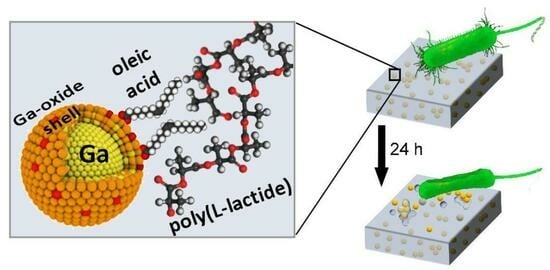
Nanogallium-poly(L-lactide) Composites with Contact Antibacterial Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Oleic-Acid-Capped Colloidal Gallium Nanoparticles
2.2. Preparation of Ga/PLA Nanocomposites
2.3. Characterization of the Nanocomposite Films
2.3.1. Quantitative Determination of Gallium
2.3.2. Morphological and Spectrophotometric Analyses
2.3.3. Crystallinity and Thermal Properties
2.3.4. Surface Wettability and Mechanical Properties
2.4. Release of Ga from the Nanocomposites in Conditions of the Antibacterial Tests
2.5. Antibacterial Tests
2.5.1. Agar Diffusion Test
2.5.2. Inhibition of Planktonic Bacterial Growth
2.5.3. Contact Antibacterial Action
2.5.4. Contact Bactericidal Action
2.5.5. Scanning Electron Microscopy of Bacteria on the Films
2.6. Cytotoxicity Tests
2.6.1. Growth of HaCaT Cells in the Presence of Films
2.6.2. Growth of HaCaT Cells on the Films
2.6.3. Scanning Electron Microscopy of HaCaT Cells on the Films
3. Results
3.1. Physico-Chemical Properties of Ga/PLA Nanocomposites
3.1.1. Appearance, Composition, and Optical Properties
3.1.2. Detailed Morphology
3.1.3. Crystallinity and Thermal Properties
3.1.4. Surface Aqueous Wettability and Young’s Modulus
3.2. Antibacterial Properties of Ga/PLA Nanocomposites against Pseudomonas aeruginosa
3.2.1. Inhibition of Planktonic and Colonial Growth
3.2.2. Contact Antibacterial Action in a Nutrient Medium
3.2.3. Contact Bactericidal Action of Ga/PLA2.4% in PBS
3.3. Cytocompatibility of Ga/PLA Nanocomposites with HaCaT Keratinocytes
3.4. Ga Release from Ga/PLA Nanocomposites in Bacterial Growth Medium
4. Discussion
- (a)
- Ga/OA NPs enhance the degradation of PLA. This is expected as a result of the higher thermal conductivity of the metallic liquid Ga core [27], leading to a higher temperature in the vicinity of Ga/OA NPs and catalytic activity of the gallium oxide/hydroxide surface layer, which could accelerate the hydrolytic degradation of PLA as with ZnO, CaO or silicate nanofillers [47,97,98], with the additional possible enhancement by a local change in pH due to the dissolution of Ga, as in the case of Mg [99].
- (b)
- Weak interactions between Ga/OA NPs and PLA enable the easy separation of these two nanocomposite components, especially when additional stronger interactions with water and other liquid broth components are possible. Water helps in this polymer chain rearrangement with its plasticizing effect on PLA (it enhances segmental mobility) [100], especially at a raised temperature (37 °C), where the above-mentioned higher thermal conductivity of metallic (Ga) nanofiller also plays an important role in exposing the nanoparticles from the Ga/PLA nanocomposite. In addition, we need to consider a possible fraction of uncovered Ga/OA NPs at the surface, for which only the neighboring PLA chains would need to move away to eject the NPs.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Sinclair, R.G. The Case for Polylactic Acid as a Commodity Packaging Plastic. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, 5, 585–597. [Google Scholar] [CrossRef]
- Oyama, H.T.; Tanaka, Y.; Kadosaka, A. Rapid controlled hydrolytic degradation of poly(l-lactic acid) by blending with poly(aspartic acid-co-l-lactide). Polym. Degrad. Stab. 2009, 94, 1419–1426. [Google Scholar] [CrossRef]
- Bergsma, J.E.; de Bruijn, W.C.; Rozema, F.R.; Bos, R.R.M.; Boering, G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomoterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kerry, J.P. Crop-based biodegradable packaging and its environmental implications. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 1–25. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of poly lactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID pollution: Impact of COVID-19 pandemic on global plastic waste footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef]
- Spennemann, D.H.R. COVID Face Masks: Policy Shift Results in Increased Littering. Sustainability 2021, 13, 9875. [Google Scholar] [CrossRef]
- Kiel, A.; Kaltschmidt, B.P.; Asghari, E.; Hütten, A.; Kaltschmidt, B.; Kaltschmidt, C. Bacterial Biofilm Formation on Nano-Copper Added PLA Suited for 3D Printed Face Masks. Microorganisms 2022, 10, 439. [Google Scholar] [CrossRef]
- Wang, Z.; An, C.; Chen, X.; Lee, K.; Zhang, B.; Feng, Q. Disposable masks release microplastics to the aqueous environment with exacerbation by natural weathering. J. Hazard. Mater. 2021, 417, 126036. [Google Scholar] [CrossRef]
- Sun, J.; Yang, S.; Zhou, G.J.; Zhang, K.; Lu, Y.; Jin, Q.; Lam, P.K.S.; Leung, K.M.Y.; He, Y. Release of Microplastics from Discarded Surgical Masks and Their Adverse Impacts on the Marine Copepod Tigriopus japonicus. Environ. Sci. Technol. Lett. 2021, 8, 1065–1070. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Wang, S.; Yeo, C.C.J.; Li, J.; Ni, X.P.; Jiang, L.; Xue, K.; Li, Z.; Fei, X.; Zhu, Q.; et al. Polylactic acid face masks: Are these the sustainable solutions in times of COVID-19 pandemic? Sci. Total Environ. 2022, 807, 151084. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Mardis, N.J. Emerging Technology and Applications of 3D Printing in the Medical Field. Mo Med. 2018, 115, 368–373. [Google Scholar]
- Flanagan, S.T.; Ballard, D.H. 3D Printed Face Shields: A Community Response to the COVID-19 Global Pandemic. Acad. Radiol. 2020, 27, 905–906. [Google Scholar] [CrossRef]
- Swennen, G.R.J.; Pottel, L.; Haers, P.E. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int. J. Oral Maxillofac. Surg. 2020, 49, 673–677. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Hall, D.C.; Palmer, P.; Ji, H.F.; Ehrlich, G.D.; Król, J.E. Bacterial Biofilm Growth on 3D-Printed Materials. Front. Microbiol. 2021, 12, 646303. [Google Scholar] [CrossRef]
- Shao, L.; Xi, Y.; Weng, Y. Recent Advances in PLA-Based Antibacterial Food Packaging and Its Applications. Molecules 2022, 27, 5953. [Google Scholar] [CrossRef]
- Park, A.M.; Khadka, S.; Sato, F.; Omura, S.; Fujita, M.; Hashiwaki, K.; Tsunoda, I. Bacterial and fungal isolation from face masks under the COVID-19 pandemic. Sci. Rep. 2022, 12, 11361. [Google Scholar] [CrossRef]
- Lee, D.E. Bacterial contamination on the inner surface of the masks used by clinicians in a dental clinic. Dent. Med. Probl. 2022, 59, 177–185. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, H. Biodegradable Polylactic Acid Polymer with Nisin for Use in Antimicrobial Food Packaging. J. Food Sci. 2008, 73, M127–M134. [Google Scholar] [CrossRef]
- Jin, T.; Liu, L.; Zhang, H.; Hicks, K. Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against Listeria monocytogenes. Int. J. Food Sci. Technol. 2009, 44, 322–329. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Development of Active Antimicrobial Fiber-Based Chitosan Polysaccharide Nanostructures using Electrospinning. Eng. Life Sci. 2008, 8, 303–314. [Google Scholar] [CrossRef]
- Lima, M.; Teixeira-Santos, R.; Gomes, L.C.; Faria, S.I.; Valcarcel, J.; Vázquez, J.A.; Cerqueira, M.A.; Pastrana, L.; Bourbon, A.I.; Mergulhão, F.J. Development of chitosan-based surfaces to prevent singleand dual-species biofilms of staphylococcus aureus and pseudomonas aeruginosa. Molecules 2021, 26, 4378. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A.; Buonocore, G.G.; Incoronato, A.L.; Massaro, A.; Panza, O. Active packaging by extrusion processing of recyclable and biodegradable polymers. J. Food Eng. 2009, 93, 1–6. [Google Scholar] [CrossRef]
- Yahyaoui, M.; Gordobil, O.; Herrera Díaz, R.; Abderrabba, M.; Labidi, J. Development of novel antimicrobial films based on poly(lactic acid) and essential oils. React. Funct. Polym. 2016, 109, 1–8. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Fouad, H.; Jawaid, M. Development of active agents filled polylactic acid films for food packaging application. Int. J. Biol. Macromol. 2020, 163, 1451–1457. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Erem, A.D.; Ozcan, G.; Erem, H.; Skrifvars, M. Antimicrobial activity of poly(l-lactide acid)/silver nanocomposite fibers. Text. Res. J. 2013, 83, 2111–2117. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Iordache, F.; Vasile, B.S.; Holban, A.M. Bioactive surfaces of polylactide and silver nanoparticles for the prevention of microbial contamination. Materials 2020, 13, 768. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of Inorganic Nanoparticles Reinforcement on Its Performance and Food Packaging Applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef]
- Demchenko, V.; Kobylinskyi, S.; Iurzhenko, M.; Riabov, S.; Vashchuk, A.; Rybalchenko, N.; Zahorodnia, S.; Naumenko, K.; Demchenko, O.; Adamus, G.; et al. Nanocomposites based on polylactide and silver nanoparticles and their antimicrobial and antiviral applications. React. Funct. Polym. 2022, 170, 105096. [Google Scholar] [CrossRef]
- Škrlová, K.; Rybková, Z.; Stachurová, T.; Zagora, J.; Malachová, K.; Měřinská, D.; Gabor, R.; Havlíček, M.; Muñoz-Bonilla, A.; Fernández-García, M.; et al. Long-term antimicrobial effect of polylactide-based composites suitable for biomedical use. Polym. Test. 2022, 116, 107760. [Google Scholar] [CrossRef]
- Murariu, M.; Paint, Y.; Murariu, O.; Raquez, J.M.; Bonnaud, L.; Dubois, P. Current progress in the production of PLA–ZnO nanocomposites: Beneficial effects of chain extender addition on key properties. J. Appl. Polym. Sci. 2015, 132, 42480. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. LWT 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Mousa, H.M.; Abdal-Hay, A.; Bartnikowski, M.; Mohamed, I.M.A.; Yasin, A.S.; Ivanovski, S.; Park, C.H.; Kim, C.S. A Multifunctional Zinc Oxide/Poly(Lactic Acid) Nanocomposite Layer Coated on Magnesium Alloys for Controlled Degradation and Antibacterial Function. ACS Biomater. Sci. Eng. 2018, 4, 2169–2180. [Google Scholar] [CrossRef]
- Swaroop, C.; Shukla, M. Development of blown polylactic acid-MgO nanocomposite films for food packaging. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105482. [Google Scholar] [CrossRef]
- Aničić, N.; Kurtjak, M.; Jeverica, S.; Suvorov, D.; Vukomanović, M. Antimicrobial polymeric composites with embedded nanotextured magnesium oxide. Polymers 2021, 13, 2183. [Google Scholar] [CrossRef]
- Loyo, C.; Moreno-Serna, V.; Fuentes, J.; Amigo, N.; Sepúlveda, F.A.; Ortiz, J.A.; Rivas, L.M.; Ulloa, M.T.; Benavente, R.; Zapata, P.A. PLA/CaO nanocomposites with antimicrobial and photodegradation properties. Polym. Degrad. Stab. 2022, 197, 109865. [Google Scholar] [CrossRef]
- Li, F.; Liu, F.; Huang, K.; Yang, S. Advancement of Gallium and Gallium-Based Compounds as Antimicrobial Agents. Front. Bioeng. Biotechnol. 2022, 10, 827960. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanović, M.; Kramer, L.; Suvorov, D. Biocompatible nano-gallium/hydroxyapatite nanocomposite with antimicrobial activity. J. Mater. Sci. Mater. Med. 2016, 27, 170. [Google Scholar] [CrossRef]
- Li, H.; Qiao, R.; Davis, T.P.; Tang, S.Y. Biomedical Applications of Liquid Metal Nanoparticles: A Critical Review. Biosensors 2020, 10, 196. [Google Scholar] [CrossRef]
- Wang, D.; Gao, C.; Wang, W.; Sun, M.; Guo, B.; Xie, H.; He, Q. Shape-Transformable, Fusible Rodlike Swimming Liquid Metal Nanomachine. ACS Nano 2018, 12, 10212–10220. [Google Scholar] [CrossRef]
- Sun, X.; Sun, M.; Liu, M.; Yuan, B.; Gao, W.; Rao, W.; Liu, J. Shape tunable gallium nanorods mediated tumor enhanced ablation through near-infrared photothermal therapy. Nanoscale 2019, 11, 2655–2667. [Google Scholar] [CrossRef]
- Chen, X.M.; Fei, G.T.; Zheng, K. The solid state phase transition of gallium particles and its size dependence. J. Phys. Condens. Matter 2009, 21, 245403. [Google Scholar] [CrossRef]
- He, H.; Fei, G.T.; Cui, P.; Zheng, K.; Liang, L.M.; Li, Y.; Zhang, L.D. Relation between size and phase structure of gallium: Differential scanning calorimeter experiments. Phys. Rev. B 2005, 72, 073310. [Google Scholar] [CrossRef]
- Di Cicco, A. Phase Transitions in Confined Gallium Droplets. Phys. Rev. Lett. 1998, 81, 2942–2945. [Google Scholar] [CrossRef]
- Di Cicco, A.; Fusari, S.; Stizza, S. Phase transitions and undercooling in confined gallium. Philos. Mag. B 1999, 79, 2113–2120. [Google Scholar] [CrossRef]
- Li, X.F.; Fei, G.T.; Chen, X.M.; Zhang, Y.; Zheng, K.; Liu, X.L.; Zhang, L.D. Size-temperature phase diagram of gallium. Europhys. Lett. 2011, 94, 16001. [Google Scholar] [CrossRef]
- Yarema, M.; Wörle, M.; Rossell, M.D.; Erni, R.; Caputo, R.; Protesescu, L.; Kravchyk, K.V.; Dirin, D.N.; Lienau, K.; von Rohr, F.; et al. Monodisperse Colloidal Gallium Nanoparticles: Synthesis, Low Temperature Crystallization, Surface Plasmon Resonance and Li-Ion Storage. J. Am. Chem. Soc. 2014, 136, 12422–12430. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Gallium. In Separation, Preconcentration and Spectrophotometry in Inorganic Analysis; Analytical Spectroscopy Library; Elsevier: Amsterdam, The Netherlands, 2000; Volume 10, pp. 198–203. ISBN 9780444505248. [Google Scholar]
- Füllbrandt, M.; Purohit, P.J.; Schönhals, A. Combined FTIR and Dielectric Investigation of Poly (vinyl acetate) Adsorbed on Silica Particles. Macromolecules 2013, 46, 4626–4632. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Klonos, P.A.; Kyritsis, A.; Tziolas, A.; Avgeropoulos, A.; Papageorgiou, G.Z.; Bikiaris, D.N. Interfacial interactions, crystallization and molecular mobility in nanocomposites of Poly(lactic acid) filled with new hybrid inclusions based on graphene oxide and silica nanoparticles. Polymer 2019, 166, 1–12. [Google Scholar] [CrossRef]
- Turner, J.F., II; Riga, A.; Connor, A.O.; Zhang, J.; Collis, J. Characterization of Drawn and Undrawn Poly-L-Lactide Films by Differential Scanning Calorimetry. J. Therm. Anal. Calorim. 2004, 75, 257–268. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Du, M.; Yu, Q.; Thurn-Albrecht, T.; Schick, C.; Androsch, R. Bulk Enthalpy of Melting of Poly (l-lactic acid) (PLLA) Determined by Fast Scanning Chip Calorimetry. Macromol. Rapid Commun. 2022, 43, 2200148. [Google Scholar] [CrossRef]
- Lan, Q.; Li, Y. Mesophase-mediated crystallization of poly(l -lactide): Deterministic pathways to nanostructured morphology and superstructure control. Macromolecules 2016, 49, 7387–7399. [Google Scholar] [CrossRef]
- Miklavič, Š.; Kogovšek, P.; Hodnik, V.; Korošec, J.; Kladnik, A.; Anderluh, G.; Gutierrez-Aguirre, I.; Maček, P.; Butala, M. The Pseudomonas aeruginosa RhlR-controlled aegerolysin RahU is a low-affinity rhamnolipid-binding protein. FEMS Microbiol. Lett. 2015, 362, fnv069. [Google Scholar] [CrossRef]
- Collins, C.H.; Lyne, P.M.; Grange, J.M.; Falkinham, J.O., III (Eds.) Collins and Lyne’s Microbiological Methods, 8th ed.; Arnold: London, UK, 2004; ISBN 0340808969. [Google Scholar]
- ISO 22196:2011(E); Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2011.
- Sokolova, A.I.; Pavlova, E.R.; Khramova, Y.V.; Klinov, D.V.; Shaitan, K.V.; Bagrov, D.V. Imaging human keratinocytes grown on electrospun mats by scanning electron microscopy. Microsc. Res. Tech. 2019, 82, 544–549. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M. Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Shukla, N.; Liu, C.; Jones, P.M.; Weller, D. FTIR study of surfactant bonding to FePt nanoparticles. J. Magn. Magn. Mater. 2003, 266, 178–184. [Google Scholar] [CrossRef]
- Wu, N.; Fu, L.; Su, M.; Aslam, M.; Wong, K.C.; Dravid, V.P. Interaction of Fatty Acid Monolayers with Cobalt Nanoparticles. Nano Lett. 2004, 4, 383–386. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Don, K.L.; Chang, W.K.; Hyun, G.C.; Young, H.K.; Young, S.K. Synthesis of magnetic nanoparticles of Fe3O4 and CoFe2O4 and their surface modification by surfactant adsorption. Bull. Korean Chem. Soc. 2006, 27, 237–242. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Klonos, P.A.; Terzopoulou, Z.; Psochia, E.; Sanusi, O.M.; Hocine, N.A.; Benelfellah, A.; Giliopoulos, D.; Triantafyllidis, K.; Kyritsis, A.; et al. Comparative study of crystallization, semicrystalline morphology, and molecular mobility in nanocomposites based on polylactide and various inclusions at low filler loadings. Polymer 2021, 217, 123457. [Google Scholar] [CrossRef]
- Črešnar, K.P.; Zemljič, L.F.; Papadopoulos, L.; Terzopoulou, Z.; Zamboulis, A.; Klonos, P.A.; Bikiaris, D.N.; Kyritsis, A.; Pissis, P. Effects of Ag, ZnO and TiO2 nanoparticles at low contents on the crystallization, semicrystalline morphology, interfacial phenomena and segmental dynamics of PLA. Mater. Today Commun. 2021, 27, 102192. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of poly(L-lactide): Molecular weight dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Kai, W.; Dong, T.; Inoue, Y. Polymorphic transition in disordered poly(L-lactide) crystals induced by annealing at elevated temperatures. Macromolecules 2008, 41, 4296–4304. [Google Scholar] [CrossRef]
- Chen, X.; Kalish, J.; Hsu, S.L. Structure evolution of α′-phase poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1446–1454. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Sato, H.; Tsuji, H.; Noda, I.; Yan, S.; Ozaki, Y. Crystal modifications and thermal behavior of poly(L-lactic acid) revealed by infrared spectroscopy. Macromolecules 2005, 38, 8012–8021. [Google Scholar] [CrossRef]
- Syazwan, M.; Sasaki, T. Rapid crystallization and mesophase formation of poly(L-lactic acid) during precipitation from a solution. E-Polymers 2018, 18, 331–337. [Google Scholar] [CrossRef]
- Lizundia, E.; Petisco, S.; Sarasua, J.R. Phase-structure and mechanical properties of isothermally melt-and cold-crystallized poly (L-lactide). J. Mech. Behav. Biomed. Mater. 2013, 17, 242–251. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Yu, Q.; Petzold, A.; Thurn-Albrecht, T.; Glüge, R.; Altenbach, H.; Androsch, R. Young’s modulus of the different crystalline phases of poly (L-lactic acid). J. Mech. Behav. Biomed. Mater. 2023, 137, 105546. [Google Scholar] [CrossRef]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of melting of α′- and α-crystals of poly(L-lactic acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- Androsch, R.; Zhuravlev, E.; Schick, C. Solid-state reorganization, melting and melt-recrystallization of conformationally disordered crystals (α’-phase) of poly (l-lactic acid). Polymer 2014, 55, 4932–4941. [Google Scholar] [CrossRef]
- Pande, S.; Shitut, S.; Freund, L.; Westermann, M.; Bertels, F.; Colesie, C.; Bischofs, I.B.; Kost, C. Metabolic cross-feeding via intercellular nanotubes among bacteria. Nat. Commun. 2015, 6, 6238. [Google Scholar] [CrossRef]
- Mazia, D.; Schatten, G.; Sale, W. Adhesion of cells to surfaces coated with polylysine. J. Cell Biol. 1975, 66, 198–200. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, X.; Li, J.; Liu, H.; Han, N.; Zhang, X. Poly-l-Lactic Acid/Graphene Electrospun Composite Nanofibers for Wearable Sensors. Energy Technol. 2020, 8, 1901252. [Google Scholar] [CrossRef]
- Subbuvel, M.; Kavan, P. Preparation and characterization of polylactic acid/fenugreek essential oil/curcumin composite films for food packaging applications. Int. J. Biol. Macromol. 2022, 194, 470–483. [Google Scholar] [CrossRef]
- Klonos, P.; Terzopoulou, Z.; Koutsoumpis, S.; Zidropoulos, S.; Kripotou, S.; Papageorgiou, G.Z.; Bikiaris, D.N.; Kyritsis, A.; Pissis, P. Rigid amorphous fraction and segmental dynamics in nanocomposites based on poly(L–lactic acid) and nano-inclusions of 1–3D geometry studied by thermal and dielectric techniques. Eur. Polym. J. 2016, 82, 16–34. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, S.; Xiao, Y.; Yu, X.; Li, X.; Wu, P. Shape memory effect of poly(d,l-lactide)/Fe3O4 nanocomposites by inductive heating of magnetite particles. Colloids Surf. B Biointerfaces 2009, 71, 67–72. [Google Scholar] [CrossRef]
- Klonos, P.; Pissis, P. Effects of interfacial interactions and of crystallization on rigid amorphous fraction and molecular dynamics in polylactide/silica nanocomposites: A methodological approach. Polymer 2017, 112, 228–243. [Google Scholar] [CrossRef]
- Shalom, H.; Sui, X.M.; Elianov, O.; Brumfeld, V.; Rosentsveig, R.; Pinkas, I.; Feldman, Y.; Kampf, N.; Wagner, H.D.; Lachman, N.; et al. Nanocomposite of poly(L-lactic acid) with inorganic nanotubes of WS 2. Lubricants 2019, 7, 28. [Google Scholar] [CrossRef]
- Salas-Papayanopolos, H.; Morales-Cepeda, A.B.; Wood-Adams, P.; Sanchez, S.; Lafleur, P.G.; Vazquez, H.P. Crystallization effect of poly(L-lactic acid)/silver nanocomposites blends, on barrier and mechanical properties using glyceryl triacetate as plasticizer. Polym. Bull. 2023, 80, 5273–5290. [Google Scholar] [CrossRef]
- Zhu, A.; Diao, H.; Rong, Q.; Cai, A. Preparation and Properties of Polylactide–Silica Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2866–2873. [Google Scholar] [CrossRef]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C 2015, 57, 314–320. [Google Scholar] [CrossRef]
- Yin, H.; Chow, G.M. Effects of oleic acid surface coating on the properties of nickel ferrite nanoparticles/PLA composites. J. Biomed. Mater. Res.—Part A 2009, 91, 331–341. [Google Scholar] [CrossRef]
- Barton, A.J.; Sagers, R.D.; Pitt, W.G. Measurement of bacterial growth rates on polymers. J. Biomed. Mater. Res. 1996, 32, 271–278. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. Polylactide-Layered Silicate Nanocomposite: A Novel Biodegradable Material. Nano Lett. 2002, 2, 1093–1096. [Google Scholar] [CrossRef]
- Trujillo, S.; Lizundia, E.; Vilas, J.L.; Salmeron-Sanchez, M. PLLA/ZnO nanocomposites: Dynamic surfaces to harness cell differentiation. Colloids Surf. B Biointerfaces 2016, 144, 152–160. [Google Scholar] [CrossRef]
- Zeynivandnejad, M.; Moradi, M.; Sadeghi, A. Mechanical, physical, and degradation properties of 3D printed PLA + Mg composites. J. Manuf. Process. 2023, 101, 234–244. [Google Scholar] [CrossRef]
- Vyavahare, O.; Ng, D.; Hsu, S.L. Analysis of structural rearrangements of poly(lactic acid) in the presence of water. J. Phys. Chem. B 2014, 118, 4185–4193. [Google Scholar] [CrossRef]
- Noor, H.; Satti, S.M.; ud Din, S.; Farman, M.; Hasan, F.; Khan, S.; Badshah, M.; Shah, A.A. Insight on esterase from Pseudomonas aeruginosa strain S3 that depolymerize poly(lactic acid) (PLA) at ambient temperature. Polym. Degrad. Stab. 2020, 174, 109096. [Google Scholar] [CrossRef]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence Factors of Pseudomonas aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef]
- Vukomanović, M.; Gazvoda, L.; Kurtjak, M.; Maček-Kržmanc, M.; Spreitzer, M.; Tang, Q.; Wu, J.; Ye, H.; Chen, X.; Mattera, M.; et al. Filler-Enhanced Piezoelectricity of Poly- L -Lactide and Its Use as a Functional Ultrasound-Activated Biomaterial. Small 2023, 19, 2301981. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Cheeseman, S.; Frias-De-Diego, A.; Hong, H.; Yang, J.; Jung, W.; Yin, H.; Murdoch, B.J.; Scholle, F.; Crook, N.; et al. A Liquid Metal Mediated Metallic Coating for Antimicrobial and Antiviral Fabrics. Adv. Mater. 2021, 33, 2104298. [Google Scholar] [CrossRef]
- Mätzler, C. MATLAB Functions for Mie Scattering and Absorption. IAP Res. Rep. 2002, 2002–08, 1139–1151. [Google Scholar]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 1998; ISBN 9780471293408. [Google Scholar]
- Knight, M.W.; Coenen, T.; Yang, Y.; Brenny, B.J.M.; Losurdo, M.; Brown, A.S.; Everitt, H.O.; Polman, A. Gallium Plasmonics: Deep Subwavelength Spectroscopic Imaging of Single and Interacting Gallium Nanoparticles. ACS Nano 2015, 9, 2049–2060. [Google Scholar] [CrossRef]
- Hutchinson, M.H.; Dorgan, J.R.; Knauss, D.M.; Hait, S.B. Optical properties of polylactides. J. Polym. Environ. 2006, 14, 119–124. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: Hoboken, NJ, USA, 2005; ISBN 1118311655. [Google Scholar]
- Sinclair, R.G.; McKay, A.F.; Myers, G.S.; Norman Jones, R. The Infrared Absorption Spectra of Unsaturated Fatty Acids and Esters. J. Am. Chem. Soc. 1952, 74, 2578–2585. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: London, UK, 1991; ISBN 0124511600. [Google Scholar]
- Thistlethwaite, P.J.; Gee, M.L.; Wilson, D. Diffuse reflectance infrared fourier transform spectroscopic studies of the adsorption of oleate/oleic acid onto zirconia. Langmuir 1996, 12, 6487–6491. [Google Scholar] [CrossRef]
- Kostevšek, N.; Hudoklin, S.; Kreft, M.E.; Serša, I.; Sepe, A.; Jagličić, Z.; Vidmar, J.; Ščančar, J.; Šturm, S.; Kobe, S.; et al. Magnetic interactions and: In vitro study of biocompatible hydrocaffeic acid-stabilized Fe-Pt clusters as MRI contrast agents. RSC Adv. 2018, 8, 14694–14704. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Dobson, K.D.; McQuillan, A.J. In situ infrared spectroscopic analysis of the adsorption of aliphatic carboxylic acids to TiO2, ZrO2, Al2O3, and Ta2O5 from aqueous solutions. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 1999, 55, 1395–1405. [Google Scholar] [CrossRef]













| PLA | Ga/PLA0.4% | Ga/PLA0.8% | Ga/PLA2.4% | |
|---|---|---|---|---|
| Tg [°C] | 69.1 | 70.0 | 68.0 | 70.4 |
| ΔCp,g [J/(gK)] | 1.00 | 0.66 | 0.87 | 0.77 |
| Tcc [°C] | 110.9 | 103.4 | 98.3 | 94.8 |
| ΔHcc [J/g] | 32.7 | 22.9 | 10.1 | 15.4 |
| Tα’→α [°C] | 165.6 | 163.1 | 163.1 | 162.0 |
| ΔHα’→α [J/g] | 2.4 | 8.1 | 4.5 | 12.6 |
| Tm [°C] | 180.5 | 179.4 | 181.0 | 178.3 |
| ΔHm [J/g] | 45.6 | 38.0 | 49.6 | 35.9 |
| Xc, DSC [%] | 12.4 | 14.7 | 39.2 | 20.4 |
| Xc, FTIR [%] | 15.0 ± 0.7 | 18 ± 4 | 30 ± 1 ** | 20 ± 2 |
| θH2O [°] | 70 ± 4 | 75 ± 3 ** | 83 ± 6 ** | 89 ± 7 ** |
| E [GPa] | 1.6 ± 0.3 | 3.9 ± 0.4 * | 2.5 ± 0.6 | 2.4 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurtjak, M.; Maček Kržmanc, M.; Spreitzer, M.; Vukomanović, M. Nanogallium-poly(L-lactide) Composites with Contact Antibacterial Action. Pharmaceutics 2024, 16, 228. https://doi.org/10.3390/pharmaceutics16020228
Kurtjak M, Maček Kržmanc M, Spreitzer M, Vukomanović M. Nanogallium-poly(L-lactide) Composites with Contact Antibacterial Action. Pharmaceutics. 2024; 16(2):228. https://doi.org/10.3390/pharmaceutics16020228
Chicago/Turabian StyleKurtjak, Mario, Marjeta Maček Kržmanc, Matjaž Spreitzer, and Marija Vukomanović. 2024. "Nanogallium-poly(L-lactide) Composites with Contact Antibacterial Action" Pharmaceutics 16, no. 2: 228. https://doi.org/10.3390/pharmaceutics16020228