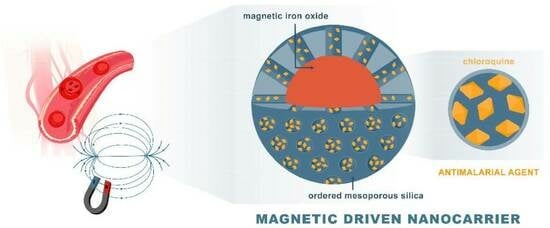
Magnetic Mesoporous Silica for Targeted Drug Delivery of Chloroquine: Synthesis, Characterization, and In Vitro Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of CQ-Loaded Magnetic Mesoporous Silica
2.3. Characterization
2.4. Dissolution/Release Assays
2.5. Cell Culture and MTT Viability Test
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Chaves, J.B.; Portugal Tavares de Moraes, B.; Regina Ferrarini, S.; Noé da Fonseca, F.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Potential of Nanoformulations in Malaria Treatment. Front. Pharmacol. 2022, 13, 999300. [Google Scholar] [CrossRef]
- Menard, D.; Dondorp, A. Antimalarial Drug Resistance: A Threat to Malaria Elimination. Cold Spring Harb. Perspect. Med. 2017, 7, a025619. [Google Scholar] [CrossRef]
- Wicht, K.J.; Mok, S.; Fidock, D.A. Molecular Mechanisms of Drug Resistance in Plasmodium Falciparum Malaria. Annu. Rev. Microbiol. 2020, 74, 431–454. [Google Scholar] [CrossRef]
- Elmi, T.; Ardestani, M.S.; Motevalian, M.; Hesari, A.K.; Hamzeh, M.S.; Zamani, Z.; Tabatabaie, F. Antiplasmodial Effect of Nano Dendrimer G2 Loaded with Chloroquine in Mice Infected with Plasmodium Berghei. Acta Parasitol. 2022, 67, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Soniran, O.T.; Idowu, O.A.; Ajayi, O.L.; Olubi, I.C. Comparative Study on the Effects of Chloroquine and Artesunate on Histopathological Damages Caused by Plasmodium Berghei in Four Vital Organs of Infected Albino Mice. Malar. Res. Treat. 2012, 2012, 960758. [Google Scholar] [CrossRef] [PubMed]
- Macedo, T.S.; Villarreal, W.; Couto, C.C.; Moreira, D.R.M.; Navarro, M.; Machado, M.; Prudêncio, M.; Batista, A.A.; Soares, M.B.P. Platinum(II)-Chloroquine Complexes Are Antimalarial Agents against Blood and Liver Stages by Impairing Mitochondrial Function. Metallomics 2017, 9, 1548–1561. [Google Scholar] [CrossRef] [PubMed]
- Prasad Raiguru, B.; Panda, J.; Mohapatra, S.; Nayak, S. Recent Developments in the Synthesis of Hybrid Antimalarial Drug Discovery. Bioorg. Chem. 2023, 139, 106706. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A Boon to Drug Delivery, Therapeutics, Diagnostics and Imaging. Nanomedicine 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, A.; Asadi, A.; Gurushankar, K.; Shayan, T.K.; Sarvestani, F.A. Importance of Nano Medicine and New Drug Therapies for Cancer. Adv. Pharm. Bull. 2021, 11, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Biosca, A.; Cabanach, P.; Abdulkarim, M.; Gumbleton, M.; Gómez-Canela, C.; Ramírez, M.; Bouzón-Arnáiz, I.; Avalos-Padilla, Y.; Borros, S.; Fernàndez-Busquets, X. Zwitterionic Self-Assembled Nanoparticles as Carriers for Plasmodium Targeting in Malaria Oral Treatment. J. Control. Release 2021, 331, 364–375. [Google Scholar] [CrossRef]
- Avitabile, E.; Senes, N.; D’Avino, C.; Tsamesidis, I.; Pinna, A.; Medici, S.; Pantaleo, A. The Potential Antimalarial Efficacy of Hemocompatible Silver Nanoparticles from Artemisia Species against P. Falciparum Parasite. PLoS ONE 2020, 15, e0238532. [Google Scholar] [CrossRef]
- Varela-Aramburu, S.; Ghosh, C.; Goerdeler, F.; Priegue, P.; Moscovitz, O.; Seeberger, P.H. Targeting and Inhibiting Plasmodium Falciparum Using Ultra-Small Gold Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 43380–43387. [Google Scholar] [CrossRef]
- Wu, K.W.; Sweeney, C.; Dudhipala, N.; Lakhani, P.; Chaurasiya, N.D.; Tekwani, B.L.; Majumdar, S. Primaquine Loaded Solid Lipid Nanoparticles (SLN), Nanostructured Lipid Carriers (NLC), and Nanoemulsion (NE): Effect of Lipid Matrix and Surfactant on Drug Entrapment, in Vitro Release, and Ex Vivo Hemolysis. AAPS PharmSciTech 2021, 22, 240. [Google Scholar] [CrossRef] [PubMed]
- da Silva de Barros, A.O.; Portilho, F.L.; Dos Santos Matos, A.P.; Ricci-Junior, E.; Alencar, L.M.R.; Dos Santos, C.C.; Paumgartten, F.J.R.; Iram, S.H.; Mazier, D.; Franetich, J.F.; et al. Preliminary Studies on Drug Delivery of Polymeric Primaquine Microparticles Using the Liver High Uptake Effect Based on Size of Particles to Improve Malaria Treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112275. [Google Scholar] [CrossRef]
- Owonubi, S.J.; Aderibigbe, B.A.; Mukwevho, E.; Sadiku, E.R.; Ray, S.S. Characterization and in Vitro Release Kinetics of Antimalarials from Whey Protein-Based Hydrogel Biocomposites. Int. J. Ind. Chem. 2018, 9, 39–52. [Google Scholar] [CrossRef]
- Hirayama, H.; Amolegbe, S.A.; Islam, M.S.; Rahman, M.A.; Goto, N.; Sekine, Y.; Hayami, S. Encapsulation and Controlled Release of an Antimalarial Drug Using Surface Functionalized Mesoporous Silica Nanocarriers. J. Mater. Chem. B 2021, 9, 5043–5046. [Google Scholar] [CrossRef] [PubMed]
- Amolegbe, S.A.; Hirano, Y.; Adebayo, J.O.; Ademowo, O.G.; Balogun, E.A.; Obaleye, J.A.; Krettli, A.U.; Yu, C.; Hayami, S. Mesoporous Silica Nanocarriers Encapsulated Antimalarials with High Therapeutic Performance. Sci. Rep. 2018, 8, 3078. [Google Scholar] [CrossRef] [PubMed]
- Santos De Oliveira, R.; Funk, N.L.; Dos Santos, J.; Viana De Oliveira, T.; Gadelha De Oliveira, E.; Petzhold, C.L.; Haas Costa, T.M.; Benvenutti, E.V.; Deon, M.; Carlos, R.; et al. Bioadhesive 3D-Printed Skin Drug Delivery Polymeric Films: From the Drug Loading in Mesoporous Silica to the Manufacturing Process. Pharmaceutics 2022, 15, 20. [Google Scholar] [CrossRef]
- Hate, S.S.; Reutzel-Edens, S.M.; Taylor, L.S. Interplay of Adsorption, Supersaturation and the Presence of an Absorptive Sink on Drug Release from Mesoporous Silica-Based Formulations. Pharm. Res. 2020, 37, 163. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska-Bartkowiak, M.; Dudziak, G.; Gras, R.; Sikorski, R.; Radhakrishnan, R.; Gubbins, K.E. Freezing Behavior in Porous Glasses and MCM-41. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 523–529. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Jiang, N.; Wang, J.; Liang, H.; Liu, D.; Yang, N.; Sang, X.; Feng, Y.; Chen, R.; et al. Enhanced Antimalarial Efficacy Obtained by Targeted Delivery of Artemisinin in Heparin-Coated Magnetic Hollow Mesoporous Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 287–297. [Google Scholar] [CrossRef]
- Nawwab Al-Deen, F.; Ma, C.; Xiang, S.D.; Selomulya, C.; Plebanski, M.; Coppel, R.L. On the Efficacy of Malaria DNA Vaccination with Magnetic Gene Vectors. J. Control. Release 2013, 168, 10–17. [Google Scholar] [CrossRef]
- Nawwab Al-Deen, F.M.; Xiang, S.D.; Ma, C.; Wilson, K.; Coppel, R.L.; Selomulya, C.; Plebanski, M. Magnetic Nanovectors for the Development of DNA Blood-Stage Malaria Vaccines. Nanomaterials 2017, 7, 30. [Google Scholar] [CrossRef]
- Reimer, P.; Balzer, T. Ferucarbotran (Resovist): A New Clinically Approved RES-Specific Contrast Agent for Contrast-Enhanced MRI of the Liver: Properties, Clinical Development, and Applications. Eur. Radiol. 2003, 13, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J.; Hussain, S.M.; Krestin, G.P. Superparamagnetic Iron Oxide Contrast Agents: Physicochemical Characteristics and Applications in MR Imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hsu, J.C.; Koo, H.; Cormode, D.P. Repurposing Ferumoxytol: Diagnostic and Therapeutic Applications of an FDA-Approved Nanoparticle. Theranostics 2022, 12, 796–816. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- de Souza, L.V.; da Rosa, D.S.; Tkachenko, O.S.; de Araujo Gomes, A.; Costa, T.M.H.; Arenas, L.T.; Benvenutti, E.V. The Role Silica Pore Structure Plays in the Performance of Modified Carbon Paste Electrodes. Ionics 2019, 25, 3259–3268. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-in Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Langford, J.I.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Hillier, S. Accurate Quantitative Analysis of Clay and Other Minerals in Sandstones by XRD: Comparison of a Rietveld and a Reference Intensity Ratio (RIR) Method and the Importance of Sample Preparation. Clay Min. 2000, 35, 291–302. [Google Scholar] [CrossRef]
- Kim, W.; Suh, C.Y.; Cho, S.W.; Roh, K.M.; Kwon, H.; Song, K.; Shon, I.J. A New Method for the Identification and Quantification of Magnetite-Maghemite Mixture Using Conventional X-ray Diffraction Technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Sijo, A.K.; Jha, V.K.; Kaykan, L.S.; Dutta, D.P. Structure and Cation Distribution in Superparamagnetic NiCrFeO4 Nanoparticles Using Mössbauer Study. J. Magn. Magn. Mater. 2020, 497, 166047. [Google Scholar] [CrossRef]
- Batlle, X.; Labarta, A. Finite-Size Effects in Fine Particles: Magnetic and Transport Properties. J. Phys. D Appl. Phys. 2002, 35, 201. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic Iron Oxide Nanoparticles for Drug Delivery: Applications and Characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Polla, M.B.; Nicolini, J.L.; Venturini, J.; da Cas Viegas, A.; Zen Vasconcellos, M.A.; Montedo, O.R.K.; Arcaro, S. Low-Temperature Sol–Gel Synthesis of Magnetite Superparamagnetic Nanoparticles: Influence of Heat Treatment and Citrate–Nitrate Equivalence Ratio. Ceram. Int. 2023, 49, 7322–7332. [Google Scholar] [CrossRef]
- Da Costa, G.M.; Blanco-Andujar, C.; De Grave, E.; Pankhurst, Q.A. Magnetic Nanoparticles for in Vivo Use: A Critical Assessment of Their Composition. J. Phys. Chem. B 2014, 118, 11738–11746. [Google Scholar] [CrossRef]
- Harres, A.; Mikhov, M.; Skumryev, V.; De Andrade, A.M.H.; Schmidt, J.E.; Geshev, J. Criteria for Saturated Magnetization Loop. J. Magn. Magn. Mater. 2016, 402, 76–82. [Google Scholar] [CrossRef]
- Shokrollahi, H. A Review of the Magnetic Properties, Synthesis Methods and Applications of Maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Pourhasan-Kisomi, R.; Shirini, F.; Golshekan, M. Introduction of Organic/Inorganic Fe3O4@MCM-41@Zr-Piperazine Magnetite Nanocatalyst for the Promotion of the Synthesis of Tetrahydro-4H-Chromene and Pyrano[2,3-d]Pyrimidinone Derivatives. Appl. Organomet. Chem. 2018, 32, e4371. [Google Scholar] [CrossRef]
- Xie, W.; Zang, X. Immobilized Lipase on Core-Shell Structured Fe3O4-MCM-41 Nanocomposites as a Magnetically Recyclable Biocatalyst for Interesterification of Soybean Oil and Lard. Food Chem. 2016, 194, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Hajian, R.; Ehsanikhah, A. Manganese Porphyrin Immobilized on Magnetic MCM-41 Nanoparticles as an Efficient and Reusable Catalyst for Alkene Oxidations with Sodium Periodate. Chem. Phys. Lett. 2018, 691, 146–154. [Google Scholar] [CrossRef]
- Jaroniec, M.; Jaroniec, C.P.; Kruk, M.; Ryoo, R. Adsorption and Thermogravimetric Methods for Monitoring Surface and Structural Changes in Ordered Mesoporous Silicas Induced by Their Chemical Modification. Adsorption 1999, 5, 313–317. [Google Scholar] [CrossRef]
- La-Salvia, N.; Lovón-Quintana, J.J.; Lovón, A.S.P.; Valença, G.P. Influence of Aluminum Addition in the Framework of MCM-41 Mesoporous Molecular Sieve Synthesized by Non-Hydrothermal Method in an Alkali-Free System. Mater. Res. 2017, 20, 1461–1469. [Google Scholar] [CrossRef]
- Florey, K. Analytical Profiles of Drug Substances; Academic Press: Cambridge, MA, USA, 1984; Volume 13, ISBN 0122608135. [Google Scholar]
- Heikkilä, T.; Salonen, J.; Tuura, J.; Kumar, N.; Salmi, T.; Murzin, D.Y.; Hamdy, M.S.; Mul, G.; Laitinen, L.; Kaukonen, A.M.; et al. Evaluation of Mesoporous TCPSi, MCM-41, SBA-15, and TUD-1 Materials as API Carriers for Oral Drug Delivery. Drug Deliv. 2007, 14, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Charnay, C.; Bégu, S.; Tourné-Péteilh, C.; Nicole, L.; Lerner, D.A.; Devoisselle, J.M. Inclusion of Ibuprofen in Mesoporous Templated Silica: Drug Loading and Release Property. Eur. J. Pharm. Biopharm. 2004, 57, 533–540. [Google Scholar] [CrossRef]
- Müller, R.H.; Hespeler, D.; Jin, N.; Pyo, S.M. SmartPearls—Novel Physically Stable Amorphous Delivery System for Poorly Soluble Dermal Actives. Int. J. Pharm. 2019, 555, 314–321. [Google Scholar] [CrossRef]
- Shah, P.; Rajput, S.J. Investigation of in Vitro Permeability and in Vivo Pharmacokinetic Behavior of Bare and Functionalized MCM-41 and MCM-48 Mesoporous Silica Nanoparticles: A Burst and Controlled Drug Release System for Raloxifene. Drug Dev. Ind. Pharm. 2019, 45, 587–602. [Google Scholar] [CrossRef]
- Padmaa Paarakh, M.; Ani Jose, P.; Setty, C.M.; Christoper, G.V.P. Release kinetics-concepts and applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar]
- Kozakevych, R.B.; Bolbukh, Y.M.; Tertykh, V.A. Controlled Release of Diclofenac Sodium from Silica-Chitosan Composites. World J. Nano Sci. Eng. 2013, 3, 69–78. [Google Scholar] [CrossRef]
- da Silva, J.D.; Gomes, M.V.; Cabral, L.M.; de Sousa, V.P. Evaluation of the in Vitro Release and Permeation of Cordia Verbenacea DC Essential Oil from Topical Dosage Forms. J. Drug Deliv. Sci. Technol. 2019, 53, 101173. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, H.; Zhang, Z.; Wen, B.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Applications and Biocompatibility of Mesoporous Silica Nanocarriers in the Field of Medicine. Front. Pharmacol. 2022, 13, 829796. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.K.M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Pegylated Mesoporous Silica Nanoparticles (Mcm-41): A Promising Carrier for the Targeted Delivery of Fenbendazole into Prostrate Cancer Cells. Pharmaceutics 2021, 13, 1605. [Google Scholar] [CrossRef]
- Varache, M.; Bezverkhyy, I.; Saviot, L.; Bouyer, F.; Baras, F.; Bouyer, F. Optimization of MCM-41 Type Silica Nanoparticles for Biological Applications: Control of Size and Absence of Aggregation and Cell Cytotoxicity. J. Non. Cryst. Solids. 2015, 408, 87–97. [Google Scholar] [CrossRef]
- Quan, G.; Pan, X.; Wang, Z.; Wu, Q.; Li, G.; Dian, L.; Chen, B.; Wu, C. Lactosaminated Mesoporous Silica Nanoparticles for Asialoglycoprotein Receptor Targeted Anticancer Drug Delivery. J. Nanobiotechnol. 2015, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, S.; Chowdhury, S.; Pandey, B.; Sil, P.C. Targeted Delivery of Quercetin Loaded Mesoporous Silica Nanoparticles to the Breast Cancer Cells. Biochim. Biophys. Acta. Gen. Subj. 2016, 1860, 2065–2075. [Google Scholar] [CrossRef]
- Lv, Y.; Cao, Y.; Li, P.; Liu, J.; Chen, H.; Hu, W.; Zhang, L. Ultrasound-Triggered Destruction of Folate-Functionalized Mesoporous Silica Nanoparticle-Loaded Microbubble for Targeted Tumor Therapy. Adv. Heal. Mater 2017, 6, 1700354. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Lee, C.C.; Lin, H.M. Hyaluronidase-Responsive Mesoporous Silica Nanoparticles with Dual-Imaging and Dual-Target Function. Cancers 2019, 11, 697. [Google Scholar] [CrossRef]








| Sample | SBET (±5 m2 g−1) | Pore Volume (±0.001 cm3 g−1) | Main Pore Size a (nm) |
|---|---|---|---|
| magMCM | 624 | 0.649 | 2.7 |
| magMCM-CQ | 467 | 0.346 | 1.9 |
| Time | Non-Encapsulated CQ (% CQ Released) | magMCM-CQ (% CQ Released) |
|---|---|---|
| 3 | 17.3 | 58.8 |
| 90 | 65.9 | 95.5 |
| 180 | 94.1 | 99.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Andrade, R.; Schmidt, R.d.C.d.R.; Gomes, L.S.; Colina-Vegas, L.; Hinrichs, R.; Vasconcellos, M.A.Z.; Costa, T.M.H.; Deon, M.; Villarreal, W.; Benvenutti, E.V. Magnetic Mesoporous Silica for Targeted Drug Delivery of Chloroquine: Synthesis, Characterization, and In Vitro Evaluation. Pharmaceutics 2024, 16, 357. https://doi.org/10.3390/pharmaceutics16030357
de Andrade R, Schmidt RdCdR, Gomes LS, Colina-Vegas L, Hinrichs R, Vasconcellos MAZ, Costa TMH, Deon M, Villarreal W, Benvenutti EV. Magnetic Mesoporous Silica for Targeted Drug Delivery of Chloroquine: Synthesis, Characterization, and In Vitro Evaluation. Pharmaceutics. 2024; 16(3):357. https://doi.org/10.3390/pharmaceutics16030357
Chicago/Turabian Stylede Andrade, Rafaela, Rita de Cássia dos Reis Schmidt, Leonardo Santos Gomes, Legna Colina-Vegas, Ruth Hinrichs, Marcos Antônio Zen Vasconcellos, Tania Maria Haas Costa, Monique Deon, Wilmer Villarreal, and Edilson Valmir Benvenutti. 2024. "Magnetic Mesoporous Silica for Targeted Drug Delivery of Chloroquine: Synthesis, Characterization, and In Vitro Evaluation" Pharmaceutics 16, no. 3: 357. https://doi.org/10.3390/pharmaceutics16030357