.D. Distribution of sulphur compounds in sewage sludge treatment. Environ. Eng. Sci. 2008, 25, 879–886. [Google Scholar] [CrossRef]
- Rusín, J.; Chamrádová, K.; Basinas, P. Two-stage psychrophilic anaerobic digestion of food waste: Comparison to conventional single-stage mesophilic process. Waste Mang. 2021, 119, 172–182. [Google Scholar] [CrossRef]
- Zan, F.; Hao, T. Sulfate in anaerobic co-digester accelerates methane production from food waste and waste activated sludge. Bioresour. Technol. 2020, 298, 122536. [Google Scholar] [CrossRef]
- Raketh, M.; Jariyaboon, R.; Kongjan, P.; Trably, E.; Reungsang, A.; Sripitak, B.; Chotisuwan, S. Sulfate removal using rubber wood ash to enhance biogas production from sulfate-rich wastewater generated from a concentrated latex factory. Biochem. Eng. J. 2021, 173, 108084. [Google Scholar] [CrossRef]
- Drosg, B. Process Monitoring in Biogas Plants; IEA Bioenergy: Paris, France, 2013. [Google Scholar]
- Genfu, W. Hydrogen sulfide-producing kinetics of Shewanella oneidensis in sulfite and thiosulfate respiration. Process. Biochem. 2020, 93, 21–27. [Google Scholar] [CrossRef]
- Federation, W.E.; Association, A. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Gu, J.; Liu, R.; Cheng, Y.; Stanisavljevic, N.; Li, L.; Djatkov, D.; Peng, X.; Wang, X. Anaerobic co-digestion of food waste and sewage sludge under mesophilic and thermophilic conditions: Focusing on synergistic effects on methane production. Bioresour. Technol. 2020, 301, 122765. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, H.; Zhang, Y.; Liu, C. Improve biogas production from low-organic-content sludge through high-solids anaerobic co-digestion with food waste. Bioresour. Technol. 2016, 219, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Heo, N.H.; Park, S.C.; Kang, H. Effects of mixture ratio and hydraulic retention time on single-stage anaerobic co-digestion of food waste and waste activated sludge. J. Environ. Sci. Health Part A 2004, 39, 1739–1756. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Jeong, C.M.; Kim, N.J.; Kim, M.I.; Chang, H.N. On-site removal of H2S from biogas produced by food waste using an aerobic sludge biofilter for steam reforming processing. Biotechnol. Bioprocess Eng. 2010, 15, 505–511. [Google Scholar] [CrossRef]
- Lili, H.; Yan, Z.; Xibin, S.; Meng, H.; Weifang, L.; Xiaoyu, N.; Yuangang, W.; Zhiqiang, L. Odors emission characteristics in methanogenic stage of two-phase anaerobic fermentation of kitchen waste. Chin. J. Environ. Eng. 2014, 8, 4386–4392. [Google Scholar]
- Tian, G.; Xi, J.; Yeung, M.; Ren, G. Characteristics and mechanisms of H2S production in anaerobic digestion of food waste. Sci. Total Environ. 2020, 724, 137977. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, M.; Niu, J.; Fu, X.; Pei, X.; Chang, X.; Wei, L.; Liu, R.; Chen, G.-H.; Jiang, F. Roles of sulfite and internal recirculation on organic compound removal and the microbial community structure of a sulfur cycle-driven biological wastewater treatment process. Chemosphere 2019, 226, 825–833. [Google Scholar] [CrossRef]
- Yongsiri, C.; Hvitved-Jacobsen, T.; Vollertsen, J.; Tanaka, N. Introducing the emission process of hydrogen sulfide to a sewer process model (WATS). Water Sci. Technol. 2003, 47, 85–92. [Google Scholar] [CrossRef]
- Kim, M.; Zhang, Z.G.; Okano, H.; Yan, D.L.; Groisman, A.; Hwa, T. Need-based activation of ammonium uptake in Escherichia coli. Mol. Syst. Biol. 2012, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Benabdallah El Hadj, T.; Astals, S.; Gali, A.; Mace, S.; Mata-Alvarez, J. Ammonia influence in anaerobic digestion of OFMSW. Water Sci. Technol. 2009, 59, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Ahring, B. Anaerobic thermophilic digestion of manure at different ammonia loads: Effect of temperature. Water Res. 1994, 28, 727–731. [Google Scholar] [CrossRef]
- Hu, C.; Cao, W.; Wang, M.; Hou, F.; Yu, A.; Xie, H.; Lou, J.; Sun, C.; Liu, R. Essential regulators of iron chemical speciation distributions in anaerobic digestion of pretreated food waste: Organic volatile fatty acids or inorganic acid radicals? Bioresour. Technol. 2019, 293, 122051. [Google Scholar]
- Drewnowski, J.; Makinia, J. The role of biodegradable particulate and colloidal organic compounds in biological nutrient removal activated sludge systems. Int. J. Environ. Sci. Technol. 2014, 11, 1973–1988. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wang, X.C.; Hu, Y.; Pu, Y.; Huang, J.; Ngo, H.H.; Zeng, Y.; Li, Y. Nutrients removal performance and sludge properties using anaerobic fermentation slurry from food waste as an external carbon source for wastewater treatment. Bioresour. Technol. 2019, 271, 125–135. [Google Scholar] [CrossRef]
- Cao, S.; Qian, T.; Zhou, Y. New insights on the sludge fermentation liquid driven denitrification: Evaluation of the system performance and effluent organic matter (EfOM). Bioresour. Technol. 2019, 289, 121621. [Google Scholar] [CrossRef]
- Liu, F.; Tian, Y.; Ding, Y.; Li, Z. The use of fermentation liquid of wastewater primary sedimentation sludge as supplemental carbon source for denitrification based on enhanced anaerobic fermentation. Bioresour. Technol. 2016, 219, 6–13. [Google Scholar] [CrossRef]
- Pereira, A.D.; de Almeida Fernandes, L.; Castro, H.M.C.; Leal, C.D.; Carvalho, B.G.P.; Dias, M.F.; Nascimento, A.M.A.; de Lemos Chernicharo, C.A.; de Araujo, J.C. Nitrogen removal from food waste digestate using partial nitritation-anammox process: Effect of different aeration strategies on performance and microbial community dynamics. J. Environ. Manag. 2019, 251, 109562. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, H.; Yan, Q.; He, R.; Zhang, G. Enhanced denitrification at biocathode facilitated with biohydrogen production in a three-chambered bioelectrochemical system (BES) reactor. Chem. Eng. J. 2017, 312, 360–366. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.J.; Xie, P.; Yuan, Y.; Zhou, X.; Wang, A.-J.; Lee, D.-J.; Ren, N.-Q. Pyrosequencing reveals microbial community dynamics in integrated simultaneous desulfurization and denitrification process at different influent nitrate concentrations. Chemosphere 2017, 171, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.D.; Cabezas, A.; Etchebehere, C.; Chernicharo, C.A.d.L.; de Araújo, J.C. Microbial communities in anammox reactors: A review. Environ. Technol. Rev. 2017, 6, 74–93. [Google Scholar] [CrossRef]
- Wang, B.; Ma, J.; Zhang, L.; Su, Y.; Xie, Y.; Ahmad, Z.; Xie, B. The synergistic strategy and microbial ecology of the anaerobic co-digestion of food waste under the regulation of domestic garbage classification in China. Sci. Total Environ. 2021, 765, 144632. [Google Scholar] [CrossRef] [PubMed]
- Mustakhimov, I.; Kalyuzhnaya, M.G.; Lidstrom, M.E.; Chistoserdova, L. Insights into denitrification in Methylotenera mobilis from denitrification pathway and methanol metabolism mutants. J. Bacteriol. 2013, 195, 2207–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Eugenio, G.; Fardeau, M.-L.; Cayol, J.-L.; Patel, B.K.; Thomas, P.; Macarie, H.; Garcia, J.-L.; Ollivier, B. Sporanaerobacter acetigenes gen. nov., sp. nov., a novel acetogenic, facultatively sulfur-reducing bacterium. Int. J. Syst. Evol. Microbiol. 2002, 52, 1217–1223. [Google Scholar] [PubMed] [Green Version]
- Wang, J.; Lei, Z.; Wang, L.; Yang, S.; Zhao, Y.; Li, Y.-Y.; Chen, R. Insight into using up-flow anaerobic sludge blanket-anammox to remove nitrogen from an anaerobic membrane reactor during mainstream wastewater treatment. Bioresour. Technol. 2020, 314, 123710. [Google Scholar] [CrossRef] [PubMed]
- Kindaichi, T.; Nierychlo, M.; Kragelund, C.; Nielsen, J.L.; Nielsen, P.H. High and stable substrate specificities of microorganisms in enhanced biological phosphorus removal plants. Environ. Microbiol. 2013, 15, 1821–1831. [Google Scholar] [CrossRef]
- Lee, J.; Kim, E.; Han, G.; Tongco, J.V.; Shin, S.G.; Hwang, S. Microbial communities underpinning mesophilic anaerobic digesters treating food wastewater or sewage sludge: A full-scale study. Bioresour. Technol. 2018, 259, 388–397. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Zhang, Y.B.; Zhao, H.M.; Quan, X. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell. Bioresour. Technol. 2015, 200, 235–244. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Q.; Li, Z.L.; Ma, X.D.; Hou, Y.N.; Ren, N.Q.; Wang, A.J. Relationship between functional bacteria in a denitrification desulfurization system under autotrophic, heterotrophic, and mixotrophic conditions. Water Res. 2020, 188, 116526. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.B. Nitrification in marine systems. Nitrogen Mar. Environ. 2008, 2, 199–261. [Google Scholar]
- Siles, J.; Brekelmans, J.; Martín, M.; Chica, A.; Martín, A. Impact of ammonia and sulphate concentration on thermophilic anaerobic digestion. Bioresour. Technol. 2010, 101, 9040–9048. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E.; Stewart, F.J.; Thamdrup, B.; De Brabandere, L.; Dalsgaard, T.; Delong, E.F.; Revsbech, N.P.; Ulloa, O. A cryptic sulfur cycle in oxygen-minimum–zone waters off the Chilean coast. Science 2010, 330, 1375–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominika, G.; Joanna, M.; Jacek, M. Sulfate reducing ammonium oxidation (SULFAMMOX) process under anaerobic conditions. Environ. Technol. Innov. 2021, 22, 101416. [Google Scholar] [CrossRef]










| Parameter | SS:FW | ||||||
|---|---|---|---|---|---|---|---|
| 10:0 | 8:2 | 7:3 | 5:5 | 3:7 | 2:8 | 0:10 | |
| CMY | 5.95 | 6.75 | 10.73 | 12.84 | 14.81 | 15.12 | 19.57 |
| P | 6.04 | 6.48 | 10.14 | 11.75 | 14.04 | 14.56 | 19.37 |
| Rm | 0.27 | 0.27 | 0.50 | 1.13 | 0.81 | 1.30 | 1.04 |
| λ | 3.28 | 1.11 | 2.00 | 3.26 | 2.85 | 2.12 | 2.65 |
| Adj R2 | 0.99 | 0.98 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 |
| Sample | OTUs | Shannon | Chao | Ace | Coverage |
|---|---|---|---|---|---|
| SS | 904 | 5.05 | 1004.96 | 993.88 | 0.99 |
| Inoculum | 889 | 3.75 | 1142.05 | 1137.53 | 0.99 |
| R-1 | 991 | 4.22 | 1223.69 | 1213.37 | 0.99 |
| R-2 | 878 | 3.81 | 1170.19 | 1156.09 | 0.99 |
| R-3 | 880 | 3.83 | 1120.64 | 1146.37 | 0.99 |
| R-4 | 861 | 3.78 | 1203.01 | 1176.86 | 0.99 |
| R-5 | 810 | 3.78 | 1034.32 | 1053.54 | 0.99 |
| R-6 | 871 | 3.77 | 1170.07 | 1219.51 | 0.99 |
| R-7 | 946 | 4.19 | 1183.03 | 1151.82 | 0.99 |
| Sample | OTUs | Shannon | Chao | Ace | Coverage |
|---|---|---|---|---|---|
| SS | 81 | 2.07 | 82.5 | 82.31 | 0.99 |
| Inoculum | 85 | 2.12 | 107.67 | 100.11 | 0.99 |
| R-1 | 63 | 2.30 | 74 | 80.71 | 0.99 |
| R-2 | 77 | 2.20 | 89.0 | 94.09 | 0.99 |
| R-3 | 82 | 2.26 | 88.5 | 93.61 | 0.99 |
| R-4 | 70 | 2.15 | 75.14 | 76.29 | 0.99 |
| R-5 | 75 | 2.26 | 105 | 92.76 | 0.99 |
| R-6 | 90 | 2.13 | 96 | 97.19 | 0.99 |
| R-7 | 73 | 2.30 | 80.5 | 79.65 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, P.; Zhang, Y.; Ge, X.; Qian, Z. Temporal Variation of Nitrogen and Sulfur Species of Food Waste and Sludge during Anaerobic Co-Digestion. Sustainability 2022, 14, 4982. https://doi.org/10.3390/su14094982
Kang P, Zhang Y, Ge X, Qian Z. Temporal Variation of Nitrogen and Sulfur Species of Food Waste and Sludge during Anaerobic Co-Digestion. Sustainability. 2022; 14(9):4982. https://doi.org/10.3390/su14094982
Chicago/Turabian StyleKang, Pengzhou, Yuxiu Zhang, Xiaopeng Ge, and Zhi Qian. 2022. "Temporal Variation of Nitrogen and Sulfur Species of Food Waste and Sludge during Anaerobic Co-Digestion" Sustainability 14, no. 9: 4982. https://doi.org/10.3390/su14094982