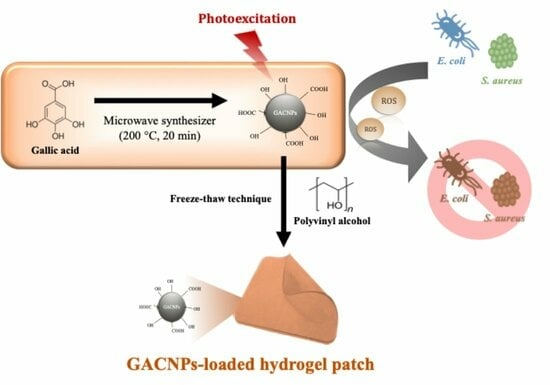
Photodynamic Antibacterial Therapy of Gallic Acid-Derived Carbon-Based Nanoparticles (GACNPs): Synthesis, Characterization, and Hydrogel Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GACNPs
2.3. Characterization of GACNPs
2.4. Antibacterial Activity of GACNPs
2.4.1. Preparation of Bacterial Suspensions
2.4.2. Minimum Inhibitory Concentration
2.4.3. Minimum Bactericidal Concentration
2.4.4. Photodynamic Antibacterial Activity of GACNPs
2.4.5. Detection of the Formation of Singlet Oxygen (1O2) of GACNPs
2.5. Preparation of Hydrogels
2.5.1. Preparation of Blank Hydrogels
2.5.2. Preparation of GACNP-Loaded Hydrogels
2.6. Characterization of Hydrogels
2.6.1. Chemical Properties
2.6.2. Mechanical Properties
2.6.3. Water Content
2.6.4. Water Absorption
2.6.5. Stability Study
2.7. Antibacterial Activity of GACNP-Loaded Hydrogel Patches
2.7.1. Quantitative Method
2.7.2. Qualitative Method (Disc Diffusion Assay)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of GACNPs
3.2. Characterization of GACNPs
3.3. Photodynamic Antibacterial Activity of GACNPs
3.4. Detection of the Formation of Singlet Oxygen (1O2) of GACNPs
3.5. Chemical Properties of the Hydrogels
3.6. Mechanical Properties of the Hydrogels
3.7. Water Content and Water Absorption
3.8. Stability Study
3.9. Antibacterial Activity of GACNP-Loaded Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Design and Synthesis of Novel Antimicrobial Agents. Antibiotics 2023, 12, 628. [Google Scholar] [CrossRef]
- Di Luca, M.; Marzo, T. Development of Effective Antibacterial Treatment: Lessons from the Past and Novel Approaches. Antibiotics 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chu, Z.; Jiang, Y.; Xu, L.; Qian, H.; Wang, Y.; Wang, W. Recent advances on nanomaterials for antibacterial treatment of oral diseases. Mater. Today Bio 2023, 20, 100635. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Ellah, N.H.A.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Xiu, W.; Shan, J.; Yang, K.; Xiao, H.; Yuwen, L.; Wang, L. Recent development of nanomedicine for the treatment of bacterial biofilm infections. View 2020, 2, 20200065. [Google Scholar] [CrossRef]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Bin Emran, T.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ.-Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M. Antibacterial Activity of Nanomaterials. Nanomaterials 2018, 8, 359. [Google Scholar] [CrossRef]
- Maťátková, O.; Michailidu, J.; Miškovská, A.; Kolouchová, I.; Masák, J.; Čejková, A. Antimicrobial properties and applications of metal nanoparticles biosynthesized by green methods. Biotechnol. Adv. 2022, 58, 107905. [Google Scholar] [CrossRef] [PubMed]
- Sweet, M.; Singleton, I. Silver nanoparticles: A microbial perspective. Adv. Appl. Microbiol. 2011, 77, 115–133. [Google Scholar] [PubMed]
- dos Santos, M.C.; Maynart, M.C.; Aveiro, L.R.; da Paz, E.C.; dos Santos Pinheiro, V. Carbon-Based Materials: Recent Advances. Challenges, and Perspectives. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2018, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care-An Updated Review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Shah, S.Z.A.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Ben Khedher, N.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Li, X.; Yu, L.; He, M.; Chen, C.; Yu, Z.; Jiang, S.; Wang, Y.; Li, L.; Li, B.; Wang, G.; et al. Review on carbon dots: Synthesis and application in biology field. BMEMat 2023, 1, e12045. [Google Scholar] [CrossRef]
- Manzoor, S.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Srivastava, S.; Bashir, I.; Khan, S.A. Carbon dots applications for development of sustainable technologies for food safety: A comprehensive review. Appl. Food Res. 2023, 3, 100263. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Yu, S.; Jiang, C. Fluorescent carbon dots: Rational synthesis, tunable optical properties and analytical applications. RSC Adv. 2017, 7, 40973–40989. [Google Scholar] [CrossRef]
- Thanayutsiri, T.; Patrojanasophon, P.; Opanasopit, P.; Ngawhirunpat, T.; Plianwong, S.; Rojanarata, T. Rapid synthesis of chitosan-capped gold nanoparticles for analytical application and facile recovery of gold from laboratory waste. Carbohydr. Polym. 2020, 250, 116983. [Google Scholar] [CrossRef]
- Cutrim, E.S.; Vale, A.A.; Manzani, D.; Barud, H.S.; Rodríguez-Castellón, E.; Santos, A.P.; Alcântara, A.C. Preparation, characterization and in vitro anticancer performance of nanoconjugate based on carbon quantum dots and 5-Fluorouracil. Mater. Sci. Eng. C 2021, 120, 111781. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Ma, Y.; Che, M.; Zhang, B.; Zhang, Y.; Li, Y.; Zhang, W.; Sang, S. Fluorescent carbon dots as carriers for intracellular doxorubicin delivery and track. J. Drug Deliv. Sci. Technol. 2019, 49, 527–533. [Google Scholar] [CrossRef]
- Ross, S.; Wu, R.S.; Wei, S.C.; Ross, G.M.; Chang, H.T. The analytical and biomedical applications of carbon dots and their future theranostic potential: A review. J. Food Drug Anal. 2020, 28, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.W.; Lee, S.L.; Chang, C.J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Kadouh, H.; Zhou, K. The antimicrobial, mechanical, physical and structural properties of chitosan–gallic acid films. LWT-Food Sci. Technol. 2014, 57, 83–89. [Google Scholar] [CrossRef]
- Zhang, O.L.; Niu, J.Y.; Yin, I.X.; Yu, O.Y.; Mei, M.L.; Chu, C.H. Antibacterial Properties of the Antimicrobial Peptide Gallic Acid-Polyphemusin I (GAPI). Antibiotics 2023, 12, 1350. [Google Scholar] [CrossRef]
- Rodrigues, G.R.; López-Abarrategui, C.; de la Serna Gómez, I.; Dias, S.C.; Otero-González, A.J.; Franco, O.L. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef]
- Singh, R.; Sreedharan, S.; Singh, S. The Role of Nanotechnology in Combating Multi-Drug Resistant Bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef]
- Jelinkova, P.; Mazumdar, A.; Sur, V.P.; Kociova, S.; Dolezelikova, K.; Jimenez, A.M.J.; Koudelkova, Z.; Mishra, P.K.; Smerkova, K.; Heger, Z.; et al. Nanoparticle-drug conjugates treating bacterial infections. J. Control. Release 2019, 307, 166–185. [Google Scholar] [CrossRef]
- Shamsi, S.; Ghafor, A.A.H.A.; Norjoshukrudin, N.H.; Ng, I.M.J.; Abdullah, S.N.S.; Sarchio, S.N.E.; Yasin, F.M.; Gani, S.A.; Desa, M.N.M. Stability, Toxicity, and Antibacterial Potential of Gallic Acid-Loaded Graphene Oxide (GAGO) Against Methicillin-Resistant Staphylococcus aureus (MRSA) Strains. Int. J. Nanomed. 2022, 17, 5781–5807. [Google Scholar] [CrossRef]
- Dediu, V.; Ghitman, J.; Gradisteanu Pircalabioru, G.; Chan, K.H.; Iliescu, F.S.; Iliescu, C. Trends in Photothermal Nanostructures for Antimicrobial Applications. Int. J. Mol. Sci. 2023, 24, 9375. [Google Scholar] [CrossRef]
- Wei, G.; Yang, G.; Wang, Y.; Jiang, H.; Fu, Y.; Yue, G.; Ju, R. Phototherapy-based combination strategies for bacterial infection treatment. Theranostics 2020, 10, 12241–12262. [Google Scholar] [CrossRef] [PubMed]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.I.; Costa-Almeida, R.; Gonçalves, I.C.; Magalhães, F.D.; Pinto, A.M. Carbon nanomaterials for phototherapy of cancer and microbial infections. Carbon 2022, 190, 194–244. [Google Scholar] [CrossRef]
- George, B.P.; Chota, A.; Sarbadhikary, P.; Abrahamse, H. Fundamentals and applications of metal nanoparticle- enhanced singlet oxygen generation for improved cancer photodynamic therapy. Front. Chem. 2022, 10, 964674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, F.; Zhang, S.; An, Y.; Sun, S. Preparation of N-doped yellow carbon dots and N, P co-doped red carbon dots for bioimaging and photodynamic therapy of tumors. New J. Chem. 2019, 43, 6332–6342. [Google Scholar] [CrossRef]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Budimir, M.; Marković, Z.; Vajdak, J.; Jovanović, S.; Kubat, P.; Humpoliček, P.; Mičušik, M.; Danko, M.; Barras, A.; Milivojević, D.; et al. Enhanced visible light-triggered antibacterial activity of carbon quantum dots/polyurethane nanocomposites by gamma rays induced pre-treatment. Radiat. Phys. Chem. 2021, 185, 109499. [Google Scholar] [CrossRef]
- Jana, D.; Wang, D.; Rajendran, P.; Bindra, A.K.; Guo, Y.; Liu, J.; Pramanik, M.; Zhao, Y. Hybrid Carbon Dot Assembly as a Reactive Oxygen Species Nanogenerator for Ultrasound-Assisted Tumor Ablation. JACS AU 2021, 1, 2328–2338. [Google Scholar] [CrossRef]
- Li, Q.; Shen, X.; Xing, D. Carbon quantum dots as ROS-generator and -scavenger: A comprehensive review. Dye. Pigment. 2023, 208, 110784. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Janczura, M.; Sip, S.; Cielecka-Piontek, J. The Development of Innovative Dosage Forms of the Fixed-Dose Combination of Active Pharmaceutical Ingredients. Pharmaceutics 2022, 14, 834. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Demeter, M.; Scarisoreanu, A.; Calina, I. State of the Art of Hydrogel Wound Dressings Developed by Ionizing Radiation. Gels 2023, 9, 55. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Waresindo, W.X.; Luthfianti, H.R.; Priyanto, A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Freeze–thaw hydrogel fabrication method: Basic principles, synthesis parameters, properties, and biomedical applications. Mater. Res. Express 2023, 10, 024003. [Google Scholar] [CrossRef]
- Li, X.; Han, W.; He, G.; Yang, J.; Li, J.; Ma, H.; Wang, S. Hydrogel-Transformable Antioxidant Poly-gamma-Glutamic Acid/Polyethyleneimine Hemostatic Powder for Efficient Wound Hemostasis. Gels 2024, 10, 68. [Google Scholar] [CrossRef]
- Omidi, M.; Yadegari, A.; Tayebi, L. Wound dressing application of pH-sensitive carbon dots/chitosan hydrogel. RSC Adv. 2017, 7, 10638–10649. [Google Scholar] [CrossRef]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; González, E.A.S.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Cui, F.; Sun, J.; Ji, J.; Yang, X.; Wei, K.; Xu, H.; Gu, Q.; Zhang, Y.; Sun, X. Carbon dots-releasing hydrogels with antibacterial activity, high biocompatibility, and fluorescence performance as candidate materials for wound healing. J. Hazard. Mater. 2021, 406, 124330. [Google Scholar] [CrossRef]
- Cui, T.; Fan, Y.; Liu, Y.; Fan, X.; Sun, Y.; Cheng, G.; Cheng, J. Antibacterial Activity and Mechanism of Self-Assembly Spermidine-Capped Carbon Dots against Staphylococcus aureus. Foods 2023, 13, 67. [Google Scholar] [CrossRef]
- Feng, J.; Chen, S.; Yu, Y.-L.; Wang, J.-H. Red-emission hydrophobic porphyrin structure carbon dots linked with transferrin for cell imaging. Talanta 2020, 217, 121014. [Google Scholar] [CrossRef]
- He, G.; Shu, M.; Yang, Z.; Ma, Y.; Huang, D.; Xu, S.; Wang, Y.; Hu, N.; Zhang, Y.; Xu, L. Microwave formation and photoluminescence mechanisms of multi-states nitrogen doped carbon dots. Appl. Surf. Sci. 2017, 422, 257–265. [Google Scholar] [CrossRef]
- Papaioannou, N.; Titirici, M.M.; Sapelkin, A. Investigating the Effect of Reaction Time on Carbon Dot Formation, Structure, and Optical Properties. ACS Omega 2019, 4, 21658–21665. [Google Scholar] [CrossRef] [PubMed]
- Kraus, J.; Kortus, J. A theoretical investigation into gallic acid pyrolysis. J. Comput. Chem. 2022, 43, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, S.; Wu, X.; Boulanger, N.; Gracia-Espino, E.; Wågberg, T.; Edman, L.; Wang, J. Carbon nanodots: A metal-free, easy-to-synthesize, and benign emitter for light-emitting electrochemical cells. Nano Res. 2022, 15, 5610–5618. [Google Scholar] [CrossRef]
- Yuan, F.; He, P.; Xi, Z.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. Highly efficient and stable white LEDs based on pure red narrow bandwidth emission triangular carbon quantum dots for wide-color gamut backlight displays. Nano Res. 2019, 12, 1669–1674. [Google Scholar] [CrossRef]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.; Chen, A.; et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.; Xu, J.; Zhao, Y. Therapeutic applications of low-toxicity spherical nanocarbon materials. NPG Asia Mater. 2014, 6, e84. [Google Scholar] [CrossRef]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Omer, K.M.; Hawaiz, F.E. Deep insights to explain the mechanism of carbon dot formation at various reaction times using the hydrothermal technique: FT-IR, (13)C-NMR, (1)H-NMR, and UV-visible spectroscopic approaches. RSC Adv. 2023, 13, 14340–14349. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Gharpure, A.; Vander Wal, R.L.; Kollar, J.; Herd, C.R. Effect of Fuel Composition on Carbon Black Formation Pathways. Appl. Sci. 2022, 12, 2569. [Google Scholar] [CrossRef]
- Tucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Dorniani, D.; Saifullah, B.; Barahuie, F.; Arulselvan, P.; Bin Hussein, M.Z.; Fakurazi, S.; Twyman, L.J. Graphene Oxide-Gallic Acid Nanodelivery System for Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A deep investigation into the structure of carbon dots. Carbon 2021, 173, 433–447. [Google Scholar] [CrossRef]
- Siddique, A.B.; Pramanick, A.K.; Chatterjee, S.; Ray, M. Amorphous Carbon Dots and their Remarkable Ability to Detect 2,4,6-Trinitrophenol. Sci. Rep. 2018, 8, 9770. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.; Liu, Y.; Xiao, Y.; Hu, H.; Liang, Y.; Zheng, M. Surface chemical functionality of carbon dots: Influence on the structure and energy storage performance of the layered double hydroxide. RSC Adv. 2021, 11, 10785–10793. [Google Scholar] [CrossRef]
- Pandey, S.; Mewada, A.; Thakur, M.; Tank, A.; Sharon, M. Cysteamine hydrochloride protected carbon dots as a vehicle for the efficient release of the anti-schizophrenic drug haloperidol. RSC Adv. 2013, 3, 26290–26296. [Google Scholar] [CrossRef]
- Senel, B.; Demir, N.; Buyukkoroglu, G.; Yildiz, M. Graphene quantum dots: Synthesis, characterization, cell viability, genotoxicity for biomedical applications. Saudi Pharm. J. 2019, 27, 846–858. [Google Scholar] [CrossRef]
- Mosquera, J.; Garcia, I.; Liz-Marzan, L.M. Cellular Uptake of Nanoparticles versus Small Molecules: A Matter of Size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, W.; Chen, J.; Huang, X.; Liu, Y.; Zhang, X.; Shu, W.; Lei, B.; Zhang, H. Antibacterial Activity and Synergetic Mechanism of Carbon Dots against Gram-Positive and -Negative Bacteria. ACS Appl. Bio Mater. 2021, 4, 6937–6945. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Balachandran, M. Antibacterial efficiency of carbon dots against Gram-positive and Gram-negative bacteria: A review. J. Environ. Chem. Eng. 2021, 9, 106821. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Jameei, A.; Garai, A.; Saha, R.; Karande, A.A.; Chakravarty, A.R. Mitochondria-localizing BODIPY-copper(ii) conjugates for cellular imaging and photo-activated cytotoxicity forming singlet oxygen. Dalton. Trans. 2018, 47, 5019–5030. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Abroshan, H.; Kauffman, D.R.; Li, G. Au38S2(SAdm)20 Photocatalyst for One-Step Selective Aerobic Oxidations. ACS Catal. 2017, 7, 3368–3374. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, W. Dipole-Dipole and H-Bonding Interactions Significantly Enhance the Multifaceted Mechanical Properties of Thermoresponsive Shape Memory Hydrogels. Adv. Funct. Mater. 2015, 25, 471–480. [Google Scholar] [CrossRef]
- Miao, L.; Wang, X.; Li, S.; Tu, Y.; Hu, J.; Huang, Z.; Lin, S.; Gui, X. An Ultra-Stretchable Polyvinyl Alcohol Hydrogel Based on Tannic Acid Modified Aramid Nanofibers for Use as a Strain Sensor. Polymers 2022, 14, 3532. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Kaewpirom, S. Potassium Release Kinetics and Water Retention of Controlled-Release Fertilizers Based on Chitosan Hydrogels. J. Polym. Environ. 2010, 18, 413–421. [Google Scholar] [CrossRef]
- Pan, N.C.; Bersaneti, G.T.; Mali, S. Celligoi MAPC, Films Based on Blends of Polyvinyl Alcohol and Microbial Hyaluronic Acid. Braz. Arch. Biol. Technol. 2020, 63, e20190386. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, C.; Cheng, S.; Zhang, P.; Chen, W. Microfluidic-Based Continuous Fabrication of Ultrathin Hydrogel Films with Controllable Thickness. Polymers 2023, 15, 2905. [Google Scholar] [CrossRef]
- Selby, A.; Maldonado-Codina, C.; Derby, B. Influence of specimen thickness on the nanoindentation of hydrogels: Measuring the mechanical properties of soft contact lenses. J. Mech. Behav. Biomed. Mater. 2014, 35, 144–156. [Google Scholar] [CrossRef]
- Le, G.H.; Thanh, D.A.; My, P.T.H.; Pham, T.T.; Quan, T.T.T.; Nguyen, T.N.; Nguyen, Q.K.; Ngo, Q.A. One-step synthesis of super-absorbent nanocomposite hydrogel based on bentonite. Mater. Res. Express 2023, 10, 015001. [Google Scholar] [CrossRef]
- Li, P.; Sun, L.; Xue, S.; Qu, D.; An, L.; Wang, X.; Sun, Z. Recent advances of carbon dots as new antimicrobial agents. SmartMat 2022, 3, 226–248. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Rybenkov, V.V. Permeability barriers of Gram-negative pathogens. Ann. N. Y. Acad. Sci. 2020, 1459, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]








| Condition | Particle Size (nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|---|
| Time (min) | Temperature (°C) | |||
| 10 | 200 | 286.3 ± 1.13 | 0.301 ± 0.052 | −8.14 ± 0.30 |
| 15 | 200 | 238.9 ± 3.87 | 0.249 ± 0.011 | −16.10 ± 1.27 |
| 20 | 200 | 96.91 ± 6.78 | 0.326 ± 0.013 | −19.00 ± 1.91 |
| 20 | 175 | 139.5 ± 1.98 | 0.321 ± 0.114 | −8.82 ± 1.67 |
| 20 | 220 | 128.6 ± 3.11 | 0.280 ± 0.049 | −14.50 ± 3.32 |
| Sample | S. aureus | E. coli | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| GACNPs | 0.29 | 0.29 | 0.29 | 0.58 |
| GA solution | 1.24 | 1.24 | 1.24 | 1.24 |
| Hydrogel Patches | GACNP-Loaded Hydrogel Patches (mg/g) | Thickness of Hydrogel (mm) | Young’s Modulus (Pa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|---|---|
| - | 1.7 | 545.19 ± 0.29 | 0.35 ± 0.00 * | 647.46 ± 8.90 * | |
| Blank | - | 2.2 | 563.32 ± 7.51 | 0.21 ± 0.00 | 378.17 ± 10.56 |
| - | 2.7 | 563.66 ± 1.59 | 0.13 ± 0.01 | 218.57 ± 2.47 | |
| GACNPs | 0.5 | 1.7 | 384.59 ± 9.44 | 0.19 ± 0.03 | 413.53 ± 15.57 |
| GACNPs | 0.7 | 1.7 | 355.37 ± 13.37 | 0.14 ± 0.00 | 395.71 ± 7.00 |
| GACNPs | 0.9 | 1.7 | 351.35 ± 10.19 | 0.15 ± 0.03 | 378.98 ± 11.88 |
| Hydrogel Patches | Water Content (%) | Water Absorption (%) |
|---|---|---|
| Blank hydrogels | 82.17 ± 0.07 | 44.77 ± 6.47 |
| GACNP-loaded hydrogels | 74.70 ± 1.04 | 94.87 ± 2.90 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dechsri, K.; Suwanchawalit, C.; Patrojanasophon, P.; Opanasopit, P.; Pengnam, S.; Charoenying, T.; Taesotikul, T. Photodynamic Antibacterial Therapy of Gallic Acid-Derived Carbon-Based Nanoparticles (GACNPs): Synthesis, Characterization, and Hydrogel Formulation. Pharmaceutics 2024, 16, 254. https://doi.org/10.3390/pharmaceutics16020254
Dechsri K, Suwanchawalit C, Patrojanasophon P, Opanasopit P, Pengnam S, Charoenying T, Taesotikul T. Photodynamic Antibacterial Therapy of Gallic Acid-Derived Carbon-Based Nanoparticles (GACNPs): Synthesis, Characterization, and Hydrogel Formulation. Pharmaceutics. 2024; 16(2):254. https://doi.org/10.3390/pharmaceutics16020254
Chicago/Turabian StyleDechsri, Koranat, Cheewita Suwanchawalit, Prasopchai Patrojanasophon, Praneet Opanasopit, Supusson Pengnam, Thapakorn Charoenying, and Theerada Taesotikul. 2024. "Photodynamic Antibacterial Therapy of Gallic Acid-Derived Carbon-Based Nanoparticles (GACNPs): Synthesis, Characterization, and Hydrogel Formulation" Pharmaceutics 16, no. 2: 254. https://doi.org/10.3390/pharmaceutics16020254