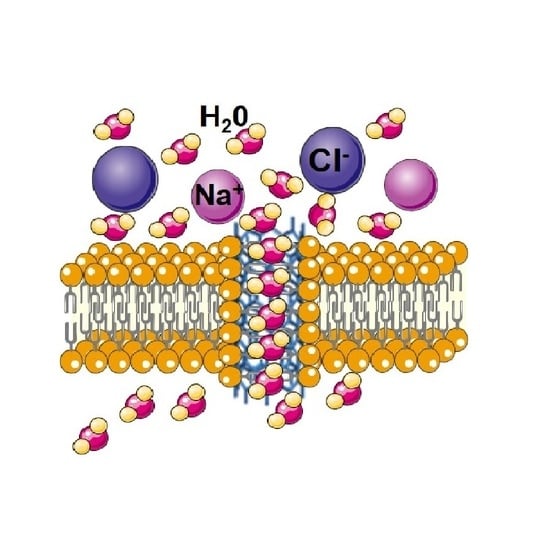
The Bionic Water Channel of Ultra-Short, High Affinity Carbon Nanotubes with High Water Permeability and Proton Selectivity
Abstract
:1. Introduction
2. Experiment Section
2.1. CNTCs Prepared by Ultrasonic Cutting
2.2. UV-vis-NIR Spectrum of CNTCs
2.3. Dynamic Light Scattering Test the Particle Size of CNTCs
2.4. Raman Spectra of CNTCs
2.5. Water Permeability Test of CNTCs
2.6. Ion Selectivity Test of CNTCs
3. Results
3.1. CNTCs Synthesis
3.2. Characterization of Water Permeability
3.3. The Selectivity of CNTCs to Protons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Werber, J.R.; Menachem, E. Permselectivity limits of biomimetic desalination membranes. Sci. Adv. 2018, 4, eaar8266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, R.; Wicaksana, F. Preparation of high performance nanofiltration (NF) membranes incorporated with aquaporin Z. J. Membr. Sci. 2014, 450, 181–188. [Google Scholar] [CrossRef]
- Li, X.; Loh, C.H.; Wang, R. Fabrication of a robust high-performance FO membrane by optimizing substrate structure and incorporating aquaporin into selective layer. J. Membr. Sci. 2016, 525, 257–268. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Wang, X. Layer-by-Layer Assembly of Aquaporin Z-Incorporated Biomimetic Membranes for Water Purification. Environ. Sci. Technol. 2015, 49, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; He, F.; Wang, B. An aquaporin-based vesicle-embedded polymeric membrane for low energy water filtration. J. Mater. Chem. A 2013, 1, 7592. [Google Scholar] [CrossRef]
- Bowen, W.R. Biomimetic separations—Learning from the early development of biological membranes. Desalination 2006, 199, 225–227. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin, Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.X.; Si, W.; Erbakan, M. Highly permeable artificial water channels that can self-assemble into two-dimensional arrays. Proc. Natl. Acad. Sci. USA 2015, 112, 9810–9815. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.B.; Chen, Z.; Tang, G. Single-molecular artificial transmembrane water channels. J. Am. Chem. Soc. 2012, 134, 8384. [Google Scholar] [CrossRef]
- Licsandru, E.; Kocsis, I.; Shen, Y.; Murail, S.; Legrand, Y.M.; Van Der Lee, A.; Tsai, D.; Baaden, M.; Kumar, M.; Barboiu, M. Salt-Excluding Artificial Water Channels Exhibiting Enhanced Dipolar Water and Proton Translocation. J. Am. Chem. Soc. 2016, 138, 5403–5409. [Google Scholar] [CrossRef]
- Sanborn, J.R.; Chen, X.; Yao, Y.C. Carbon Nanotube Porins in Amphiphilic Block Copolymers as Fully Synthetic Mimics of Biological Membranes. Adv. Mater. 2018, 30, 1803355.1–1803355.9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Zhu, B.; Liang, S. Impact of –C2H5 and –OH Functionalizations on the Water Flow Blockage in Carbon Nanotubes. J. Phys. Chem. C 2018, 122, 11807–11813. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Ning, L. Hyperfast Water Transport through Biomimetic Nanochannels from Peptide-Attached (pR)-pillar[5]arene. Small 2019, 15, 1804678. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Chen, L.; Hu, X.B. Selective artificial transmembrane channels for protons by formation of water wires. Angew. Chem. Int. Ed. 2015, 123, 12772–12776. [Google Scholar] [CrossRef]
- Xie, W.; Low, J.W.J.; Armugam, A. Regulation of Aquaporin Z osmotic permeability in ABA tri-block copolymer. AIMS Biophys. 2015, 2, 381–397. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P. Bayesian inference of phylogeny and its impact on evolutionary biology. Science 2001, 294, 2310–2314. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, H.; Steinbronn, C. Enhancement of Proton Conductance by Mutations of the Selectivity Filter of Aquaporin-1. J. Mol. Biol. 2011, 407, 607–620. [Google Scholar] [CrossRef]
- Janet, T.; Jaume, T. Can Stabilization and Inhibition of Aquaporins Contribute to Future Development of Biomimetic Membranes? Membranes 2015, 5, 352–368. [Google Scholar]
- Barboiu, M.; Gilles, A. From Natural to Bioassisted and Biomimetic Artificial Water Channel Systems. Acc. Chem. Res. 2013, 46, 2814. [Google Scholar] [CrossRef]
- Shen, Y.X.; Saboe, P.O.; Sines, I.T. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Noy, A.; Wanunu, M. A new type of artificial water channels. Nat. Nanotechnol. 2019, 15, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Hoenlein, W.; Kreupl, F.; Duesberg, G.S. Carbon nanotube applications in microelectronics. IEEE Trans. Compon. Packaging Technol. 2004, 27, 629–634. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, W.; Li, P. Improving ultrafiltration membrane performance with pre-deposited carbon nanotubes/nanofibers layers for drinking water treatment. Chemosphere 2019, 234, 545–557. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Escalada, A.; Frolov, V.A. Synthesis, lipid membrane incorporation, and ion permeability testing of carbon nanotube porins. Nat. Protoc. 2016, 11, 2029–2047. [Google Scholar] [CrossRef]
- Langecker, M. Synthetic Lipid Membrane Channels Formed by Designed DNA Nanostructures. Science 2012, 338, 932–936. [Google Scholar] [CrossRef] [Green Version]
- Tunuguntla, R.H.; Chen, X.; Belliveau, A. High-Yield Synthesis and Optical Properties of Carbon Nanotube Porins. J. Phys. Chem. C 2017, 121, 3117–3125. [Google Scholar] [CrossRef]
- Guido, P.; Micah, J.G.; Philippe, P. Competing mechanisms and scaling laws for carbon nanotube scission by ultrasonication. Proc. Natl. Acad. Sci. USA 2012, 109, 11599–11604. [Google Scholar]
- Chew, H.B.; Moon, M.W.; Lee, K.R. Compressive dynamic scission of carbon nanotubes under sonication: Fracture by atomic ejection. In Proceedings of the Royal Society A: Mathematical. Phys. Eng. Sci. 2011, 2129, 1270–1289. [Google Scholar]
- Bachilo, S.M.; Strano, M.S.; Kittrell, C.; Hauge, R.H.; Smalley, R.E.; Weisman, R.B. Structure-assigned optical spectra of singlewalled carbon nanotubes. Science 2002, 298, 1261–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Zaric, S.; Daranciang, D.; Welsher, K.; Lu, Y.; Li, X.; Dai, H. Optical properties of ultrashort semiconducting single-walled carbon nanotube capsules down to sub-10 nm. J. Am. Chem. Soc. 2008, 130, 6551–6555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opilik, L.; Bauer, T.; Schmid, T.; Stadler, J.; Zenobi, R. Nanoscale chemical imaging of segregated lipid domains using tipenhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 9978–9981. [Google Scholar] [CrossRef] [PubMed]
- Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes. Nature 2014, 514, 612–615. [CrossRef] [PubMed]
- Zuo, G.; Shen, R.; Ma, S. Transport properties of single-file water molecules inside a carbon nanotube biomimicking water channel. ACS Nano 2010, 4, 205. [Google Scholar] [CrossRef]
- Sheng, J.; Zhu, Q.; Zeng, X. Promotion of Water Channels for Enhanced Ion Transport in 14 nm Diameter Carbon Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 11009. [Google Scholar] [CrossRef]
- Murata, K.; Kaoru, M.; Hirai, T. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef]
- Kitchen, P.; Conner, A.C. Control of the Aquaporin-4 Channel Water Permeability by Structural Dynamics of Aromatic/Arginine Selectivity Filter Residues. Biochemistry 2015, 54, 6753–6755. [Google Scholar] [CrossRef]
- Montesarchio, D.; Coppola, C.; Boccalon, M. Carbohydrate-based synthetic ion transporters. Carbohyd. Res. 2012, 356, 62–74. [Google Scholar] [CrossRef]
- Tobias, D.J.; Hemminger, J.C. Getting Specific about Specific Ion Effects. Science 2008, 319, 1197–1198. [Google Scholar] [CrossRef]
- Pomès, R.; Roux, B. Free energy profiles for H+ conduction along hydrogen-bonded chains of water molecules. Biophys. J. 1998, 75, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Groot, B.L.D.; Engel, A.; Grubmüller, H. The Structure of the Aquaporin-1 Water Channel: A Comparison between Cryo-electron Microscopy and X-ray Crystallography. J. Mol. Biol. 2003, 325, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Renal Phys. 2000, 278, F13. [Google Scholar] [CrossRef] [PubMed]
- Braun-Sand, S.; Burykin, A.; Chu, Z.T. Realistic simulations of proton transport along the gramicidin channel: Demonstrating the importance of solvation effects. J. Phys. Chem. B 2005, 109, 583. [Google Scholar] [CrossRef] [PubMed]
- Wraight, C.A. Chance and design—Proton transfer in water, channels and bioenergetic proteins. Biol. Biol. Acta 2006, 1757, 886–912. [Google Scholar] [CrossRef] [Green Version]
- Dellago, C.; Hummer, G. Kinetics and Mechanism of Proton Transport across Membrane Nanopores. Phys. Rev. Lett. 2006, 97, 245901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunuguntla, R.; Allen, F.; Kim, K. Ultrafast proton transport in sub-1-nm diameter carbon nanotube porins. Biophys. J. 2016, 110, 338a. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Voth, G.A. Origins of Proton Transport Behavior from Selectivity Domain Mutations of the Aquaporin-1 Channel. Biophys. J. 2006, 90, L73–L75. [Google Scholar] [CrossRef] [Green Version]
- Nemeth-Cahalan, K.L.; Hall, J.E. pH and Calcium Regulate the Water Permeability of Aquaporin 0. J. Biol. Chem. 2000, 275, 6777–6782. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zhou, B.; Liu, J.; Zhao, H. The Bionic Water Channel of Ultra-Short, High Affinity Carbon Nanotubes with High Water Permeability and Proton Selectivity. Sustainability 2021, 13, 102. https://doi.org/10.3390/su13010102
Liu G, Zhou B, Liu J, Zhao H. The Bionic Water Channel of Ultra-Short, High Affinity Carbon Nanotubes with High Water Permeability and Proton Selectivity. Sustainability. 2021; 13(1):102. https://doi.org/10.3390/su13010102
Chicago/Turabian StyleLiu, Guangli, Bin Zhou, Jinwei Liu, and Huazhang Zhao. 2021. "The Bionic Water Channel of Ultra-Short, High Affinity Carbon Nanotubes with High Water Permeability and Proton Selectivity" Sustainability 13, no. 1: 102. https://doi.org/10.3390/su13010102