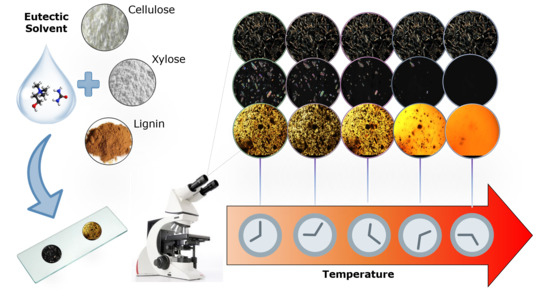
Fast and Efficient Method to Evaluate the Potential of Eutectic Solvents to Dissolve Lignocellulosic Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Eutectic Solvents Preparation
2.3. Thermogravimetric Analysis (TGA)
2.4. Dissolution of Solutes by Polarized Optical Microscopy or Simple Optical Microscopy
2.5. Dissolution Kinetics of Xylose
3. Results
3.1. α-cellulose and Microcrystalline Cellulose Dissolution
3.2. Alkaline Lignin Dissolution
3.3. Xylose Dissolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Mei, Q.; Shen, X.; Liu, H.; Han, B. Selectively transform lignin into value-added chemicals. Chin. Chem. Lett. 2019, 30, 15–24. [Google Scholar] [CrossRef]
- Van Haveren, J.; Scott, E.L.; Sanders, J. Bulk chemicals from biomass Jacco. Biofuels Bioprod. Biorefining 2008, 2, 41–57. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—a review. Cell. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Higuchi, T. Microbial degradation of lignin: Role of lignin peroxidase, manganese peroxidase, and laccase. Proc. Jpn. Acad. 2004, 80, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Bugg, T.D.H.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011, 28, 1883–1896. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology of Lignocellulose Materials, 1st ed.; Chemical Industry Press: Beijing, China, 2014; Volume 218419139, ISBN 9789400768970. [Google Scholar]
- Gandini, A.; Belgacem, M.N. Lignins as components of macromolecular materials. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 243–271. [Google Scholar]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. Natl. Renew. Energy Lab. 2011, 1–147. [Google Scholar] [CrossRef] [Green Version]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Guthrie, F. LII. On eutexia. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1884, 17, 462–482. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Cholride Urea Mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solution Chem. 2018, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R. Deep Eutectic Solvents Formed Between Choline Chloride and Carboxylic Acids. J. Am. Chem. Soc. 2004, 126, 9142. [Google Scholar] [CrossRef]
- Gorke, J.; Srienc, F.; Kazlauskas, R. Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol. Bioprocess Eng. 2010, 15, 40–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Benoit, M.; Dea Oliveiraa Vigier, K.; Barrault, J.; Jérǒme, F. Green and inexpensive choline-derived solvents for cellulose decrystallization. Chem. A Eur. J. 2012, 18, 1043–1046. [Google Scholar] [CrossRef]
- Bagh, F.S.G.; Shahbaz, K.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Zinc (II) chloride-based deep eutectic solvents for application as electrolytes: Preparation and characterization. J. Mol. Liq. 2015, 204, 76–83. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; Hayyan, M.; AlSaadi, M.A.H.; Mirghani, M.E.S.; Hashim, M.A. Functionalization of carbon nanotubes using eutectic mixtures: A promising route for enhanced aqueous dispersibility and electrochemical activity. Chem. Eng. J. 2017, 311, 326–339. [Google Scholar] [CrossRef]
- Gu, L.; Huang, W.; Tang, S.; Tian, S.; Zhang, X. A novel deep eutectic solvent for biodiesel preparation using a homogeneous base catalyst. Chem. Eng. J. 2015, 259, 647–652. [Google Scholar] [CrossRef]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153. [Google Scholar] [CrossRef]
- Makoś, P.; Słupek, E.; Gębicki, J. Extractive detoxification of feedstocks for the production of biofuels using new hydrophobic deep eutectic solvents—Experimental and theoretical studies. J. Mol. Liq. 2020, 308, 113101. [Google Scholar] [CrossRef]
- Tian, D.; Guo, Y.; Hu, J.; Yang, G.; Zhang, J.; Luo, L.; Xiao, Y.; Deng, S.; Deng, O.; Zhou, W.; et al. Acidic deep eutectic solvents pretreatment for selective lignocellulosic biomass fractionation with enhanced cellulose reactivity. Int. J. Biol. Macromol. 2020, 142, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Procentese, A.; Johnson, E.; Orr, V.; Garruto Campanile, A.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef]
- Xu, G.C.; Ding, J.C.; Han, R.Z.; Dong, J.J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. 2016, 23, 9265–9275. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Green Chemistry Cutting-edge research for a greener sustainable future Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization Unique low-molecular-weight lignin with hig. Green Chem. 2016, 18, 5071–5378. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Xia, S.-Q.; Ma, P.-S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef]
- Soares, B.; Tavares, D.J.P.; Amaral, J.L.; Silvestre, A.J.D.; Freire, C.S.R.; Coutinho, J.A.P. Enhanced Solubility of Lignin Monomeric Model Compounds and Technical Lignins in Aqueous Solutions of Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 4056–4065. [Google Scholar] [CrossRef]
- Lynam, J.G.; Kumar, N.; Wong, M.J. Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour. Technol. 2017, 238, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.-D.; Feng, G.-J.; Ye, M.; Huang, C.-M.; Zhang, Y. Significantly enhanced enzymatic hydrolysis of rice straw via a high-performance two-stage deep eutectic solvents synergistic pretreatment. Bioresour. Technol. 2017, 238, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.; Silvestre, A.J.D.; Rodrigues Pinto, P.C.; Freire, C.S.R.; Coutinho, J.A.P. Hydrotropy and Cosolvency in Lignin Solubilization with Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149. [Google Scholar] [CrossRef]
- Morais, E.S.; Mendonåa, P.V.; Coelho, J.F.J.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Deep Eutectic Solvent Aqueous Solutions as Efficient Media for the Solubilization of Hardwood Xylans. ChemSusChem 2018, 1–11. [Google Scholar] [CrossRef]
- Ji, H.; Lv, P. Mechanistic insights into the lignin dissolution behaviors of a recyclable acid hydrotrope, deep eutectic solvent (DES), and ionic liquid (IL). Green Chem. 2020, 22, 1378–1387. [Google Scholar] [CrossRef]
- Balaji, C.; Banerjee, T.; Goud, V.V. COSMO-RS based predictions for the extraction of lignin from lignocellulosic biomass using ionic liquids: Effect of cation and anion combination. J. Solut. Chem. 2012, 41, 1610–1630. [Google Scholar] [CrossRef]
- Casas, A.; Palomar, J.; Alonso, M.V.; Oliet, M.; Omar, S.; Rodriguez, F. Comparison of lignin and cellulose solubilities in ionic liquids by COSMO-RS analysis and experimental validation. Ind. Crops Prod. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Jeliński, T.; Cysewski, P. Application of a computational model of natural deep eutectic solvents utilizing the COSMO-RS approach for screening of solvents with high solubility of rutin. J. Mol. Model. 2018, 24, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic liquid as a green solvent for lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Fitzpatrick, M.; Champagne, P.; Cunningham, M.F.; Falkenburger, C. Application of optical microscopy as a screening technique for cellulose and lignin solvent systems. Can. J. Chem. Eng. 2012, 90, 1142–1152. [Google Scholar] [CrossRef]
- Zavrel, M.; Bross, D.; Funke, M.; Büchs, J.; Spiess, A.C. High-throughput screening for ionic liquids dissolving (ligno-)cellulose. Bioresour. Technol. 2009, 100, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Andanson, J.M.; Bordes, E.; Devémy, J.; Leroux, F.; Pádua, A.A.H.; Gomes, M.F.C. Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 2528–2538. [Google Scholar] [CrossRef] [Green Version]
- Dias, R.M.; Sosa, F.H.B.; da Costa, M.C. Dissolution of lignocellulosic biopolymers in ethanolamine—Based protic ionic liquids. Polym. Bull. 2019. [Google Scholar] [CrossRef]
- Prausnitz, J.M.; Lichtenthaler, R.N.; de Azevedo, E.G. Molecular Thermodynamics of Fluid-Phase Equilibria; Pearson Education: London, UK, 1998; ISBN 9780132440509. [Google Scholar]
- Dadi, A.P.; Varanasi, S.; Schall, C.A. Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol. Bioeng. 2006, 95, 904–910. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.A.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Pharm. 2019, 13, 100162. [Google Scholar] [CrossRef]
- Mayer, C.; Moritz, R.; Kirschner, C.; Borchard, W.; Maibaum, R.; Wingender, J.; Flemming, H.C. The role of intermolecular interactions: Studies on model systems for bacterial biofilms. Int. J. Biol. Macromol. 1999, 26, 3–16. [Google Scholar] [CrossRef]
- Mahkam, M. Starch-based polymeric carriers for oral-insulin delivery. J. Biomed. Mater. Res. Part A 2010, 92, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Baker, G.A.; Song, Z.; Olubajo, O.; Crittle, T.; Peters, D. Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem. 2008, 10, 696–705. [Google Scholar] [CrossRef]
- Xu, A.; Wang, J.; Wang, H. Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem. 2010, 12, 268–275. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Xun, Y.C.; You, Z.T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar]
- Wahlström, R.; Hiltunen, J.; Mariah, P.d.S.N.S.; Kruus, S.V.; Kruus, K. Comparison of three deep eutectic solvents and 1-ethyl-3-methylimidazolium acetate in the pretreatment of lignocellulose: Effect on enzyme stability, lignocellulose digestibility and one-pot hydrolysis. RSC Adv. 2016, 6, 68100–68110. [Google Scholar] [CrossRef]
- Häkkinen, R.; Abbott, A. Solvation of carbohydrates in five choline chloride-based deep eutectic solvents and the implication for cellulose solubility. Green Chem. 2019, 21, 4673–4682. [Google Scholar] [CrossRef]
- Dobbs, W.; Douce, L.; Allouche, L.; Louati, A.; Malbosc, F.; Welter, R. New ionic liquid crystals based on imidazolium salts. New J. Chem. 2006, 30, 528–532. [Google Scholar] [CrossRef]
- Malaeke, H.; Housaindokht, M.R.; Monhemi, H.; Izadyar, M. Deep eutectic solvent as an efficient molecular liquid for lignin solubilization and wood delignification. J. Mol. Liq. 2018, 263, 193–199. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Xia, Q.; Guo, B.; Wang, Q.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Lignin–Carbohydrate Complexes and Ultrafast Extraction of Lignin Oligomers from Wood Biomass by Microwave-Assisted Treatment with Deep Eutectic Solvent. ChemSusChem 2017, 10, 1692–1700. [Google Scholar] [CrossRef] [Green Version]
- Melro, E.; Alves, L.; Antunes, F.E.; Medronho, B. A brief overview on lignin dissolution. J. Mol. Liq. 2018, 265, 578–584. [Google Scholar] [CrossRef]






| Quaternary Ammonium Salt (HBA) | Hydrogen-Bonding Donors (HBD) | HBA:HBD (mol) | Abbreviation |
|---|---|---|---|
 |  | 1:2 | ChCl:EtGLY |
 | 1:2 | ChCl:GLY | |
 | 1:2 | ChCl:PROP | |
 | 1:2 | ChCl:UREA | |
 | 1:2 | ChCl:LAC | |
 | 1:1 | ChCl:OXA |
| ES | 5 wt% | 10 wt% | 15 wt% | 20 wt% | 25 wt% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | t (min) | T (°C) | t (min) | T (°C) | t (min) | T (°C) | t (min) | T (°C) | t (min) | |
| ChCl:GLY | 58.6 | 28.6 | 66.5 | 36.5 | 84.4 | 54.4 | * | * | * | * |
| ChCl:EtGLY | 50.8 | 20.8 | 56.6 | 26.5 | 63.5 | 33.5 | 68.7 | 38.7 | 77.1 | 47.1 |
| ChCl:PROP | 91.4 | 61.4 | * | * | * | * | * | * | * | * |
| ChCl:UREA | 80.4 | 50.5 | 84.4 | 54.4 | 91.3 | 61.3 | 100 | 70 | * | * |
| ChCl:LAC | 50.8 | 20.8 | 57.6 | 27.6 | 71.5 | 41.5 | 86.5 | 56.5 | * | * |
| ChCl:OXA | 49.6 | 19.6 | 59.5 | 29.5 | 72.6 | 42.6 | 82.4 | 52.4 | * | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa, F.H.B.; Dias, R.M.; da Costa Lopes, A.M.; Coutinho, J.A.P.; da Costa, M.C. Fast and Efficient Method to Evaluate the Potential of Eutectic Solvents to Dissolve Lignocellulosic Components. Sustainability 2020, 12, 3358. https://doi.org/10.3390/su12083358
Sosa FHB, Dias RM, da Costa Lopes AM, Coutinho JAP, da Costa MC. Fast and Efficient Method to Evaluate the Potential of Eutectic Solvents to Dissolve Lignocellulosic Components. Sustainability. 2020; 12(8):3358. https://doi.org/10.3390/su12083358
Chicago/Turabian StyleSosa, Filipe H. B., Rafael M. Dias, André M. da Costa Lopes, João A. P. Coutinho, and Mariana C. da Costa. 2020. "Fast and Efficient Method to Evaluate the Potential of Eutectic Solvents to Dissolve Lignocellulosic Components" Sustainability 12, no. 8: 3358. https://doi.org/10.3390/su12083358