Recent Advances in Alginate-Based Hydrogels for Cell Transplantation Applications
Abstract
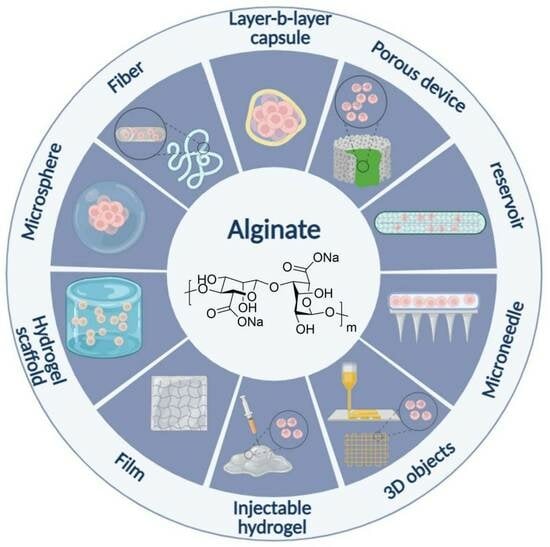
:1. Introduction
2. Alginate
3. Cell Encapsulation Techniques
3.1. Macroencapsulation
3.1.1. Device Based on Porous Membranes
3.1.2. Anisotropic 3D Scaffolds
3.1.3. Hydrogel Fibers
3.1.4. 3D Printing Devices

3.1.5. Injectable Hydrogels
Nervous System Stem Cell Transplantation Therapies
Cardiovascular Stem Cell Transplantation Therapies
Traumatic Brain Injury
Bone Regeneration
3.2. Microencapsulation
3.3. Nanocrocapsulation (Layer-by-Layer (LbL) Self-Assembly)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kharbikar, B.N.; Mohindra, P.; Desai, T.A. Biomaterials to Enhance Stem Cell Transplantation. Cell Stem Cell 2022, 29, 692–721. [Google Scholar] [CrossRef] [PubMed]
- Mitrousis, N.; Fokina, A.; Shoichet, M.S. Biomaterials for Cell Transplantation. Nat. Rev. Mater. 2018, 3, 441–456. [Google Scholar] [CrossRef]
- Iansante, V.; Mitry, R.R.; Filippi, C.; Fitzpatrick, E.; Dhawan, A. Human Hepatocyte Transplantation for Liver Disease: Current Status and Future Perspectives. Pediatr. Res. 2018, 83, 232–240. [Google Scholar] [CrossRef] [PubMed]
- de Souza, Y.E.D.M.; Chaib, E.; de Lacerda, P.G.; Crescenzi, A.; Bernal-Filho, A.; D’Albuquerque, L.A.C. Islet Transplantation in Rodents. Do Encapsulated Islets Really Work? Arq. Gastroenterol. 2011, 48, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes Derived from Human Embryonic Stem Cells in Pro-Survival Factors Enhance Function of Infarcted Rat Hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J.; Lakey, J.R.T.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Rafii, S.; Lyden, D. Therapeutic Stem and Progenitor Cell Transplantation for Organ Vascularization and Regeneration. Nat. Med. 2003, 9, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Cell Therapy for Spinal Cord Regeneration. Adv. Drug Deliv. Rev. 2008, 60, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Sortwell, C.E.; Pitzer, M.R.; Collier, T.J. Time Course of Apoptotic Cell Death within Mesencephalic Cell Suspension Grafts: Implications for Improving Grafted Dopamine Neuron Survival. Exp. Neurol. 2000, 165, 268–277. [Google Scholar] [CrossRef]
- Zhang, M.; Methot, D.; Poppa, V.; Fujio, Y.; Walsh, K.; Murry, C.E. Cardiomyocyte Grafting for Cardiac Repair: Graft Cell Death and Anti-Death Strategies. J. Mol. Cell. Cardiol. 2001, 33, 907–921. [Google Scholar] [CrossRef]
- Young, S.A.; Sherman, S.E.; Cooper, T.T.; Brown, C.; Anjum, F.; Hess, D.A.; Flynn, L.E.; Amsden, B.G. Mechanically Resilient Injectable Scaffolds for Intramuscular Stem Cell Delivery and Cytokine Release. Biomaterials 2018, 159, 146–160. [Google Scholar] [CrossRef]
- Leverett, L.B.; Hellums, J.D.; Alfrey, C.P.; Lynch, E.C. Red Blood Cell Damage by Shear Stress. Biophys. J. 1972, 12, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Mauck, R.L.; Gerecht, S. To Serve and Protect: Hydrogels to Improve Stem Cell-Based Therapies. Cell Stem Cell 2016, 18, 13–15. [Google Scholar] [CrossRef]
- Payne, S.L.; Anandakumaran, P.N.; Varga, B.V.; Morshead, C.M.; Nagy, A.; Shoichet, M.S. In Vitro Maturation of Human iPSC-Derived Neuroepithelial Cells Influences Transplant Survival in the Stroke-Injured Rat Brain. Tissue Eng. Part A 2018, 24, 351–360. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wang, F.-Y.; Chan, Y.-S.; Huang, C.; Huang, Y.-Y. Biofabricating Hollow Microneedle Array with Controllable Microstructure for Cell Transplantation. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.U.; Bolli, R. Cardiac Stem Cell Therapy for Cardiac Repair. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 324. [Google Scholar] [CrossRef]
- Li, W.; Ma, N.; Ong, L.-L.; Nesselmann, C.; Klopsch, C.; Ladilov, Y.; Furlani, D.; Piechaczek, C.; Moebius, J.M.; Lützow, K.; et al. Bcl-2 Engineered MSCs Inhibited Apoptosis and Improved Heart Function. Stem Cells 2007, 25, 2118–2127. [Google Scholar] [CrossRef]
- Weir, G.C.; Susan, B.-W. Scientific and Political Impediments to Successful Islet Transplantation. Diabetes 1997, 46, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Lin, Z.; Song, P.; Quan, D.; Bai, Y. Biomaterial-Based Schwann Cell Transplantation and Schwann Cell-Derived Biomaterials for Nerve Regeneration. Front. Cell. Neurosci. 2022, 16, 926222. [Google Scholar] [CrossRef]
- Cooke, M.J.; Vulic, K.; Shoichet, M.S. Design of Biomaterials to Enhance Stem Cell Survival When Transplanted into the Damaged Central Nervous System. Soft Matter 2010, 6, 4988–4998. [Google Scholar] [CrossRef]
- Yasuhara, T.; Kawauchi, S.; Kin, K.; Morimoto, J.; Kameda, M.; Sasaki, T.; Bonsack, B.; Kingsbury, C.; Tajiri, N.; Borlongan, C.V.; et al. Cell Therapy for Central Nervous System Disorders: Current Obstacles to Progress. CNS Neurosci. Ther. 2020, 26, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Cell Culture on a Thermo-Responsive Polymer Surface|Nature Biotechnology. Available online: https://www.nature.com/articles/nbt0990-854 (accessed on 12 December 2023).
- Zurina, I.M.; Presniakova, V.S.; Butnaru, D.V.; Timashev, P.S.; Rochev, Y.A.; Liang, X.-J. Towards Clinical Translation of the Cell Sheet Engineering: Technological Aspects. Smart Mater. Med. 2023, 4, 146–159. [Google Scholar] [CrossRef]
- Thummarati, P.; Laiwattanapaisal, W.; Nitta, R.; Fukuda, M.; Hassametto, A.; Kino-oka, M. Recent Advances in Cell Sheet Engineering: From Fabrication to Clinical Translation. Bioengineering 2023, 10, 211. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Gao, Y.; Yang, L. Cell Sheet Technology: A Promising Strategy in Regenerative Medicine. Cytotherapy 2019, 21, 3–16. [Google Scholar] [CrossRef]
- Pu, C.; Lin, R.; Liang, S.; Qiu, X.; Hou, H. Smart Surface-Based Cell Sheet Engineering for Regenerative Medicine. Trends Chem. 2023, 5, 88–101. [Google Scholar] [CrossRef]
- Ohashi, K.; Yokoyama, T.; Yamato, M.; Kuge, H.; Kanehiro, H.; Tsutsumi, M.; Amanuma, T.; Iwata, H.; Yang, J.; Okano, T.; et al. Engineering Functional Two- and Three-Dimensional Liver Systems In Vivo Using Hepatic Tissue Sheets. Nat. Med. 2007, 13, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Yamanouchi, K.; Ohashi, K.; Koike, M.; Utoh, R.; Hasegawa, H.; Muraoka, I.; Suematsu, T.; Soyama, A.; Hidaka, M.; et al. Vascularized Subcutaneous Human Liver Tissue from Engineered Hepatocyte/Fibroblast Sheets in Mice. Biomaterials 2015, 65, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.Á.P.; Geraldes Monteiro, B.; Melo, G.B.; Smith, R.L.; Cavenaghi Pereira da Silva, M.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Human Immature Dental Pulp Stem Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef]
- Khalili, M.; Zarebkohan, A.; Dianat-Moghadam, H.; Panahi, M.; Andre, H.; Alizadeh, E. Corneal Endothelial Cell Sheet Bioengineering from Neural Crest Cell-Derived Adipose Stem Cells on Novel Thermo-Responsive Elastin-Mimetic Dendrimers Decorated with RGD. Chem. Eng. J. 2022, 429, 132523. [Google Scholar] [CrossRef]
- Fan, Z.; Liao, X.; Tian, Y.; Xie, X.; Nie, Y. A Prevascularized Nerve Conduit Based on a Stem Cell Sheet Effectively Promotes the Repair of Transected Spinal Cord Injury. Acta Biomater. 2020, 101, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Tada, K.; Nakada, M.; Matsuta, M.; Tsuchiya, H. Facilitatory Effects of Artificial Nerve Filled with Adipose-Derived Stem Cell Sheets on Peripheral Nerve Regeneration: An Experimental Study. J. Orthop. Sci. 2021, 26, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Itoh, S.; Tada, K.; Matsuta, M.; Murai, A.; Tsuchiya, H. Effects of Hybridization of Decellularized Allogenic Nerves with Adipose-Derive Stem Cell Sheets to Facilitate Nerve Regeneration. Brain Res. 2020, 1746, 147025. [Google Scholar] [CrossRef] [PubMed]
- Imafuku, A.; Oka, M.; Miyabe, Y.; Sekiya, S.; Nitta, K.; Shimizu, T. Rat Mesenchymal Stromal Cell Sheets Suppress Renal Fibrosis via Microvascular Protection. Stem Cells Transl. Med. 2019, 8, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shichinohe, H.; Houkin, K.; Kuroda, S. Application of Cell Sheet Technology to Bone Marrow Stromal Cell Transplantation for Rat Brain Infarct. J. Tissue Eng. Regen. Med. 2017, 11, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Morimatsu, M.; Feng, T.; Lan, F.; Chang, D.; Wan, F.; Ling, Y. Stem Cell-Derived Cell Sheet Transplantation for Heart Tissue Repair in Myocardial Infarction. Stem Cell Res. Ther. 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, D.; Roh, J.-L. Use of a Pre-Vascularised Oral Mucosal Cell Sheet for Promoting Cutaneous Burn Wound Healing. Theranostics 2018, 8, 5703–5712. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Kajiya, M.; Motoike, S.; Yoshino, M.; Morimoto, S.; Yoshii, H.; Ogawa, T.; Sone, H.; Iwata, T.; Ouhara, K.; et al. Clumps of Mesenchymal Stem Cells/Extracellular Matrix Complexes Generated with Xeno-Free Chondro-Inductive Medium Induce Bone Regeneration via Endochondral Ossification. Biomedicines 2021, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Akahane, M.; Morita, Y.; Omokawa, S.; Nakano, K.; Kira, T.; Onishi, T.; Inagaki, Y.; Okuda, A.; Kawate, K.; et al. The Regeneration and Augmentation of Bone with Injectable Osteogenic Cell Sheet in a Rat Critical Fracture Healing Model. Injury 2015, 46, 1457–1464. [Google Scholar] [CrossRef]
- Takeuchi, R.; Kuruma, Y.; Sekine, H.; Dobashi, I.; Yamato, M.; Umezu, M.; Shimizu, T.; Okano, T. In Vivo Vascularization of Cell Sheets Provided Better Long-Term Tissue Survival than Injection of Cell Suspension. J. Tissue Eng. Regen. Med. 2016, 10, 700–710. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, F.; Zhang, J.; He, L.; Cheng, X.; Ma, Q.; Mao, T. Vitalisation of Tubular Coral Scaffolds with Cell Sheets for Regeneration of Long Bones: A Preliminary Study in Nude Mice. Br. J. Oral. Maxillofac. Surg. 2009, 47, 116–122. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Okano, T. Temperature-Responsive Polymer Modified Surface for Cell Sheet Engineering. Polymers 2012, 4, 1478–1498. [Google Scholar] [CrossRef]
- Zurina, I.M.; Presniakova, V.S.; Butnaru, D.V.; Svistunov, A.A.; Timashev, P.S.; Rochev, Y.A. Tissue Engineering Using a Combined Cell Sheet Technology and Scaffolding Approach. Acta Biomater. 2020, 113, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Hernández, R.M.; Gascón, A.R.; Igartua, M.; Pedraz, J.L. Encapsulated Cell Technology: From Research to Market. Trends Biotechnol. 2002, 20, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Kim, B.-S.; Park, I.-K.; Hoshiba, T.; Jiang, H.-L.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Design of Artificial Extracellular Matrices for Tissue Engineering. Prog. Polym. Sci. 2011, 36, 238–268. [Google Scholar] [CrossRef]
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-Based Functional Composites for Medical Applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Harper, B.A.; Barbut, S.; Lim, L.-T.; Marcone, M.F. Effect of Various Gelling Cations on the Physical Properties of “Wet” Alginate Films. J. Food Sci. 2014, 79, E562–E567. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665. [Google Scholar] [CrossRef]
- Szabó, L.; Gerber-Lemaire, S.; Wandrey, C. Strategies to Functionalize the Anionic Biopolymer Na-Alginate without Restricting Its Polyelectrolyte Properties. Polymers 2020, 12, 919. [Google Scholar] [CrossRef]
- Mahou, R.; Borcard, F.; Crivelli, V.; Montanari, E.; Passemard, S.; Noverraz, F.; Gerber-Lemaire, S.; Bühler, L.; Wandrey, C. Tuning the Properties of Hydrogel Microspheres by Adding Chemical Cross-Linking Functionality to Sodium Alginate. Chem. Mater. 2015, 27, 4380–4389. [Google Scholar] [CrossRef]
- Szabó, L.; Gonelle-Gispert, C.; Montanari, E.; Noverraz, F.; Bornet, A.; Bühler, L.H.; Gerber-Lemaire, S. Cross-Reactive Alginate Derivatives for the Production of Dual Ionic–Covalent Hydrogel Microspheres Presenting Tunable Properties for Cell Microencapsulation. ACS Appl. Polym. Mater. 2019, 1, 1326–1333. [Google Scholar] [CrossRef]
- Gao, C.; Liu, M.; Chen, J.; Zhang, X. Preparation and Controlled Degradation of Oxidized Sodium Alginate Hydrogel. Polym. Degrad. Stab. 2009, 94, 1405–1410. [Google Scholar] [CrossRef]
- Shoichet, M.S.; Li, R.H.; White, M.L.; Winn, S.R. Stability of Hydrogels Used in Cell Encapsulation: An in Vitro Comparison of Alginate and Agarose. Biotechnol. Bioeng. 1996, 50, 374–381. [Google Scholar] [CrossRef]
- Kong, X.; Chen, L.; Li, B.; Quan, C.; Wu, J. Applications of Oxidized Alginate in Regenerative Medicine. J. Mater. Chem. B 2021, 9, 2785–2801. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, M.A.; Veiseh, O.; Vegas, A.J.; McGarrigle, J.J.; Qi, M.; Marchese, E.; Omami, M.; Doloff, J.C.; Mendoza-Elias, J.; Nourmohammadzadeh, M.; et al. Alginate Encapsulation as Long-Term Immune Protection of Allogeneic Pancreatic Islet Cells Transplanted into the Omental Bursa of Macaques. Nat. Biomed. Eng. 2018, 2, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Syanda, A.M.; Kringstad, V.I.; Blackford, S.J.I.; Kjesbu, J.S.; Ng, S.S.; Ma, L.; Xiao, F.; Coron, A.E.; Rokstad, A.M.A.; Modi, S.; et al. Sulfated Alginate Reduces Pericapsular Fibrotic Overgrowth on Encapsulated cGMP-Compliant hPSC-Hepatocytes in Mice. Front. Bioeng. Biotechnol. 2022, 9, 816542. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chiu, A.; Wang, L.-H.; An, D.; Zhong, M.; Smink, A.M.; de Haan, B.J.; de Vos, P.; Keane, K.; Vegge, A.; et al. Zwitterionically Modified Alginates Mitigate Cellular Overgrowth for Cell Encapsulation. Nat. Commun. 2019, 10, 5262. [Google Scholar] [CrossRef] [PubMed]
- Noverraz, F.; Montanari, E.; Pimenta, J.; Szabó, L.; Ortiz, D.; Gonelle-Gispert, C.; Bühler, L.H.; Gerber-Lemaire, S. Antifibrotic Effect of Ketoprofen-Grafted Alginate Microcapsules in the Transplantation of Insulin Producing Cells. Bioconjug. Chem. 2018, 29, 1932–1941. [Google Scholar] [CrossRef]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.K.; Vissers, C.B.; Leach, J.K. Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels. Stem Cells Transl. Med. 2016, 5, 773–781. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Lian, C.; Li, X.; Liu, H.; Hu, L.; Kumar Kankala, R.; Chen, A.; Wang, S.; Fu, C. Study on Galactosylated Sodium Alginate for Enhancing HepG2 Cells Adhesion and 3D Printability. J. Biomater. Sci. Polym. Ed. 2023, 34, 1683–1701. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, K.B.; Raquel Maia, F.; Cruz, F.A.; Andrade, D.; Juliano, M.; Granja, P.L.; Barrias, C.C. Enzymatic, Physicochemical and Biological Properties of MMP-Sensitive Alginate Hydrogels. Soft Matter 2013, 9, 3283–3292. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Wang, J.; Lanier, O.L.; Wechsler, M.E.; Peppas, N.A.; Gu, Z. Macroencapsulation Devices for Cell Therapy. Engineering 2022, 13, 53–70. [Google Scholar] [CrossRef]
- An, D.; Chiu, A.; Flanders, J.A.; Song, W.; Shou, D.; Lu, Y.-C.; Grunnet, L.G.; Winkel, L.; Ingvorsen, C.; Christophersen, N.S.; et al. Designing a Retrievable and Scalable Cell Encapsulation Device for Potential Treatment of Type 1 Diabetes. Proc. Natl. Acad. Sci. USA 2018, 115, E263–E272. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Emerich, D.; Khademhosseini, A.; Matsumoto, S.; Hernández, R.M.; Pedraz, J.L.; Desai, T.; Calafiore, R.; de Vos, P. Engineering a Clinically Translatable Bioartificial Pancreas to Treat Type I Diabetes. Trends Biotechnol. 2018, 36, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-G.; Bottino, R.; Hawthorne, W.J. Current Status of Islet Xenotransplantation. Int. J. Surg. 2015, 23, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Wahlberg, L.U. Encapsulated Cell Biodelivery of GDNF: A Novel Clinical Strategy for Neuroprotection and Neuroregeneration in Parkinson’s Disease? Exp. Neurol. 2008, 209, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yu, J.; Wang, C.; Nguyen, N.-Y.; Walker, G.M.; Buse, J.B.; Gu, Z. Microneedles Integrated with Pancreatic Cells and Synthetic Glucose-Signal Amplifiers for Smart Insulin Delivery. Adv. Mater. 2016, 28, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Luo, Y.; Chen, H. Hydrogel-Bondable Asymmetric Planar Membranes with Hierarchical Pore Structures for Cell Scaffolding and Encapsulation. ACS Biomater. Sci. Eng. 2023, 9, 1706–1719. [Google Scholar] [CrossRef]
- An, D.; Ji, Y.; Chiu, A.; Lu, Y.-C.; Song, W.; Zhai, L.; Qi, L.; Luo, D.; Ma, M. Developing Robust, Hydrogel-Based, Nanofiber-Enabled Encapsulation Devices (NEEDs) for Cell Therapies. Biomaterials 2015, 37, 40–48. [Google Scholar] [CrossRef]
- Wang, X.; Maxwell, K.G.; Wang, K.; Bowers, D.T.; Flanders, J.A.; Liu, W.; Wang, L.-H.; Liu, Q.; Liu, C.; Naji, A.; et al. A Nanofibrous Encapsulation Device for Safe Delivery of Insulin-Producing Cells to Treat Type 1 Diabetes. Sci. Transl. Med. 2021, 13, eabb4601. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Flanders, J.A.; Wang, L.-H.; Liu, Q.; Bowers, D.T.; Wang, K.; Chiu, A.; Wang, X.; Ernst, A.U.; Shariati, K.; et al. A Safe, Fibrosis-Mitigating, and Scalable Encapsulation Device Supports Long-Term Function of Insulin-Producing Cells. Small 2022, 18, 2104899. [Google Scholar] [CrossRef]
- Sousa, J.P.M.; Stratakis, E.; Mano, J.; Marques, P.A.A.P. Anisotropic 3D Scaffolds for Spinal Cord Guided Repair: Current Concepts. Biomater. Adv. 2023, 148, 213353. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Q.; Li, W.; Su, H.; Li, S.; Zhu, Y.; Zhou, J.; Feng, Z.; Liu, Z.; Mao, S.; et al. Spinal Cord Conduits for Spinal Cord Injury Regeneration. Eng. Regen. 2023, 4, 68–80. [Google Scholar] [CrossRef]
- Prang, P.; Müller, R.; Eljaouhari, A.; Heckmann, K.; Kunz, W.; Weber, T.; Faber, C.; Vroemen, M.; Bogdahn, U.; Weidner, N. The Promotion of Oriented Axonal Regrowth in the Injured Spinal Cord by Alginate-Based Anisotropic Capillary Hydrogels. Biomaterials 2006, 27, 3560–3569. [Google Scholar] [CrossRef] [PubMed]
- Grulova, I.; Slovinska, L.; Blaško, J.; Devaux, S.; Wisztorski, M.; Salzet, M.; Fournier, I.; Kryukov, O.; Cohen, S.; Cizkova, D. Delivery of Alginate Scaffold Releasing Two Trophic Factors for Spinal Cord Injury Repair. Sci. Rep. 2015, 5, 13702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, Y.; Tang, Z.; Zou, K.; Chen, J.; Lei, Z.; Wan, X.; Liu, Y.; Zhang, H.; Wang, Y.; et al. Alginate Hydrogel Cross-Linked by Ca2+ to Promote Spinal Cord Neural Stem/Progenitor Cell Differentiation and Functional Recovery after a Spinal Cord Injuryhh. Regen. Biomater. 2022, 9, rbac057. [Google Scholar] [CrossRef]
- Liu, S.; Sandner, B.; Schackel, T.; Nicholson, L.; Chtarto, A.; Tenenbaum, L.; Puttagunta, R.; Müller, R.; Weidner, N.; Blesch, A. Regulated Viral BDNF Delivery in Combination with Schwann Cells Promotes Axonal Regeneration through Capillary Alginate Hydrogels after Spinal Cord Injury. Acta Biomater. 2017, 60, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, Y.; Zhu, M.; Wan, X.; Zhang, H.; Lei, T.; Blesch, A.; Liu, S. Anisotropic Alginate Hydrogels Promote Axonal Growth across Chronic Spinal Cord Transections after Scar Removal. ACS Biomater. Sci. Eng. 2020, 6, 2274–2286. [Google Scholar] [CrossRef]
- Günther, M.I.; Weidner, N.; Müller, R.; Blesch, A. Cell-Seeded Alginate Hydrogel Scaffolds Promote Directed Linear Axonal Regeneration in the Injured Rat Spinal Cord. Acta Biomater. 2015, 27, 140–150. [Google Scholar] [CrossRef]
- Francis, N.L.; Hunger, P.M.; Donius, A.E.; Riblett, B.W.; Zavaliangos, A.; Wegst, U.G.K.; Wheatley, M.A. An Ice-Templated, Linearly Aligned Chitosan-Alginate Scaffold for Neural Tissue Engineering. J. Biomed. Mater. Res. A 2013, 101, 3493–3503. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Prang, P.; Müller, R.; Caioni, M.; Bogdahn, U.; Kunz, W.; Weidner, N. Intrinsic and Extrinsic Determinants of Central Nervous System Axon Outgrowth into Alginate-Based Anisotropic Hydrogels. Acta Biomater. 2015, 27, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Radtke, C.; Tan, A.M.; Zhao, P.; Hamada, H.; Houkin, K.; Honmou, O.; Kocsis, J.D. BDNF-Hypersecreting Human Mesenchymal Stem Cells Promote Functional Recovery, Axonal Sprouting, and Protection of Corticospinal Neurons after Spinal Cord Injury. J. Neurosci. 2009, 29, 14932–14941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, J.; Qi, B.; Tao, W.; Wang, J.; Zhao, C.; Peng, H.; Shi, J. Multifunctional Fibers to Shape Future Biomedical Devices. Adv. Funct. Mater. 2019, 29, 1902834. [Google Scholar] [CrossRef]
- Jun, Y.; Kim, M.J.; Hwang, Y.H.; Jeon, E.A.; Kang, A.R.; Lee, S.-H.; Lee, D.Y. Microfluidics-Generated Pancreatic Islet Microfibers for Enhanced Immunoprotection. Biomaterials 2013, 34, 8122–8130. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Kang, A.R.; Lee, J.S.; Park, S.-J.; Lee, D.Y.; Moon, S.-H.; Lee, S.-H. Microchip-Based Engineering of Super-Pancreatic Islets Supported by Adipose-Derived Stem Cells. Biomaterials 2014, 35, 4815–4826. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-Long Cell-Laden Microfibres Exhibit Tissue Morphologies and Functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, F.; Okitsu, T.; Takeuchi, S. Improvement in the Mechanical Properties of Cell-Laden Hydrogel Microfibers Using Interpenetrating Polymer Networks. ACS Biomater. Sci. Eng. 2017, 3, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.U.; Wang, L.-H.; Ma, M. Interconnected Toroidal Hydrogels for Islet Encapsulation. Adv. Healthc. Mater. 2019, 8, 1900423. [Google Scholar] [CrossRef]
- Lin, H.; Du, Q.; Li, Q.; Wang, O.; Wang, Z.; Liu, K.; Elowsky, C.; Zhang, C.; Lei, Y. Hydrogel-Based Bioprocess for Scalable Manufacturing of Human Pluripotent Stem Cell-Derived Neural Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 29238–29250. [Google Scholar] [CrossRef]
- Akkouch, A.; Yu, Y.; Ozbolat, I.T. Microfabrication of Scaffold-Free Tissue Strands for Three-Dimensional Tissue Engineering. Biofabrication 2015, 7, 031002. [Google Scholar] [CrossRef]
- Wei, D.; Charlton, L.; Glidle, A.; Qi, N.; Dobson, P.S.; Dalby, M.J.; Fan, H.; Yin, H. Dynamically Modulated Core–Shell Microfibers to Study the Effect of Depth Sensing of Matrix Stiffness on Stem Cell Fate. ACS Appl. Mater. Interfaces 2021, 13, 37997–38006. [Google Scholar] [CrossRef]
- Lin, H.; Li, Q.; Wang, O.; Rauch, J.; Harm, B.; Viljoen, H.J.; Zhang, C.; Van Wyk, E.; Zhang, C.; Lei, Y. Automated Expansion of Primary Human T Cells in Scalable and Cell-Friendly Hydrogel Microtubes for Adoptive Immunotherapy. Adv. Healthc. Mater. 2018, 7, 1701297. [Google Scholar] [CrossRef]
- Lin, H.; Qiu, X.; Du, Q.; Li, Q.; Wang, O.; Akert, L.; Wang, Z.; Anderson, D.; Liu, K.; Gu, L.; et al. Engineered Microenvironment for Manufacturing Human Pluripotent Stem Cell-Derived Vascular Smooth Muscle Cells. Stem Cell Rep. 2019, 12, 84–97. [Google Scholar] [CrossRef]
- Lin, H.; Li, Q.; Du, Q.; Wang, O.; Wang, Z.; Akert, L.; Carlson, M.A.; Zhang, C.; Subramanian, A.; Zhang, C.; et al. Integrated Generation of Induced Pluripotent Stem Cells in a Low-Cost Device. Biomaterials 2019, 189, 23–36. [Google Scholar] [CrossRef]
- Jorgensen, M.; Ramesh, P.; Toro, M.; Evans, E.; Moskwa, N.; Zhang, X.; Sharfstein, S.T.; Larsen, M.; Xie, Y. Alginate Hydrogel Microtubes for Salivary Gland Cell Organization and Cavitation. Bioengineering 2022, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Fannon, O.M.; Bithell, A.; Whalley, B.J.; Delivopoulos, E. A Fiber Alginate Co-Culture Platform for the Differentiation of mESC and Modeling of the Neural Tube. Front. Neurosci. 2021, 14, 524346. [Google Scholar] [PubMed]
- Watanabe, T.; Okitsu, T.; Ozawa, F.; Nagata, S.; Matsunari, H.; Nagashima, H.; Nagaya, M.; Teramae, H.; Takeuchi, S. Millimeter-Thick Xenoislet-Laden Fibers as Retrievable Transplants Mitigate Foreign Body Reactions for Long-Term Glycemic Control in Diabetic Mice. Biomaterials 2020, 255, 120162. [Google Scholar] [CrossRef]
- Wang, L.-H.; Marfil-Garza, B.A.; Ernst, A.U.; Pawlick, R.L.; Pepper, A.R.; Okada, K.; Epel, B.; Viswakarma, N.; Kotecha, M.; Flanders, J.A.; et al. Inflammation-Induced Subcutaneous Neovascularization for the Long-Term Survival of Encapsulated Islets without Immunosuppression. Nat. Biomed. Eng. 2023, 1–19. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Gu, H.; Ge, Y.; Wu, X.; Zuo, F.; Du, Q.; Lei, Y.; Wang, Z.; Lin, H. Comparative Study of Differentiating Human Pluripotent Stem Cells into Vascular Smooth Muscle Cells in Hydrogel-Based Culture Methods. Regen. Ther. 2023, 22, 39–49. [Google Scholar] [CrossRef]
- Naficy, S.; Dehghani, F.; Chew, Y.V.; Hawthorne, W.J.; Le, T.Y.L. Engineering a Porous Hydrogel-Based Device for Cell Transplantation. ACS Appl. Bio Mater. 2020, 3, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Evron, Y.; Colton, C.K.; Ludwig, B.; Weir, G.C.; Zimermann, B.; Maimon, S.; Neufeld, T.; Shalev, N.; Goldman, T.; Leon, A.; et al. Long-Term Viability and Function of Transplanted Islets Macroencapsulated at High Density Are Achieved by Enhanced Oxygen Supply. Sci. Rep. 2018, 8, 6508. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, F.; Nagata, S.; Oda, H.; Yabe, S.G.; Okochi, H.; Takeuchi, S. Lotus-Root-Shaped Cell-Encapsulated Construct as a Retrieval Graft for Long-Term Transplantation of Human iPSC-Derived β-Cells. iScience 2021, 24, 102309. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-H.; Jang, J.; Choi, Y.-J.; Shim, J.-H.; Cho, D.-W. 3D-Printed Drug/Cell Carrier Enabling Effective Release of Cyclosporin a for Xenogeneic Cell-Based Therapy. Cell Transplant. 2015, 24, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Pandita, V.; Kumar, A.; Parihar, D.S.; Puranik, N.; Bajpai, T.; Khan, R. 3D Printing: Advancement in Biogenerative Engineering to Combat Shortage of Organs and Bioapplicable Materials. Regen. Eng. Transl. Med. 2022, 8, 173–199. [Google Scholar] [CrossRef]
- Liu, X.; Carter, S.-S.D.; Renes, M.J.; Kim, J.; Rojas-Canales, D.M.; Penko, D.; Angus, C.; Beirne, S.; Drogemuller, C.J.; Yue, Z.; et al. Development of a Coaxial 3D Printing Platform for Biofabrication of Implantable Islet-Containing Constructs. Adv. Healthc. Mater. 2019, 8, 1801181. [Google Scholar] [CrossRef]
- Samadi, A.; Moammeri, A.; Pourmadadi, M.; Abbasi, P.; Hosseinpour, Z.; Farokh, A.; Shamsabadipour, A.; Heydari, M.; Mohammadi, M.R. Cell Encapsulation and 3D Bioprinting for Therapeutic Cell Transplantation. ACS Biomater. Sci. Eng. 2023, 9, 1862–1890. [Google Scholar] [CrossRef] [PubMed]
- Accolla, R.P.; Simmons, A.M.; Stabler, C.L. Integrating Additive Manufacturing Techniques to Improve Cell-Based Implants for the Treatment of Type 1 Diabetes. Adv. Healthc. Mater. 2022, 11, 2200243. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.B.; Park, Y.-W.; Lee, D.Y. 3D Bioprinting for Artificial Pancreas Organ. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Noh, I., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 355–374. ISBN 9789811304453. [Google Scholar]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of Tissue Engineering Scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef]
- Zhang, Q.; Gonelle-Gispert, C.; Li, Y.; Geng, Z.; Gerber-Lemaire, S.; Wang, Y.; Buhler, L. Islet Encapsulation: New Developments for the Treatment of Type 1 Diabetes. Front. Immunol. 2022, 13, 869984. [Google Scholar]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D Printing Process and Application of Sodium Alginate Based Hydrogels in Soft Tissue Engineering: A Review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef]
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Villa, R.; Saenz del Burgo, L.; Alvarez, M.; Pedraz, J.L. 3D Printed Polyamide Macroencapsulation Devices Combined with Alginate Hydrogels for Insulin-Producing Cell-Based Therapies. Int. J. Pharm. 2019, 566, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Joy, T.; Thomas, L.V. 3D-Printed Polyurethane Immunoisolation Bags with Controlled Pore Architecture for Macroencapsulation of Islet Clusters Encapsulated in Alginate Gel. Prog. Biomater. 2023, 12, 13–24. [Google Scholar] [CrossRef]
- Paez-Mayorga, J.; Capuani, S.; Farina, M.; Lotito, M.L.; Niles, J.A.; Salazar, H.F.; Rhudy, J.; Esnaola, L.; Chua, C.Y.X.; Taraballi, F.; et al. Enhanced In Vivo Vascularization of 3D-Printed Cell Encapsulation Device Using Platelet-Rich Plasma and Mesenchymal Stem Cells. Adv. Healthc. Mater. 2020, 9, 2000670. [Google Scholar] [CrossRef]
- Adamo, F.; Farina, M.; Thekkedath, U.R.; Grattoni, A.; Sesana, R. Mechanical Characterization and Numerical Simulation of a Subcutaneous Implantable 3D Printed Cell Encapsulation System. J. Mech. Behav. Biomed. Mater. 2018, 82, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Saenz del Burgo, L.; Ciriza, J.; Espona-Noguera, A.; Illa, X.; Cabruja, E.; Orive, G.; Hernández, R.M.; Villa, R.; Pedraz, J.L.; Alvarez, M. 3D Printed Porous Polyamide Macrocapsule Combined with Alginate Microcapsules for Safer Cell-Based Therapies. Sci. Rep. 2018, 8, 8512. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xia, Y.; Zhang, L.; Xu, T.; Yi, Y.; Chen, J.; Liu, Z.; Yang, L.; Chen, S.; Zhou, X.; et al. Loading Neural Stem Cells on Hydrogel Scaffold Improves Cell Retention Rate and Promotes Functional Recovery in Traumatic Brain Injury. Mater. Today Bio 2023, 19, 100606. [Google Scholar] [CrossRef]
- Marchioli, G.; Luca, A.D.; de Koning, E.; Engelse, M.; Van Blitterswijk, C.A.; Karperien, M.; Van Apeldoorn, A.A.; Moroni, L. Hybrid Polycaprolactone/Alginate Scaffolds Functionalized with VEGF to Promote de Novo Vessel Formation for the Transplantation of Islets of Langerhans. Adv. Healthc. Mater. 2016, 5, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-G.; Kim, H.; Kwon, J.; Choi, Y.-J.; Jang, J.; Cho, D.-W. Application of 3D Bioprinting in the Prevention and the Therapy for Human Diseases. Signal Transduct. Target. Ther. 2021, 6, 177. [Google Scholar] [CrossRef]
- Khalil, S.; Nam, J.; Sun, W. Multi-nozzle Deposition for Construction of 3D Biopolymer Tissue Scaffolds. Rapid Prototyp. J. 2005, 11, 9–17. [Google Scholar] [CrossRef]
- Gao, Q.; He, Y.; Fu, J.; Liu, A.; Ma, L. Coaxial Nozzle-Assisted 3D Bioprinting with Built-in Microchannels for Nutrients Delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lode, A.; Gelinsky, M. Direct Plotting of Three-Dimensional Hollow Fiber Scaffolds Based on Concentrated Alginate Pastes for Tissue Engineering. Adv. Healthc. Mater. 2013, 2, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D Bioprinting of Functional Islets of Langerhans in an Alginate/Methylcellulose Hydrogel Blend. Adv. Healthc. Mater. 2019, 8, 1801631. [Google Scholar] [CrossRef]
- Abadpour, S.; Niemi, E.M.; Orrhult, L.S.; Hermanns, C.; de Vries, R.; Nogueira, L.P.; Haugen, H.J.; Josefsen, D.; Krauss, S.; Gatenholm, P.; et al. Adipose-Derived Stromal Cells Preserve Pancreatic Islet Function in a Transplantable 3D Bioprinted Scaffold. Adv. Healthc. Mater. 2023, 12, 2300640. [Google Scholar] [CrossRef]
- Dutta, S.D.; Hexiu, J.; Patel, D.K.; Ganguly, K.; Lim, K.-T. 3D-Printed Bioactive and Biodegradable Hydrogel Scaffolds of Alginate/Gelatin/Cellulose Nanocrystals for Tissue Engineering. Int. J. Biol. Macromol. 2021, 167, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, L.K.; Huebner, P.; Fisher, M.B.; Spang, J.T.; Starly, B.; Shirwaiker, R.A. 3D-Bioprinting of Polylactic Acid (PLA) Nanofiber–Alginate Hydrogel Bioink Containing Human Adipose-Derived Stem Cells. ACS Biomater. Sci. Eng. 2016, 2, 1732–1742. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Kim, J.; Shim, I.K.; Hwang, D.G.; Lee, Y.N.; Kim, M.; Kim, H.; Kim, S.-W.; Lee, S.; Kim, S.C.; Cho, D.-W.; et al. 3D Cell Printing of Islet-Laden Pancreatic Tissue-Derived Extracellular Matrix Bioink Constructs for Enhancing Pancreatic Functions. J. Mater. Chem. B 2019, 7, 1773–1781. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-Ink Properties and Printability for Extrusion Printing Living Cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Wüst, S.; Godla, M.E.; Müller, R.; Hofmann, S. Tunable Hydrogel Composite with Two-Step Processing in Combination with Innovative Hardware Upgrade for Cell-Based Three-Dimensional Bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Ooi, H.W.; Mota, C.; ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol–Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Sun, W. Bioprinting Endothelial Cells With Alginate for 3D Tissue Constructs. J. Biomech. Eng. 2009, 131, 111002. [Google Scholar] [CrossRef]
- Idaszek, J.; Volpi, M.; Paradiso, A.; Nguyen Quoc, M.; Górecka, Ż.; Klak, M.; Tymicki, G.; Berman, A.; Wierzbicki, M.; Jaworski, S.; et al. Alginate-Based Tissue-Specific Bioinks for Multi-Material 3D-Bioprinting of Pancreatic Islets and Blood Vessels: A Step towards Vascularized Pancreas Grafts. Bioprinting 2021, 24, e00163. [Google Scholar] [CrossRef]
- Krishna, D.V.; Sankar, M.R. Extrusion Based Bioprinting of Alginate Based Multicomponent Hydrogels for Tissue Regeneration Applications: State of the Art. Mater. Today Commun. 2023, 35, 105696. [Google Scholar] [CrossRef]
- Ribezzi, D.; Pinos, R.; Bonetti, L.; Cellani, M.; Barbaglio, F.; Scielzo, C.; Farè, S. Design of a Novel Bioink Suitable for the 3D Printing of Lymphoid Cells. Front. Biomater. Sci. 2023, 2, 1081065. [Google Scholar]
- Duin, S.; Bhandarkar, S.; Lehmann, S.; Kemter, E.; Wolf, E.; Gelinsky, M.; Ludwig, B.; Lode, A. Viability and Functionality of Neonatal Porcine Islet-like Cell Clusters Bioprinted in Alginate-Based Bioinks. Biomedicines 2022, 10, 1420. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Enhe, J.; Yao, B.; Wang, Y.; Zhu, D.; Li, Z.; Song, W.; Duan, X.; Yuan, X.; et al. Bioactive Nanoparticle Reinforced Alginate/Gelatin Bioink for the Maintenance of Stem Cell Stemness. Mater. Sci. Eng. C 2021, 126, 112193. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cardoso, J.C.; Manoharan, V.; Cristino, A.L.; Bhise, N.S.; Araujo, W.A.; Zorlutuna, P.; Vrana, N.E.; Ghaemmaghami, A.M.; Dokmeci, M.R.; et al. Direct-Write Bioprinting of Cell-Laden Methacrylated Gelatin Hydrogels. Biofabrication 2014, 6, 024105. [Google Scholar] [CrossRef]
- Salg, G.A.; Poisel, E.; Neulinger-Munoz, M.; Gerhardus, J.; Cebulla, D.; Bludszuweit-Philipp, C.; Vieira, V.; Nickel, F.; Herr, I.; Blaeser, A.; et al. Toward 3D-Bioprinting of an Endocrine Pancreas: A Building-Block Concept for Bioartificial Insulin-Secreting Tissue. J. Tissue Eng. 2022, 13, 20417314221091033. [Google Scholar] [CrossRef]
- Parvaneh, S.; Kemény, L.; Ghaffarinia, A.; Yarani, R.; Veréb, Z. Three-Dimensional Bioprinting of Functional β-Islet-like Constructs. Int. J. Bioprinting 2023, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Buitinga, M.; Janeczek Portalska, K.; Cornelissen, D.-J.; Plass, J.; Hanegraaf, M.; Carlotti, F.; de Koning, E.; Engelse, M.; van Blitterswijk, C.; Karperien, M.; et al. Coculturing Human Islets with Proangiogenic Support Cells to Improve Islet Revascularization at the Subcutaneous Transplantation Site. Tissue Eng. Part. A 2016, 22, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended Alginate/Collagen Hydrogels Promote Neurogenesis and Neuronal Maturation. Mater. Sci. Eng. C 2019, 104, 109904. [Google Scholar] [CrossRef] [PubMed]
- Najafi, R.; Chahsetareh, H.; Pezeshki-Modaress, M.; Aleemardani, M.; Simorgh, S.; Davachi, S.M.; Alizadeh, R.; Asghari, A.; Hassanzadeh, S.; Bagher, Z. Alginate Sulfate/ECM Composite Hydrogel Containing Electrospun Nanofiber with Encapsulated Human Adipose-Derived Stem Cells for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2023, 238, 124098. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, G.; van Gurp, L.; van Krieken, P.P.; Stamatialis, D.; Engelse, M.; van Blitterswijk, C.A.; Karperien, M.B.J.; de Koning, E.; Alblas, J.; Moroni, L.; et al. Fabrication of Three-Dimensional Bioplotted Hydrogel Scaffolds for Islets of Langerhans Transplantation. Biofabrication 2015, 7, 025009. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Q.; Xie, S.; Shan, X.; Cai, Z. In Vitro and in Vivo Biocompatibility Evaluation of a 3D Bioprinted Gelatin-Sodium Alginate/Rat Schwann-Cell Scaffold. Mater. Sci. Eng. C 2020, 109, 110530. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Chen, D.; Xie, Y.; Tai, C.; Wang, L.; Wang, P.; Wang, B. Three-Dimensional Bioprinting Sodium Alginate/Gelatin Scaffold Combined with Neural Stem Cells and Oligodendrocytes Markedly Promoting Nerve Regeneration after Spinal Cord Injury. Regen. Biomater. 2022, 9, rbac038. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Ernst, A.U.; An, D.; Datta, A.K.; Epel, B.; Kotecha, M.; Ma, M. A Bioinspired Scaffold for Rapid Oxygenation of Cell Encapsulation Systems. Nat. Commun. 2021, 12, 5846. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, T.; Singh, N. 3D Bioprinted Alginate-Silk-Based Smart Cell-Instructive Scaffolds for Dual Differentiation of Human Mesenchymal Stem Cells. ACS Appl. Bio Mater. 2022, 5, 2870–2879. [Google Scholar] [CrossRef]
- Shrestha, P.; Regmi, S.; Jeong, J.-H. Injectable Hydrogels for Islet Transplantation: A Concise Review. J. Pharm. Investig. 2020, 50, 29–45. [Google Scholar] [CrossRef]
- Tsur-Gang, O.; Ruvinov, E.; Landa, N.; Holbova, R.; Feinberg, M.S.; Leor, J.; Cohen, S. The Effects of Peptide-Based Modification of Alginate on Left Ventricular Remodeling and Function after Myocardial Infarction. Biomaterials 2009, 30, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Seif-Naraghi, S.B.; Salvatore, M.A.; Schup-Magoffin, P.J.; Hu, D.P.; Christman, K.L. Design and Characterization of an Injectable Pericardial Matrix Gel: A Potentially Autologous Scaffold for Cardiac Tissue Engineering. Tissue Eng. Part. A 2010, 16, 2017–2027. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, W. The Functional Behavior of a Macrophage/Fibroblast Co-Culture Model Derived from Normal and Diabetic Mice with a Marine Gelatin–Oxidized Alginate Hydrogel. Biomaterials 2010, 31, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.Y.; Shinde, U.P.; Yeon, B.; Jeong, B. Recent Progress of in Situ Formed Gels for Biomedical Applications. Prog. Polym. Sci. 2013, 38, 672–701. [Google Scholar] [CrossRef]
- Xu, Q.; Sigen, A.; Gao, Y.; Guo, L.; Creagh-Flynn, J.; Zhou, D.; Greiser, U.; Dong, Y.; Wang, F.; Tai, H.; et al. A Hybrid Injectable Hydrogel from Hyperbranched PEG Macromer as a Stem Cell Delivery and Retention Platform for Diabetic Wound Healing. Acta Biomater. 2018, 75, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Khodadadi Yazdi, M.; Youssefi Azarfam, M.; Zare, M.; Ramsey, J.D.; Seidi, F.; Reza Saeb, M.; Ramakrishna, S.; Mozafari, M. Injectable Cell-Laden Hydrogels for Tissue Engineering: Recent Advances and Future Opportunities. Tissue Eng. Part. A 2021, 27, 821–843. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and Biodegradable Hydrogels: Gelation, Biodegradation and Biomedical Applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Yang, J.-A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In Situ-Forming Injectable Hydrogels for Regenerative Medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Phelps, E.A.; Headen, D.M.; Taylor, W.R.; Thulé, P.M.; García, A.J. Vasculogenic Bio-Synthetic Hydrogel for Enhancement of Pancreatic Islet Engraftment and Function in Type 1 Diabetes. Biomaterials 2013, 34, 4602–4611. [Google Scholar] [CrossRef]
- Phelps, E.A.; Enemchukwu, N.O.; Fiore, V.F.; Sy, J.C.; Murthy, N.; Sulchek, T.A.; Barker, T.H.; García, A.J. Maleimide Cross-Linked Bioactive PEG Hydrogel Exhibits Improved Reaction Kinetics and Cross-Linking for Cell Encapsulation and In Situ Delivery. Adv. Mater. 2012, 24, 64–70. [Google Scholar] [CrossRef]
- Weaver, J.D.; Headen, D.M.; Aquart, J.; Johnson, C.T.; Shea, L.D.; Shirwan, H.; García, A.J. Vasculogenic Hydrogel Enhances Islet Survival, Engraftment, and Function in Leading Extrahepatic Sites. Sci. Adv. 2017, 3, e1700184. [Google Scholar] [CrossRef]
- Anamizu, M.; Tabata, Y. Design of Injectable Hydrogels of Gelatin and Alginate with Ferric Ions for Cell Transplantation. Acta Biomater. 2019, 100, 184–190. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate Hydrogels for Bone Tissue Engineering, from Injectables to Bioprinting: A Review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable Alginate Hydrogels for Cell Delivery in Tissue Engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Fernandez, L.; Ochoa, I.; Saenz del Burgo, L.; Pedraz, J.L. Tunable Injectable Alginate-Based Hydrogel for Cell Therapy in Type 1 Diabetes Mellitus. Int. J. Biol. Macromol. 2018, 107, 1261–1269. [Google Scholar] [CrossRef]
- Yin, N.; Han, Y.; Xu, H.; Gao, Y.; Yi, T.; Yao, J.; Dong, L.; Cheng, D.; Chen, Z. VEGF-Conjugated Alginate Hydrogel Prompt Angiogenesis and Improve Pancreatic Islet Engraftment and Function in Type 1 Diabetes. Mater. Sci. Eng. C 2016, 59, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving Viability of Stem Cells During Syringe Needle Flow Through the Design of Hydrogel Cell Carriers. Tissue Eng. Part. A 2012, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, R.; Matsukawa, M.; Nakagawa, K.; Isomura, E.; Kuwahara, T.; Nii, T.; Tanaka, S.; Tabata, Y. Efficient Cell Transplantation Combining Injectable Hydrogels with Control Release of Growth Factors. Regen. Ther. 2021, 18, 372–383. [Google Scholar] [CrossRef]
- Fang, R.; Tian, W.; Chen, X. Synthesis of Injectable Alginate Hydrogels with Muscle-Derived Stem Cells for Potential Myocardial Infarction Repair. Appl. Sci. 2017, 7, 252. [Google Scholar] [CrossRef]
- Sudhadevi, T.; Resmi, R.; Chandrababu, K.; Joseph, J.; Joseph, R.; John, A.; Abraham, A. Neural Tissue Engineering with Rat Adipose-Derived Mesenchymal Stem Cells: The Role of an Injectable, Resorbable Hydrogel Scaffold Derived from Oxidized Alginate and Gelatin. ACS Appl. Bio Mater. 2023, 6, 1742–1754. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, K.; Ma, S.; Liu, T.; Yao, M.; Li, J.; Wang, X.; Guan, F. Preparing an Injectable Hydrogel with Sodium Alginate and Type I Collagen to Create Better MSCs Growth Microenvironment. E-Polym. 2019, 19, 87–91. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Li, Y.; Wang, Y.; Yao, M.; Zhang, K.; Chen, Z.; Yue, H.; Shi, J.; Guan, F.; et al. Sodium Alginate/Collagen Hydrogel Loaded with Human Umbilical Cord Mesenchymal Stem Cells Promotes Wound Healing and Skin Remodeling. Cell Tissue Res. 2021, 383, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cabrerizo, P.; Saludas, L.; Prósper, F.; Abizanda, G.; Echanove-González de Anleo, M.; Ruiz-Villalba, A.; Garbayo, E.; Blanco-Prieto, M.J. Development of an Injectable Alginate-Collagen Hydrogel for Cardiac Delivery of Extracellular Vesicles. Int. J. Pharm. 2022, 629, 122356. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, J.; Huang, T.; Zhang, Z.; Xing, Q.; Zhou, X.; Zhang, K.; Yao, M.; Cheng, T.; Wang, X.; et al. Sodium Alginate/Collagen/Stromal Cell-Derived Factor-1 Neural Scaffold Loaded with BMSCs Promotes Neurological Function Recovery after Traumatic Brain Injury. Acta Biomater. 2021, 131, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.A.; Kipke, D.R. Flow Properties of Liquid Calcium Alginate Polymer Injected through Medical Microcatheters for Endovascular Embolization. J. Biomed. Mater. Res. 2002, 61, 533–540. [Google Scholar] [CrossRef]
- Walker, P.A.; Jimenez, F.; Gerber, M.H.; Aroom, K.R.; Shah, S.K.; Harting, M.T.; Gill, B.S.; Savitz, S.I.; Cox, C.S. Effect of Needle Diameter and Flow Rate on Rat and Human Mesenchymal Stromal Cell Characterization and Viability. Tissue Eng. Part. C Methods 2010, 16, 989–997. [Google Scholar] [CrossRef]
- Roche, E.T.; Hastings, C.L.; Lewin, S.A.; Shvartsman, D.E.; Brudno, Y.; Vasilyev, N.V.; O’Brien, F.J.; Walsh, C.J.; Duffy, G.P.; Mooney, D.J. Comparison of Biomaterial Delivery Vehicles for Improving Acute Retention of Stem Cells in the Infarcted Heart. Biomaterials 2014, 35, 6850–6858. [Google Scholar] [CrossRef]
- Marquardt, L.M.; Heilshorn, S.C. Design of Injectable Materials to Improve Stem Cell Transplantation. Curr. Stem Cell Rep. 2016, 2, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhang, T.; Kong, W.; Fu, C.; Chang, Y.; Li, H.; Yang, X.; Pan, S. A Dual-Drug Enhanced Injectable Hydrogel Incorporated with Neural Stem Cells for Combination Therapy in Spinal Cord Injury. Chem. Eng. J. 2022, 427, 130906. [Google Scholar] [CrossRef]
- Sitoci-Ficici, K.H.; Matyash, M.; Uckermann, O.; Galli, R.; Leipnitz, E.; Later, R.; Ikonomidou, C.; Gelinsky, M.; Schackert, G.; Kirsch, M. Non-Functionalized Soft Alginate Hydrogel Promotes Locomotor Recovery after Spinal Cord Injury in a Rat Hemimyelonectomy Model. Acta Neurochir. 2018, 160, 449–457. [Google Scholar] [CrossRef]
- Grijalvo, S.; Nieto-Díaz, M.; Maza, R.M.; Eritja, R.; Díaz, D.D. Alginate Hydrogels as Scaffolds and Delivery Systems to Repair the Damaged Spinal Cord. Biotechnol. J. 2019, 14, 1900275. [Google Scholar] [CrossRef] [PubMed]
- Jarrah, R.; Sammak, S.E.; Onyedimma, C.; Ghaith, A.K.; Moinuddin, F.M.; Bhandarkar, A.R.; Siddiqui, A.; Madigan, N.; Bydon, M. The Role of Alginate Hydrogels as a Potential Treatment Modality for Spinal Cord Injury: A Comprehensive Review of the Literature. Neurospine 2022, 19, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Xie, Z.; Chen, K.; Ma, M.; Huang, Y.; Li, M.; Cai, Z.; Wang, P.; Shen, H. Injectable Hydrogels for Spinal Cord Injury Repair. Eng. Regen. 2022, 3, 407–419. [Google Scholar] [CrossRef]
- Xing, S.; Yan, M.; Yang, Y.; Wang, Y.; Hu, X.; Ma, B.; Kang, X. Diacerein Loaded Poly (Styrene Sulfonate) and Carbon Nanotubes Injectable Hydrogel: An Effective Therapy for Spinal Cord Injury Regeneration. J. Clust. Sci. 2023, 34, 565–576. [Google Scholar] [CrossRef]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The Influence of Hydrogel Modulus on the Proliferation and Differentiation of Encapsulated Neural Stem Cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.A.; Pomrenke, R.D.; McLane, J.S.; Schaub, N.J.; DeSimone, E.K.; Ligon, L.A.; Gilbert, R.J. An Injectable, Calcium Responsive Composite Hydrogel for the Treatment of Acute Spinal Cord Injury. ACS Appl. Mater. Interfaces 2014, 6, 1424–1438. [Google Scholar] [CrossRef]
- Zhu, S.; Ying, Y.; Wu, Q.; Ni, Z.; Huang, Z.; Cai, P.; Tu, Y.; Ying, W.; Ye, J.; Zhang, R.; et al. Alginate Self-Adhesive Hydrogel Combined with Dental Pulp Stem Cells and FGF21 Repairs Hemisection Spinal Cord Injury via Apoptosis and Autophagy Mechanisms. Chem. Eng. J. 2021, 426, 130827. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial Ischemia/Reperfusion Injury: Mechanisms of Injury and Implications for Management (Review). Exp. Ther. Med. 2022, 23, 430. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive Summary: Heart Disease and Stroke Statistics—2010 Update. Circulation 2010, 121, 948–954. [Google Scholar] [CrossRef]
- Majka, M.; Sułkowski, M.; Badyra, B.; Musiałek, P. Concise Review: Mesenchymal Stem Cells in Cardiovascular Regeneration: Emerging Research Directions and Clinical Applications. Stem Cells Transl. Med. 2017, 6, 1859–1867. [Google Scholar] [CrossRef]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human Embryonic-Stem-Cell-Derived Cardiomyocytes Regenerate Non-Human Primate Hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, Z.; Luo, R.; Jiang, Q.; Yang, L.; Wang, Y. Advances in Injectable Hydrogel Strategies for Heart Failure Treatment. Adv. Healthc. Mater. 2023, 12, 2300029. [Google Scholar] [CrossRef] [PubMed]
- Cattelan, G.; Guerrero Gerbolés, A.; Foresti, R.; Pramstaller, P.P.; Rossini, A.; Miragoli, M.; Caffarra Malvezzi, C. Alginate Formulations: Current Developments in the Race for Hydrogel-Based Cardiac Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Landa, N.; Miller, L.; Feinberg, M.S.; Holbova, R.; Shachar, M.; Freeman, I.; Cohen, S.; Leor, J. Effect of Injectable Alginate Implant on Cardiac Remodeling and Function After Recent and Old Infarcts in Rat. Circulation 2008, 117, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Leor, J.; Tuvia, S.; Guetta, V.; Manczur, F.; Castel, D.; Willenz, U.; Petneh, Ö.; Landa, N.; Feinberg, M.S.; Konen, E.; et al. Intracoronary Injection of In Situ Forming Alginate Hydrogel Reverses Left Ventricular Remodeling After Myocardial Infarction in Swine. J. Am. Coll. Cardiol. 2009, 54, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gu, Y.; Du, K.T.; Mihardja, S.; Sievers, R.E.; Lee, R.J. The Effect of Injected RGD Modified Alginate on Angiogenesis and Left Ventricular Function in a Chronic Rat Infarct Model. Biomaterials 2009, 30, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Wall, S.T.; Klepach, D.; Ge, L.; Zhang, Z.; Lee, R.J.; Hinson, A.; Gorman, J.H.; Gorman, R.C.; Guccione, J.M. Algisyl-LVRTM with Coronary Artery Bypass Grafting Reduces Left Ventricular Wall Stress and Improves Function in the Failing Human Heart. Int. J. Cardiol. 2013, 168, 2022–2028. [Google Scholar] [CrossRef]
- Ruvinov, E.; Cohen, S. Alginate Biomaterial for the Treatment of Myocardial Infarction: Progress, Translational Strategies, and Clinical Outlook: From Ocean Algae to Patient Bedside. Adv. Drug Deliv. Rev. 2016, 96, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, C.; Zhang, J.; Wang, Z.; Guo, X.; Wei, Y. Injectable Conductive Hydrogel Containing Black Phosphorus Nanosheets Inhibits the Oxidative Stress-Inflammation Chain during Myocardial Infarction. ACS Appl. Nano Mater. 2023, 6, 16749–16767. [Google Scholar] [CrossRef]
- Panda, N.C.; Zuckerman, S.T.; Mesubi, O.O.; Rosenbaum, D.S.; Penn, M.S.; Donahue, J.K.; Alsberg, E.; Laurita, K.R. Improved Conduction and Increased Cell Retention in Healed MI Using Mesenchymal Stem Cells Suspended in Alginate Hydrogel. J. Interv. Card. Electrophysiol. 2014, 41, 117–127. [Google Scholar] [CrossRef]
- Curley, C.J.; Dolan, E.B.; Otten, M.; Hinderer, S.; Duffy, G.P.; Murphy, B.P. An Injectable Alginate/Extra Cellular Matrix (ECM) Hydrogel towards Acellular Treatment of Heart Failure. Drug Deliv. Transl. Res. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sanganalmath, S.K.; Bolli, R. Cell Therapy for Heart Failure. Circ. Res. 2013, 113, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac Tissue Engineering. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef]
- Hattori, F.; Fukuda, K. Strategies for Ensuring That Regenerative Cardiomyocytes Function Properly and in Cooperation with the Host Myocardium. Exp. Mol. Med. 2010, 42, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Christman, K.L.; Vardanian, A.J.; Fang, Q.; Sievers, R.E.; Fok, H.H.; Lee, R.J. Injectable Fibrin Scaffold Improves Cell Transplant Survival, Reduces Infarct Expansion, and Induces Neovasculature Formation in Ischemic Myocardium. J. Am. Coll. Cardiol. 2004, 44, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Wollert, K.C.; Meyer, G.P.; Menke, A.; Arseniev, L.; Hertenstein, B.; Ganser, A.; Knapp, W.H.; Drexler, H. Monitoring of Bone Marrow Cell Homing Into the Infarcted Human Myocardium. Circulation 2005, 111, 2198–2202. [Google Scholar] [CrossRef]
- Levit, R.D.; Landázuri, N.; Phelps, E.A.; Brown, M.E.; García, A.J.; Davis, M.E.; Joseph, G.; Long, R.; Safley, S.A.; Suever, J.D.; et al. Cellular Encapsulation Enhances Cardiac Repair. J. Am. Heart Assoc. 2013, 2, e000367. [Google Scholar] [CrossRef]
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for Cardiac Regeneration: From Seaweed to Clinical Trials. Glob. Cardiol. Sci. Pract. 2016, 2016, e201604. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Young, S.; Hamilton, A.; Amsden, B.G.; Flynn, L.E. Mesenchymal Stem Cell Delivery Strategies to Promote Cardiac Regeneration Following Ischemic Injury. Biomaterials 2014, 35, 3956–3974. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Xu, Y.; Long, L.; Hu, X.; Zhang, J.; Wang, Y. Injectable Polyaniline Nanorods/Alginate Hydrogel with AAV9-Mediated VEGF Overexpression for Myocardial Infarction Treatment. Biomaterials 2023, 296, 122088. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.; Mashayekhan, S.; Baheiraei, N.; Pourjavadi, A. Biohybrid Oxidized Alginate/Myocardial Extracellular Matrix Injectable Hydrogels with Improved Electromechanical Properties for Cardiac Tissue Engineering. Int. J. Biol. Macromol. 2021, 180, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Karimi Hajishoreh, N.; Baheiraei, N.; Naderi, N.; Salehnia, M. Reduced Graphene Oxide Facilitates Biocompatibility of Alginate for Cardiac Repair. J. Bioact. Compat. Polym. 2020, 35, 363–377. [Google Scholar] [CrossRef]
- Liang, W.; Chen, J.; Li, L.; Li, M.; Wei, X.; Tan, B.; Shang, Y.; Fan, G.; Wang, W.; Liu, W. Conductive Hydrogen Sulfide-Releasing Hydrogel Encapsulating ADSCs for Myocardial Infarction Treatment. ACS Appl. Mater. Interfaces 2019, 11, 14619–14629. [Google Scholar] [CrossRef] [PubMed]
- Sodha, N.R.; Clements, R.T.; Feng, J.; Liu, Y.; Bianchi, C.; Horvath, E.M.; Szabo, C.; Stahl, G.L.; Sellke, F.W. Hydrogen Sulfide Therapy Attenuates the Inflammatory Response in a Porcine Model of Myocardial Ischemia/Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 2009, 138, 977–984. [Google Scholar] [CrossRef]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Kim, S.-W.; Park, J.; Park, J.; Kim, S.; Kim, Y.S.; Ahn, Y.; Jung, D.-W.; Williams, D.R.; Lee, J.Y. Anti-Oxidant Activity Reinforced Reduced Graphene Oxide/Alginate Microgels: Mesenchymal Stem Cell Encapsulation and Regeneration of Infarcted Hearts. Biomaterials 2019, 225, 119513. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, Y.; Yang, H.; Lu, K.; Zhang, H.; Wang, Y.; Wang, J.; Ruan, L.; Shen, Z.; Yu, Q.; et al. An Injectable Alginate/Fibrin Hydrogel Encapsulated with Cardiomyocytes and VEGF for Myocardial Infarction Treatment. J. Mater. Sci. Technol. 2023, 143, 198–206. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-Y.; Gao, G.-Y.; Feng, J.-F.; Mao, Q.; Chen, L.-G.; Yang, X.-F.; Liu, J.-F.; Wang, Y.-H.; Qiu, B.-H.; Huang, X.-J. Traumatic Brain Injury in China. Lancet Neurol. 2019, 18, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Tsang, K.K.-T.; Whitfield, P.C. Traumatic Brain Injury: Review of Current Management Strategies. Br. J. Oral. Maxillofac. Surg. 2012, 50, 298–308. [Google Scholar] [CrossRef]
- Xu, C.; Fu, F.; Li, X.; Zhang, S. Mesenchymal Stem Cells Maintain the Microenvironment of Central Nervous System by Regulating the Polarization of Macrophages/Microglia after Traumatic Brain Injury. Int. J. Neurosci. 2017, 127, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, Z.; Zhou, J.; Xing, Q.; Ma, S.; Li, Q.; Zhang, Y.; Yao, M.; Wang, X.; Li, Q.; et al. Potential Application of an Injectable Hydrogel Scaffold Loaded with Mesenchymal Stem Cells for Treating Traumatic Brain Injury. J. Mater. Chem. B 2018, 6, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; He, Y.; Wang, A.; Pan, J.; Xu, C.; Fu, D.; Ye, Q.; Wu, F. Development of an Integrated Device Utilizing Exosome-Hyaluronic Acid-Based Hydrogel and Investigation of Its Osteogenic and Angiogenic Characteristics. Mater. Des. 2024, 237, 112565. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Gionet-Gonzales, M.; Vorwald, C.E.; Bohannon, L.K.; Clark, K.; Galuppo, L.D.; Leach, J.K. Injectable Mineralized Microsphere-Loaded Composite Hydrogels for Bone Repair in a Sheep Bone Defect Model. Biomaterials 2019, 197, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Lei, X.; Miao, S.; Zhang, S.; Cheng, P.; Song, Y.; Wu, H.; Gao, Y.; Bi, L.; et al. Cell-Loaded Injectable Gelatin/Alginate/LAPONITE® Nanocomposite Hydrogel Promotes Bone Healing in a Critical-Size Rat Calvarial Defect Model. RSC Adv. 2020, 10, 25652–25661. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Diao, C.; Liang, L. Engineering an Alginate/β-Glycerophosphate/Dextran Injectable Hydrogel-Delivery for Cardiac Therapies after Acute Myocardial Infarctions. Mater. Express 2021, 11, 846–853. [Google Scholar] [CrossRef]
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C.; et al. Incorporation of Small Extracellular Vesicles in Sodium Alginate Hydrogel as a Novel Therapeutic Strategy for Myocardial Infarction. Theranostics 2019, 9, 7403–7416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Liu, S.; Luo, W.; Wang, G.; Zhu, Z.; Ma, Q.; Liu, Y.; Wang, L.; Lu, S.; et al. Application of Vancomycin-Impregnated Calcium Sulfate Hemihydrate/Nanohydroxyapatite/Carboxymethyl Chitosan Injectable Hydrogels Combined with BMSC Sheets for the Treatment of Infected Bone Defects in a Rabbit Model. BMC Musculoskelet. Disord. 2022, 23, 557. [Google Scholar] [CrossRef] [PubMed]
- Sevari, S.P.; Shahnazi, F.; Chen, C.; Mitchell, J.C.; Ansari, S.; Moshaverinia, A. Bioactive Glass-Containing Hydrogel Delivery System for Osteogenic Differentiation of Human Dental Pulp Stem Cells. J. Biomed. Mater. Res. A 2020, 108, 557–564. [Google Scholar] [CrossRef]
- de Vos, P. Historical Perspectives and Current Challenges in Cell Microencapsulation. In Cell Microencapsulation: Methods and Protocols; Opara, E.C., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 3–21. ISBN 978-1-4939-6364-5. [Google Scholar]
- Xu, M.; Qin, M.; Cheng, Y.; Niu, X.; Kong, J.; Zhang, X.; Huang, D.; Wang, H. Alginate Microgels as Delivery Vehicles for Cell-Based Therapies in Tissue Engineering and Regenerative Medicine. Carbohydr. Polym. 2021, 266, 118128. [Google Scholar] [CrossRef]
- Lopez-Mendez, T.B.; Santos-Vizcaino, E.; Pedraz, J.L.; Orive, G.; Hernandez, R.M. Cell Microencapsulation Technologies for Sustained Drug Delivery: Latest Advances in Efficacy and Biosafety. J. Control. Release 2021, 335, 619–636. [Google Scholar] [CrossRef]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell Encapsulation Within Alginate Microcapsules: Immunological Challenges and Outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of Alginate Microspheres in Therapeutics Delivery and Cell Culture: Past, Present and Future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Eskandari, M.; Ghazali, Z.S.; Ghazali, H.S. Cell Encapsulation in Alginate-Based Microgels Using Droplet Microfluidics; a Review on Gelation Methods and Applications. Biomed. Phys. Eng. Express 2022, 8, 022001. [Google Scholar] [CrossRef] [PubMed]
- Len’shina, N.A.; Konev, A.N.; Baten’kin, A.A.; Bardina, P.S.; Cherkasova, E.I.; Kashina, A.V.; Zagainova, E.V.; Zagainov, V.E.; Chesnokov, S.A. Alginate Functionalization for the Microencapsulation of Insulin Producing Cells. Polym. Sci. Ser. B 2021, 63, 640–656. [Google Scholar] [CrossRef]
- Basta, G.; Montanucci, P.; Calafiore, R. Microencapsulation of Cells and Molecular Therapy of Type 1 Diabetes Mellitus: The Actual State and Future Perspectives between Promise and Progress. J. Diabetes Investig. 2021, 12, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mendez, T.B.; Santos-Vizcaino, E.; Pedraz, J.L.; Hernandez, R.M.; Orive, G. Cell Microencapsulation Technologies for Sustained Drug Delivery: Clinical Trials and Companies. Drug Discov. Today 2021, 26, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Liu, B.; Zhang, H.; Ryu, S.; Kuss, M.; Shukla, D.; Hu, G.; Shi, W.; Jiang, X.; Lei, Y.; et al. Controllable Fabrication of Alginate/Poly-L-Ornithine Polyelectrolyte Complex Hydrogel Networks as Therapeutic Drug and Cell Carriers. Acta Biomater. 2022, 138, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xie, L.; Zhang, Q.; Wang, G.; Zhang, S.; Jiang, M.; Zhang, R.; Yang, T.; Hu, X.; Yang, Z.; et al. Gelatin Methacryloyl-Alginate Core-Shell Microcapsules as Efficient Delivery Platforms for Prevascularized Microtissues in Endodontic Regeneration. Acta Biomater. 2022, 144, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Somo, S.I.; Langert, K.A.; Yang, C.-S.; Vaicik, M.K.; Ibarra, V.; Appel, A.A.; Akar, B.; Cheng, M.-H.; Brey, E.M. Synthesis and Evaluation of Dual Crosslinked Alginate Microbeads. Acta Biomater. 2018, 65, 53–65. [Google Scholar] [CrossRef]
- Distler, T.; Kretzschmar, L.; Schneidereit, D.; Girardo, S.; Goswami, R.; Friedrich, O.; Detsch, R.; Guck, J.; Boccaccini, A.R.; Budday, S. Mechanical Properties of Cell- and Microgel Bead-Laden Oxidized Alginate-Gelatin Hydrogels. Biomater. Sci. 2021, 9, 3051–3068. [Google Scholar] [CrossRef] [PubMed]
- Samsonchi, Z.; Karimi, H.; Izadi, Z.; Baei, P.; Najarasl, M.; Kazemi Ashtiani, M.; Mohammadi, J.; Moazenchi, M.; Tahamtani, Y.; Baharvand, H.; et al. Transplantation of Islet-Containing Microcapsules Modified with Constitutional Isomers of Sulfated Alginate in Diabetic Mice to Mitigate Fibrosis for Long-Term Glycemic Control. Chem. Eng. J. 2022, 432, 134298. [Google Scholar] [CrossRef]
- Hosseinzadeh, Z.; Alemzadeh, I.; Vossoughi, M. Investigation of Encapsulation of Pancreatic Beta Cells and Curcumin within Alginate Microcapsules. Can. J. Chem. Eng. 2024, 102, 561–573. [Google Scholar] [CrossRef]
- Enck, K.; Tamburrini, R.; Deborah, C.; Gazia, C.; Jost, A.; Khalil, F.; Alwan, A.; Orlando, G.; Opara, E.C. Effect of Alginate Matrix Engineered to Mimic the Pancreatic Microenvironment on Encapsulated Islet Function. Biotechnol. Bioeng. 2021, 118, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Crisóstomo, J.; Araújo, F.; Granja, P.; Barrias, C.; Sarmento, B.; Seiça, R. Increasing Levels of Insulin Secretion in Bioartificial Pancreas Technology: Co-Encapsulation of Beta Cells and Nanoparticles Containing GLP-1 in Alginate Hydrogels. Health Technol. 2020, 10, 885–890. [Google Scholar] [CrossRef]
- Sivan, S.S.; Bonstein, I.; Marmor, Y.N.; Pelled, G.; Gazit, Z.; Amit, M. Encapsulation of Human-Bone-Marrow-Derived Mesenchymal Stem Cells in Small Alginate Beads Using One-Step Emulsification by Internal Gelation: In Vitro, and In Vivo Evaluation in Degenerate Intervertebral Disc Model. Pharmaceutics 2022, 14, 1179. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Y.; Zhong, W.; Li, B.; Mequanint, K.; Luo, G.; Xing, M. Biomedical Applications of Layer-by-Layer Self-Assembly for Cell Encapsulation: Current Status and Future Perspectives. Adv. Healthc. Mater. 2019, 8, 1800939. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.; Sun, A.M. Microencapsulated Islets as Bioartificial Endocrine Pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Hatami, J.; Mano, J.F. Coating Strategies Using Layer-by-Layer Deposition for Cell Encapsulation. Chem.–Asian J. 2016, 11, 1753–1764. [Google Scholar] [CrossRef]
- Zhi, Z.-L.; Khan, F.; Pickup, J.C. Multilayer Nanoencapsulation: A Nanomedicine Technology for Diabetes Research and Management. Diabetes Res. Clin. Pract. 2013, 100, 162–169. [Google Scholar] [CrossRef]
- Xue, Z.; Mei, D.; Zhang, L. Advances in Single-Cell Nanoencapsulation and Applications in Diseases. J. Microencapsul. 2022, 39, 481–494. [Google Scholar] [CrossRef]
- Schneider, S.; Feilen, P.J.; Slotty, V.; Kampfner, D.; Preuss, S.; Berger, S.; Beyer, J.; Pommersheim, R. Multilayer Capsules: A Promising Microencapsulation System for Transplantation of Pancreatic Islets. Biomaterials 2001, 22, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Rangasami, V.K.; Asawa, K.; Teramura, Y.; Le Blanc, K.; Nilsson, B.; Hilborn, J.; Varghese, O.P.; Oommen, O.P. Biomimetic Polyelectrolyte Coating of Stem Cells Suppresses Thrombotic Activation and Enhances Its Survival and Function. Biomater. Adv. 2023, 147, 213331. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.M. Surface Electrostatic-Charge Measurements on Islet and Zymogen Granules: Effect of Calcium Ions. Diabetologia 1974, 10, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, L.; Huo, D.; Li, Y.; Wu, Y.; Zeng, L.; Cheng, P.; Xing, M.; Zeng, W.; Zhu, C. A VEGF Delivery System Targeting MI Improves Angiogenesis and Cardiac Function Based on the Tropism of MSCs and Layer-by-Layer Self-Assembly. Biomaterials 2017, 127, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guan, T.; Zhang, X.; Wang, Z.; Wang, M.; Zhong, W.; Feng, H.; Xing, M.; Kong, J. The Effect of Layer-by-Layer Assembly Coating on the Proliferation and Differentiation of Neural Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.-L.; Kerby, A.; King, A.J.F.; Jones, P.M.; Pickup, J.C. Nano-Scale Encapsulation Enhances Allograft Survival and Function of Islets Transplanted in a Mouse Model of Diabetes. Diabetologia 2012, 55, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Hu, Q.; Chen, T.; Yang, Y.; Zhang, C.; Zhong, J.; Yin, Y.; Jiang, X.; Zhou, X.; Wang, S.; et al. Transplantation of Layer-by-Layer Assembled Neural Stem Cells Tethered with Vascular Endothelial Growth Factor Reservoir Promotes Neurogenesis and Angiogenesis after Ischemic Stroke in Mice. Appl. Mater. Today 2022, 28, 101548. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Fang, Y.; Huang, H.; You, X.; Wu, J. Advances in Encapsulation and Delivery Strategies for Islet Transplantation. Adv. Healthc. Mater. 2021, 10, 2100965. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Cui, W.; Chaikof, E.L. Layer-by-Layer Assembly of a Conformal Nanothin PEG Coating for Intraportal Islet Transplantation. Nano Lett. 2008, 8, 1940–1948. [Google Scholar] [CrossRef]
- Wilson, J.T.; Cui, W.; Kozlovskaya, V.; Kharlampieva, E.; Pan, D.; Qu, Z.; Krishnamurthy, V.R.; Mets, J.; Kumar, V.; Wen, J.; et al. Cell Surface Engineering with Polyelectrolyte Multilayer Thin Films. J. Am. Chem. Soc. 2011, 133, 7054–7064. [Google Scholar] [CrossRef] [PubMed]
- Krol, S.; del Guerra, S.; Grupillo, M.; Diaspro, A.; Gliozzi, A.; Marchetti, P. Multilayer Nanoencapsulation. New Approach for Immune Protection of Human Pancreatic Islets. Nano Lett. 2006, 6, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Teramura, Y.; Kaneda, Y.; Iwata, H. Islet-Encapsulation in Ultra-Thin Layer-by-Layer Membranes of Poly(Vinyl Alcohol) Anchored to Poly(Ethylene Glycol)–Lipids in the Cell Membrane. Biomaterials 2007, 28, 4818–4825. [Google Scholar] [CrossRef] [PubMed]
- Gattás-Asfura, K.M.; Stabler, C.L. Bioorthogonal Layer-by-Layer Encapsulation of Pancreatic Islets via Hyperbranched Polymers. ACS Appl. Mater. Interfaces 2013, 5, 9964–9974. [Google Scholar] [CrossRef] [PubMed]
- Kizilel, S.; Scavone, A.; Liu, X.; Nothias, J.-M.; Ostrega, D.; Witkowski, P.; Millis, M. Encapsulation of Pancreatic Islets Within Nano-Thin Functional Polyethylene Glycol Coatings for Enhanced Insulin Secretion. Tissue Eng. Part. A 2010, 16, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Bhaiji, T.; Zhi, Z.-L.; Pickup, J.C. Improving Cellular Function and Immune Protection via Layer-by-Layer Nanocoating of Pancreatic Islet β-Cell Spheroids Cocultured with Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 2012, 100A, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Rao, K.P. Pectin–Gelatin and Alginate–Gelatin Complex Coacervation for Controlled Drug Delivery: Influence of Anionic Polysaccharides and Drugs Being Encapsulated on Physicochemical Properties of Microcapsules. Carbohydr. Polym. 2010, 80, 808–816. [Google Scholar] [CrossRef]
- Lin, B.; Wang, J.; Miao, Y.; Liu, Y.; Jiang, W.; Fan, Z.; Darabi, M.-A.; Hu, Z.; Xing, M. Cytokine Loaded Layer-by-Layer Ultrathin Matrices to Deliver Single Dermal Papilla Cells for Spot-by-Spot Hair Follicle Regeneration. J. Mater. Chem. B 2016, 4, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Ru, L.; Wu, N.; Wei, K.; Zeng, Y.; Li, Q.; Weng, C.; Ren, C.; Ren, B.; Huo, D.; Li, Y.; et al. Improving Cell Survival and Engraftment in Vivo via Layer-by-Layer Nanocoating of hESC-Derived RPE Cells. Stem Cell Res. Ther. 2020, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Tan, G.; Hosseini, H.; Nagiel, A. Subretinal Transplantation of Embryonic Stem Cell–Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFc1–ORSFc9. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.W.; Wang, L.; Li, S.Y.; Zhao, C.J.; Hao, J.; Li, Q.Y.; Zhao, T.T.; Wu, W.; Wang, Y.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium Transplants as a Potential Treatment for Wet Age-Related Macular Degeneration. Cell Discov. 2018, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In Vitro Cytotoxicity Testing of Polycations: Influence of Polymer Structure on Cell Viability and Hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Turtoi, M.; Suflet, D.M.; Dinu, M.V.; Darie-Nita, R.N.; Anghelache, M.; Calin, M.; Constantin, M. Alginate/Poloxamer Hydrogel Obtained by Thiol-Acrylate Photopolymerization for the Alleviation of the Inflammatory Response of Human Keratinocytes. Int. J. Biol. Macromol. 2021, 180, 418–431. [Google Scholar] [CrossRef]
- Genç, H.; Hazur, J.; Karakaya, E.; Dietel, B.; Bider, F.; Groll, J.; Alexiou, C.; Boccaccini, A.R.; Detsch, R.; Cicha, I. Differential Responses to Bioink-Induced Oxidative Stress in Endothelial Cells and Fibroblasts. Int. J. Mol. Sci. 2021, 22, 2358. [Google Scholar] [CrossRef] [PubMed]
- Sher, P.; Correia, C.R.; Costa, R.R.; Mano, J.F. Compartmentalized Bioencapsulated Liquefied 3D Macro-Construct by Perfusion-Based Layer-by-Layer Technique. RSC Adv. 2014, 5, 2511–2516. [Google Scholar] [CrossRef]
- Mansouri, S.; Merhi, Y.; Winnik, F.M.; Tabrizian, M. Investigation of Layer-by-Layer Assembly of Polyelectrolytes on Fully Functional Human Red Blood Cells in Suspension for Attenuated Immune Response. Biomacromolecules 2011, 12, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Mets, J.M.; Wilson, J.T.; Cui, W.; Chaikof, E.L. An Automated Process for Layer-by-Layer Assembly of Polyelectrolyte Multilayer Thin Films on Viable Cell Aggregates. Adv. Healthc. Mater. 2013, 2, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Gattás-Asfura, K.M.; Abuid, N.J.; Labrada, I.; Stabler, C.L. Promoting Dendrimer Self-Assembly Enhances Covalent Layer-by-Layer Encapsulation of Pancreatic Islets. ACS Biomater. Sci. Eng. 2020, 6, 2641–2651. [Google Scholar] [CrossRef]
- Tran, P.L.; Kim, J.-H.; Jung, Y.-H.; Lee, D.-C.; Choi, J.U.; Le, D.N.; Nam, J.-W.; Shrestha, M.; Kim, J.-Y.; Pham, T.T.; et al. Prolongation of Graft Survival via Layer-by-Layer Assembly of Collagen and Immunosuppressive Particles on Pancreatic Islets. Biomaterials 2022, 290, 121804. [Google Scholar] [CrossRef]
- Chen, P.; Miao, Y.; Zhang, F.; Huang, J.; Chen, Y.; Fan, Z.; Yang, L.; Wang, J.; Hu, Z. Nanoscale Microenvironment Engineering Based on Layer-by-Layer Self-Assembly to Regulate Hair Follicle Stem Cell Fate for Regenerative Medicine. Theranostics 2020, 10, 11673–11689. [Google Scholar] [CrossRef]
- Wu, N.; Ru, L.; Liu, Y. Layer-by-Layer Assembly for Steering the Survival and Immunogenicity of RPE Cells In Vitro and In Vivo. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1924. [Google Scholar]






| Materials | Cells Encapsulated+Bioactive Compound | Devices | Application | Ref |
|---|---|---|---|---|
| Alginate | pancreatic β-cells/GSA | microneedle | type 1 diabetes | [69] |
| Alginate/polyether sulfone membrane | insulin-producing cells INS-1 and BMSCs | hydrogel-composited/membrane system | type 1 diabetes | [70] |
| Zwitterionic alginates/nylon nanofiber | islets and stem cell-derived beta |  | type 1 diabetes | [73] |
| Collagen-alginate | pancreatic rat islets | microtube  | type 1 diabetes | [86] |
| Collagen-alginate | pancreatic islet cells | microtube  | type 1 diabetes | [87] |
| Alginate/ECM | pancreatic islet cells, endothelial cells, cardiomyocytes and nerve cells | core–shell microtube | e.g., type 1 diabetes | [88] |
| Alginate/ECM/polyacrylamide | mouse pancreatic β cells | core–shell microtube  | type 1 diabetes | [89] |
| Alginate | pancreatic rat islets | donut-shaped, hydrogels  | type 1 diabetes | [90] |
| Alginate/ECM | pancreatic rat islets | core–shell microtube | type 1 diabetes | [99] |
| Alginate | hSC-βs | lotus-root-shaped construct | type 1 diabetes | [104] |
| Alginate/gelatin | islet cells, T regulatory cells and EPC | 3D printed macroporous construct | type 1 diabetes | [107] |
| Polyamide/alginate | β-cells, INS1E pseudoislets | 3D printed microcapsule | type 1 diabetes | [114] |
| Alginate/GelMA | NSC | 3D printed construct | traumatic brain injury | [119] |
| Alginate/polycaprolactone | pancreatic islets and VEGF | 3D plotted scaffolds  | type 1 diabetes | [120] |
| Alginate/methylcellulose | pancreatic islets | 3D printed construct | type 1 diabetes | [125] |
| Alginate/nanofibrillated cellulose | pancreatic islets and ASCs | 3D printed construct | type 1 diabetes | [126] |
| Alginate/collagen/pdECM | human islets | 3D printed construct | type 1 diabetes | [130] |
| Alginate/dECM Alginate/fibrinogen | porcine pancreatic islets, HMSC and HUVEC | 3D printed construct | type 1 diabetes | [136] |
| Alginate/methylcellulose | NICC and BSA | 3D printed construct | type 1 diabetes | [139] |
| PCL/GelXA LAMININK-411 (alginate, GelMA, laminin) | INS1, HUVEC | 3D printed construct | type 1 diabetes | [142] |
| Alginate/collagen | iPSC | scaffold | 3D environment for the growth and development of human neurons | [145] |
| Alginate sulfate/ECM | hASCs | laminated composite scaffolds | cartilage treatment | [146] |
| Alginate/hyaluronic acid /matrigel | human islet, mouse islet, rat INS1E Β-cell | 3D printed construct | type 1 diabetes | [147] |
| Alginate/gelatin | rat Schwann cells | 3D bioprinted scaffold | peripheral nerve injury | [148] |
| Alginate/gelatin | NSCs and OLGs | 3D bioprinted scaffold | spinal cord injury | [149] |
| PVDF-HFP/alginate | INS-1 cells or islets | 3D bioprinted scaffold | type 1 diabetes | [150] |
| Alginate/silk | hMSCs | 3D bioprinted scaffold | cartilage treatment | [151] |
| Formulation | Cells Encapsulated+Bioactive Compound | Applications | Ref |
|---|---|---|---|
| Alginate/gelatin/FeCl3 | MC3T3-E1 cells | bone regeneration | [164] |
| Alginate/gelatin/CaCl2 | MDSCs | MI | [171] |
| Oxidized alginate/gelatin/sodium tetraborate | ADMSC and asiatic acid | neurological disorders | [172] |
| Alginate/collagen/calcium gluconate | MSCs | MI | [175] |
| Alginate/collagen/CaCO3 | BMSCs | traumatic brain injury | [176] |
| Alginate/PSS/CNT | HACs, diacerein | SCI | [186] |
| Alginate/CaCl2 | NSCs | SCI | [187] |
| Alginate/CaCl2 | DPSCs and FGF21 | SCI | [189] |
| Alginate/GRGDSP/CaSO4 | MSCs | MI | [202] |
| alginate/ECM/calcium carbonate | - | MI | [203] |
| Alginate/reduced graphene oxide/CaCl2 | hBM-MSCs | MI | [214] |
| Oxidized alginate/gelatin | ADSCs | MI | [215] |
| Alginate/fullerenol/calcium gluconate | BADSCs | MI | [217] |
| Alginate/reduced graphene oxide/BaCl2 | MSCs | MI | [218] |
| Alginate/fibrin/calcium gluconate | CMs and VEGF | MI | [219] |
| alginate/β-glycerophosphate/dextran | hMSCs | MI | [228] |
| Alginate/CaCl2 | MSC-derived sEVs | MI | [229] |
| Oxidized alginate/carboxymethyl chitosan | BMSCs | infected bone defects | [230] |
| Alginate/matrigel/BG MPs/CaSO4 | DPSC | craniofacial bone regeneration | [231] |
| Polyelectrolytes | Cells+Active Compound | Application | Ref |
|---|---|---|---|
| Alginate/gelatin | MSCs and VEGF | MI | [258] |
| Alginate/gelatin, chitosan, PEI, and PDL | NSCs | Nervous system disorders | [259] |
| Phosphorylcholine-derived polysaccharides/alginate | Pancreatic islets | Type 1 diabetes | [260] |
| Alginate/PLL-g-PEG | Murine pancreatic islets | Type 1 diabetes | [264] |
| Alginate/gelatin | DPC and FGF-2 | Treating hair loss diseases | [271] |
| Alginate/chitosan | L929 cells | - | [278] |
| Alginate/chitosan-graft-phosphorylcholine (CH-PC)/PLL-g-PEG | RBCs | Production of universal RBCs | [279] |
| Alginate/PLL-g-PEG | Murine pancreatic islets | Type 1 diabetes | [280] |
| Alginate/gelatin | hESC-RPE | RPE replacement therapies | [272] |
| Alginate/(PAMAM) | Pancreatic rat islets | Type 1 diabetes | [281] |
| Alginate/PD-TAMP/collagen | Pancreatic islets | Type 1 diabetes | [282] |
| Alginate/gelatin | HFSCs and TGF-β2 | Treating hair loss diseases | [283] |
| Alginate/gelatin | hESC-RPE | Treating retinal degenerative diseases | [284] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavand, A.; Noverraz, F.; Gerber-Lemaire, S. Recent Advances in Alginate-Based Hydrogels for Cell Transplantation Applications. Pharmaceutics 2024, 16, 469. https://doi.org/10.3390/pharmaceutics16040469
Kavand A, Noverraz F, Gerber-Lemaire S. Recent Advances in Alginate-Based Hydrogels for Cell Transplantation Applications. Pharmaceutics. 2024; 16(4):469. https://doi.org/10.3390/pharmaceutics16040469
Chicago/Turabian StyleKavand, Alireza, François Noverraz, and Sandrine Gerber-Lemaire. 2024. "Recent Advances in Alginate-Based Hydrogels for Cell Transplantation Applications" Pharmaceutics 16, no. 4: 469. https://doi.org/10.3390/pharmaceutics16040469