Melatonin Derivative-Conjugated Formulations of Pd(II) and Pt(II) Thiazoline Complexes on Mesoporous Silica to Enhance Cytotoxicity and Apoptosis against HeLa Cells
Abstract
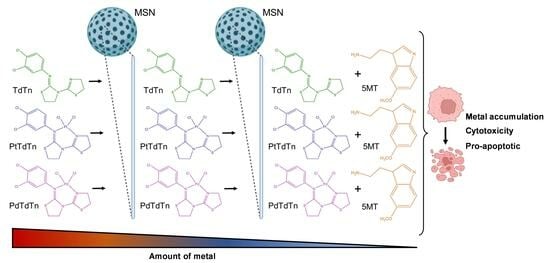
:1. Introduction
2. Materials and Methods
2.1. General Remarks on the Synthesis and Characterization of the Materials
2.2. Synthesis of the Starting Mesoporous Silica Material (MSN)
2.3. Functionalization with Silane Ligands
2.4. Incorporation of 5MT Derivative
2.5. Functionalization with the Cytotoxic Compounds
2.6. Stability Study of the Final Materials: Release Analysis
2.7. DNA Interaction Study
2.8. Cell Culture and Treatments
2.9. In Vitro Cytotoxicity Assay
2.10. Determination of Apoptosis
2.11. Cellular Uptake and Accumulation of MSNs
2.12. Statistics
3. Results and Discussion
3.1. Synthesis and Characterization of the Mesoporous Silica Materials
3.2. Cell-Free Assays: Release Analysis and DNA Interaction Study
3.3. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, R.; Singh, V.J.; Chawla, P.A. Advancements in the Use of Platinum Complexes as Anticancer Agents. Anti-Cancer Agents Med. Chem. 2021, 22, 821–835. [Google Scholar] [CrossRef]
- Fernández-Delgado, E.; de la Cruz-Martínez, F.; Galán, C.; Franco, L.; Espino, J.; Viñuelas-Zahínos, E.; Luna-Giles, F.; Bejarano, I. Pt(II) and Pd(II) Complexes with a Thiazoline Derivative Ligand: Synthesis, Structural Characterization, Antiproliferative Activity and Evaluation of pro-Apoptotic Ability in Tumor Cell Lines HT-29 and U-937. J. Inorg. Biochem. 2020, 202, 110870. [Google Scholar] [CrossRef] [PubMed]
- Rathor, S.; Bhatt, D.C.; Aamir, S.; Singh, S.K.; Kumar, V. A Comprehensive Review on Role of Nanoparticles in Therapeutic Delivery of Medicine. Pharm. Nanotechnol. 2018, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Bruckmann, F.D.S.; Nunes, F.B.; Salles, T.D.R.; Franco, C.; Cadoná, F.C.; Rhoden, B.; Schwaminger, S.; Bondarenko, L.; Da, F.; Bruckmann, S.; et al. Biological Applications of Silica-Based Nanoparticles. Magnetochemistry 2022, 8, 131. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, H.; Liu, C.G.; Kanubaddi, K.R.; Lee, C.H.; Wang, S.B.; Cui, W.; Santos, H.A.; Lin, K.L.; Chen, A.Z. Metal Species–Encapsulated Mesoporous silica Nanoparticles: Current Advancements and Latest Breakthroughs. Adv. Funct. Mater. 2019, 29, 1902652. [Google Scholar] [CrossRef]
- Díaz-García, D.; Prashar, S.; Gómez-Ruiz, S. Biological Use of Nanostructured Silica-Based Materials Functionalized with Metallodrugs: The Spanish Perspective. Int. J. Mol. Sci. 2023, 24, 2332. [Google Scholar] [CrossRef]
- Gupta, J.; Quadros, M.; Momin, M. Mesoporous silica Nanoparticles: Synthesis and Multifaceted Functionalization for Controlled Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 81, 104305. [Google Scholar] [CrossRef]
- Siddiqui, B.; Rehman, A.U.; Haq, I.U.; Al-Dossary, A.A.; Elaissari, A.; Ahmed, N. Exploiting Recent Trends for the Synthesis and Surface Functionalization of Mesoporous silica Nanoparticles towards Biomedical Applications. Int. J. Pharm. X 2022, 4, 100116. [Google Scholar] [CrossRef]
- Méndez, J.; Monteagudo, A.; Griebenow, K. Stimulus-Responsive Controlled Release System by Covalent Immobilization of an Enzyme into Mesoporous silica Nanoparticles. Bioconjug. Chem. 2012, 23, 698. [Google Scholar] [CrossRef]
- Ovejero-Paredes, K.; Díaz-García, D.; Mena-Palomo, I.; Marciello, M.; Lozano-Chamizo, L.; Morato, Y.L.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Synthesis of a Theranostic Platform Based on Fibrous Silica Nanoparticles for the Enhanced Treatment of Triple-Negative Breast Cancer Promoted by a Combination of Chemotherapeutic Agents. Biomater. Adv. 2022, 137, 212823. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Lozano, D.; Vallet-Regí, M. Mesoporous silica Nanoparticles for Targeting Subcellular Organelles. Int. J. Mol. Sci. 2020, 21, 9696. [Google Scholar] [CrossRef]
- Colilla, M.; Vallet-Regí, M. Targeted Stimuli-Responsive Mesoporous silica Nanoparticles for Bacterial Infection Treatment. Int. J. Mol. Sci. 2020, 21, 8605. [Google Scholar] [CrossRef] [PubMed]
- Torabi, M.; Aghanejad, A.; Savadi, P.; Barzegari, A.; Omidi, Y.; Barar, J. Fabrication of Mesoporous silica Nanoparticles for Targeted Delivery of Sunitinib to Ovarian Cancer Cells. BioImpacts BI 2023, 13, 255. [Google Scholar] [CrossRef]
- Song, F.; Li, Y.; Wang, S.; Zhang, L.; Chen, Q.L. Multifunctional Dual-Mesoporous silica Nanoparticles Loaded with a Protein and Dual Antitumor Drugs as a Targeted Delivery System. New J. Chem. 2019, 43, 17284–17297. [Google Scholar] [CrossRef]
- Díaz-García, D.; Fischer-Fodor, E.; Vlad, C.I.; Méndez-Arriaga, J.M.; Prashar, S.; Gómez-Ruiz, S. Study of Cancer Cell Cytotoxicity, Internalization and Modulation of Growth Factors Induced by Transferrin-Conjugated Formulations of Metallodrug-Functionalized Mesoporous silica Nanoparticles. Microporous Mesoporous Mater. 2021, 323, 111238. [Google Scholar] [CrossRef]
- Mai, Z.; Chen, J.; Hu, Y.; Liu, F.; Fu, B.; Zhang, H.; Dong, X.; Huang, W.; Zhou, W. Novel Functional Mesoporous silica Nanoparticles Loaded with Vitamin E Acetate as Smart Platforms for PH Responsive Delivery with High Bioactivity. J. Colloid Interface Sci. 2017, 508, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Ugalde-Arbizu, M.; Aguilera-Correa, J.J.; García-Almodóvar, V.; Ovejero-Paredes, K.; Díaz-García, D.; Esteban, J.; Páez, P.L.; Prashar, S.; San Sebastian, E.; Filice, M.; et al. Dual Anticancer and Antibacterial Properties of Silica-Based Theranostic Nanomaterials Functionalized with Coumarin343, Folic Acid and a Cytotoxic Organotin(IV) Metallodrug. Pharmaceutics 2023, 15, 560. [Google Scholar] [CrossRef]
- Díaz-García, D.; Montalbán-Hernández, K.; Mena-Palomo, I.; Achimas-Cadariu, P.; Rodríguez-Diéguez, A.; López-Collazo, E.; Prashar, S.; Ovejero Paredes, K.; Filice, M.; Fischer-Fodor, E.; et al. Role of Folic Acid in the Therapeutic Action of Nanostructured Porous silica Functionalized with Organotin(IV) Compounds against Different Cancer Cell Lines. Pharmaceutics 2020, 12, 512. [Google Scholar] [CrossRef]
- Niemelä, E.; Desai, D.; Nkizinkiko, Y.; Eriksson, J.E.; Rosenholm, J.M. Sugar-Decorated Mesoporous silica Nanoparticles as Delivery Vehicles for the Poorly Soluble Drug Celastrol Enables Targeted Induction of Apoptosis in Cancer Cells. Eur. J. Pharm. Biopharm. 2015, 96, 11–21. [Google Scholar] [CrossRef]
- Sodagar Taleghani, A.; Ebrahimnejad, P.; Heidarinasab, A.; Akbarzadeh, A. Sugar-Conjugated Dendritic Mesoporous silica Nanoparticles as PH-Responsive Nanocarriers for Tumor Targeting and Controlled Release of Deferasirox. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, Y.; Zhang, H.; He, W.; Zhou, Q.; Gui, S.; Wang, Y. Melatonin Inhibits Cell Growth and Migration, but Promotes Apoptosis in Gastric Cancer Cell Line, SGC7901. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2013, 88, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, S.J.; Luo, J.H.; Zhang, H.; Wang, R.X.; Liu, H.; Li, L.; Zhang, Z.G.; Zhou, R.X. Melatonin Induces the Apoptosis and Inhibits the Proliferation of Human Gastric Cancer Cells via Blockade of the AKT/MDM2 Pathway. Oncol. Rep. 2018, 39, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento Gonçalves, N.; Colombo, J.; Lopes, J.R.; Gelaleti, G.B.; Moschetta, M.G.; Sonehara, N.M.; Hellmén, E.; De Freitas Zanon, C.; Oliani, S.M.; Pires De Campos Zuccari, D.A. Effect of Melatonin in Epithelial Mesenchymal Transition Markers and Invasive Properties of Breast Cancer Stem Cells of Canine and Human Cell Lines. PLoS ONE 2016, 11, e0150407. [Google Scholar] [CrossRef]
- Estirado, S.; Fernández-Delgado, E.; Viñuelas-Zahínos, E.; Luna-Giles, F.; Rodríguez, A.B.; Pariente, J.A.; Espino, J. Pro-Apoptotic and Anti-Migration Properties of a Thiazoline-Containing Platinum(II) Complex in MDA-MB-231 Breast Cancer Cells: The Role of Melatonin as a Synergistic Agent. Antioxidants 2022, 11, 1971. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Li, Y.; Gao, J. Melatonin Increases Human Cervical Cancer HeLa Cells Apoptosis Induced by Cisplatin via Inhibition of JNK/Parkin/Mitophagy Axis. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Hevia, D.; Quiros-Gonzalez, I.; Rodriguez-Garcia, A.; Gonzalez-Menendez, P.; Cepas, V.; Gonzalez-Pola, I.; Sainz, R.M. IGFBP3 and MAPK/ERK Signaling Mediates Melatonin-Induced Antitumor Activity in Prostate Cancer. J. Pineal Res. 2017, 62, e12373. [Google Scholar] [CrossRef]
- Tai, S.Y.; Huang, S.P.; Bao, B.Y.; Wu, M.T. Urinary Melatonin-Sulfate/Cortisol Ratio and the Presence of Prostate Cancer: A Case-Control Study. Sci. Rep. 2016, 6, 29606. [Google Scholar] [CrossRef]
- Mafi, A.; Rezaee, M.; Hedayati, N.; Hogan, S.D.; Reiter, R.J.; Aarabi, M.H.; Asemi, Z. Melatonin and 5-Fluorouracil Combination Chemotherapy: Opportunities and Efficacy in Cancer Therapy. Cell Commun. Signal. CCS 2023, 21, 33. [Google Scholar] [CrossRef]
- Pariente, R.; Bejarano, I.; Rodríguez, A.B.; Pariente, J.A.; Espino, J. Melatonin Increases the Effect of 5-Fluorouracil-Based Chemotherapy in Human Colorectal Adenocarcinoma Cells in Vitro. Mol. Cell. Biochem. 2018, 440, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Carlomagno, G.; Dinicola, S.; Bizzarri, M. Soft Gel Capsules Improve Melatonin’s Bioavailability in Humans. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Sakellaropoulou, A.; Siamidi, A.; Vlachou, M. Melatonin/Cyclodextrin Inclusion Complexes: A Review. Molecules 2022, 27, 445. [Google Scholar] [CrossRef]
- Vlachou, M.; Eikosipentaki, A.; Xenogiorgis, V. Pineal Hormone Melatonin: Solubilization Studies in Model Aqueous Gastrointestinal Environments. Curr. Drug Deliv. 2006, 3, 255–265. [Google Scholar] [CrossRef]
- Chuffa, L.G.d.A.; Seiva, F.R.F.; Novais, A.A.; Simão, V.A.; Martín Giménez, V.M.; Manucha, W.; Zuccari, D.A.P.d.C.; Reiter, R.J. Melatonin-Loaded Nanocarriers: New Horizons for Therapeutic Applications. Molecules 2021, 26, 3562. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Yang, B.; Li, J.; Xu, L.; Liu, H.; Li, S.; Xu, J.; Yang, M.; Wei, M. Evaluation of Biomimetically Synthesized Mesoporous silica Nanoparticles as Drug Carriers: Structure, Wettability, Degradation, Biocompatibility and Brain Distribution. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Shi, J. In Vivo Bio-Safety Evaluations and Diagnostic/Therapeutic Applications of Chemically Designed Mesoporous silica Nanoparticles. Adv. Mater. 2013, 25, 3144–3176. [Google Scholar] [CrossRef]
- Li, R.; Zheng, W.; Yang, R.; Chen, J.; Wang, H.; Ma, L.; Zhang, H. A Silicon Particle-Based Courier Promotes Melatonin-Mediated Seed Tolerance to Nickel Toxicity in Rice. Environ. Sci. Nano 2022, 9, 2854–2868. [Google Scholar] [CrossRef]
- Chen, J.; Qin, H.; Zhang, B.; Mao, W.; Lou, L.; Shen, C.; Mao, J.; Lin, Q. Development of Melatonin Nano-Delivery Systems to Reduce Cadmium Accumulation in Rice (Oryza sativa L.) Seedlings: Insights from Photosynthetic Efficiency, Antioxidative Response and Gene Expression. Environ. Exp. Bot. 2022, 196, 104822. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wu, H.M.; Li, S.; Wang, M.Z.; Fang, L.; Liu, R.Y. Melatonin Enhances Autophagy and Decreases Apoptosis Induced by Nanosilica in RAW264.7 Cells. IUBMB Life 2019, 71, 1021–1029. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Zu, Y.; Wang, L.; Wu, W.; Deng, Y.; Zu, C.; Liu, Y. Melatonin-Loaded Silica Coated with Hydroxypropyl Methylcellulose Phthalate for Enhanced Oral Bioavailability: Preparation, and in Vitro-in Vivo Evaluation. Eur. J. Pharm. Biopharm. 2017, 112, 58–66. [Google Scholar] [CrossRef]
- Lissoni, P.; Rovelli, F.; Frassineti, A.; Fumagalli, L.; Malysheva, O.; Conti, A.; Maestroni, G. Oncostatic Activity of Pineal Neuroendocrine Treatment with the Pineal Indoles Melatonin and 5-Methoxytryptamine in Untreatable Metastatic Cancer Patients Progressing on Melatonin Alone. Neuro Endocrinol. Lett. 2000, 21, 319–323. [Google Scholar] [PubMed]
- Lissoni, P.; Malugani, F.; Brivio, F.; Piazza, A.; Vintimilla, C.; Giani, L.; Tancini, G. Total Pineal Endocrine Substitution Therapy (TPEST) as a New Neuroendocrine Palliative Treatment of Untreatable Metastatic Solid Tumor Patients: A Phase II Study. Neuroendocrinol. Lett. 2003, 24, 259–262. [Google Scholar] [PubMed]
- Lissoni, P.; Messina, G.; Rovelli, F. Cancer as the Main Aging Factor for Humans: The Fundamental Role of 5-Methoxy-Tryptamine in Reversal of Cancer-Induced Aging Processes in Metabolic and Immune Reactions by Non-Melatonin Pineal Hormones. Curr. Aging Sci. 2012, 5, 231–235. [Google Scholar] [CrossRef]
- Lissoni, P.; Malugani, F.; Bukovec, R.; Bordin, V.; Perego, M.; Mengo, S.; Ardizzoia, A.; Tancini, G. Reduction of Cisplatin-Induced Anemia by the Pineal Indole 5-Methoxytryptamine in Metastatic Lung Cancer Patients. Neuro Endocrinol. Lett. 2003, 24, 83–85. [Google Scholar]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef] [PubMed]
- Outcalt, R.J. On the Reaction of 2-Chloroethylisothiocyanate with Aromatic Amines. J. Heterocycl. Chem. 1987, 24, 1425–1428. [Google Scholar] [CrossRef]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Mesoporous silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.S.S.; Makhlouf, M.M.; Mohamed, M.A.; Desoky, M.; Mohamad, A. Promoted Catalytic Potential in Sulfides Oxidation and Biological Screening of Green Pd (II) and Co (II) Complexes of Salicylidene Isatin Hydrazone Ligand. Appl. Organomet. Chem. 2022, 36, e6688. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Choudante, P.C.; Nethi, S.K.; Díaz-García, D.; Prashar, S.; Misra, S.; Gómez-Ruiz, S.; Patra, C.R. Tin-Loaded Mesoporous silica Nanoparticles: Antineoplastic Properties and Genotoxicity Assessment. Biomater. Adv. 2022, 137, 212819. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Morelli, C.; Maris, P.; Sisci, D.; Perrotta, E.; Brunelli, E.; Perrotta, I.; Panno, M.L.; Tagarelli, A.; Versace, C.; Casula, M.F.; et al. PEG-Templated Mesoporous silica Nanoparticles Exclusively Target Cancer Cells. Nanoscale 2011, 3, 3198–3207. [Google Scholar] [CrossRef]
- Pu, X.; Li, J.; Qiao, P.; Li, M.; Wang, H.; Zong, L.; Yuan, Q.; Duan, S. Mesoporous silica Nanoparticles as a Prospective and Promising Approach for Drug Delivery and Biomedical Applications. Curr. Cancer Drug Targets 2019, 19, 285–295. [Google Scholar] [CrossRef]
- He, H.; Xiao, H.; Kuang, H.; Xie, Z.; Chen, X.; Jing, X.; Huang, Y. Synthesis of Mesoporous silica Nanoparticle-Oxaliplatin Conjugates for Improved Anticancer Drug Delivery. Colloids Surf. B Biointerfaces 2014, 117, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, H.; Zhu, C.; Hu, J.; Du, M.; Zhang, F.; Yang, D. Cisplatin and Doxorubicin Dual-Loaded Mesoporous silica Nanoparticles for Controlled Drug Delivery. RSC Adv. 2016, 6, 94160–94169. [Google Scholar] [CrossRef]
- Munaweera, I.; Shi, Y.; Koneru, B.; Patel, A.; Dang, M.H.; Di Pasqua, A.J.; Balkus, K.J. Nitric Oxide- and Cisplatin-Releasing Silica Nanoparticles for Use against Non-Small Cell Lung Cancer. J. Inorg. Biochem. 2015, 153, 23–31. [Google Scholar] [CrossRef]












| Material | %5MT a | %TdTn a | %Pt b | %Pd b |
|---|---|---|---|---|
| MSN-TdTn | - | 1.78 | - | - |
| MSN-PtTdTn | - | 11.58 | 6.80 | - |
| MSN-PdTdTn | - | 6.56 | - | 2.10 |
| MSN-5MT | 0.35 | - | - | - |
| MSN-5MT-TdTn | 0.21 | - | - | |
| MSN-5MT-PtTdTn | 1.47 | 0.86 | - | |
| MSN-5MT-PdTdTn | 1.52 | - | 0.49 |
| Material | SBET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| MSN | 1064 | 0.80 | 2.8 |
| MSN-5MT | 901 | 0.54 | 2.8 |
| MSN-TdTn | 878 | 0.43 | 2.6 |
| MSN-PtTdTn | 856 | 0.41 | 2.8 |
| MSN-PdTdTn | 818 | 0.41 | 2.7 |
| MSN-5MT-TdTn | 761 | 0.47 | 2.9 |
| MSN-5MT-PtTdTn | 825 | 0.40 | 2.9 |
| MSN-5MT-PdTdTn | 808 | 0.39 | 2.8 |
| Material | Time (h) | % Metal Release (±0.001) |
|---|---|---|
| MSN-PtTdTn | 3 | 0.044 |
| 24 | 0.044 | |
| 48 | 0.044 | |
| MSN-PdTdTn | 3 | 0.024 |
| 24 | 0.024 | |
| 48 | 0.024 | |
| MSN-5MT-PtTdTn | 3 | 0.536 |
| 24 | 0.360 | |
| 48 | 0.349 | |
| MSN-5MT-PdTdTn | 3 | 0.102 |
| 24 | 0.069 | |
| 48 | 0.126 |
| IC50 (±SD; µg/mL) | |||
|---|---|---|---|
| Material/Compound | Total | Metal | Ligand |
| MSN | <1000 | - | - |
| MSN-MP | <1000 | - | - |
| MSN-PtTdTn | 462.40 ± 16.52 | 31.44 ± 1.12 | - |
| PtTdTn | 30.96 ± 2.43 | 10.10 ± 0.79 | - |
| MSN-PdTdTn | 424.8 ± 9.59 | 8.92 ± 0.20 | - |
| PdTdTn | 20.05 ± 0.86 | 4.19 ± 0.18 | - |
| MSN-TdTn | 648.6 ± 43.26 | - | 11.55 ± 0.77 |
| TdTn | 17.12 ± 1.04 | - | 17.12 ± 1.04 |
| Cisplatin | 4.84 ± 1.01 | 3.14 ± 0.66 | - |
| IC50 (±SD; µg/mL) | ||||
|---|---|---|---|---|
| Material/Compound | Total | (Metal) | (Ligand) | (5MT) |
| MSN-MP-TEDTS | <1000 | - | - | - |
| MSN-5MT-PtTdTn | 394.90 ± 13.14 | 3.41 ± 0.11 | - | - |
| MSN-5MT-PdTdTn | 366.20 ± 8.69 | 1.79 ± 0.04 | - | - |
| MSN-5MT-TdTn | 632.9 ± 32.82 | - | 1.33 ± 0.07 | - |
| MSN-5MT | 724.7 ± 113 | - | - | 2.54 ± 0.40 |
| 5MT | 1120 ± 0.02 | - | - | 212.80 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estirado, S.; Díaz-García, D.; Fernández-Delgado, E.; Viñuelas-Zahínos, E.; Gómez-Ruiz, S.; Prashar, S.; Rodríguez, A.B.; Luna-Giles, F.; Pariente, J.A.; Espino, J. Melatonin Derivative-Conjugated Formulations of Pd(II) and Pt(II) Thiazoline Complexes on Mesoporous Silica to Enhance Cytotoxicity and Apoptosis against HeLa Cells. Pharmaceutics 2024, 16, 92. https://doi.org/10.3390/pharmaceutics16010092
Estirado S, Díaz-García D, Fernández-Delgado E, Viñuelas-Zahínos E, Gómez-Ruiz S, Prashar S, Rodríguez AB, Luna-Giles F, Pariente JA, Espino J. Melatonin Derivative-Conjugated Formulations of Pd(II) and Pt(II) Thiazoline Complexes on Mesoporous Silica to Enhance Cytotoxicity and Apoptosis against HeLa Cells. Pharmaceutics. 2024; 16(1):92. https://doi.org/10.3390/pharmaceutics16010092
Chicago/Turabian StyleEstirado, Samuel, Diana Díaz-García, Elena Fernández-Delgado, Emilio Viñuelas-Zahínos, Santiago Gómez-Ruiz, Sanjiv Prashar, Ana B. Rodríguez, Francisco Luna-Giles, José A. Pariente, and Javier Espino. 2024. "Melatonin Derivative-Conjugated Formulations of Pd(II) and Pt(II) Thiazoline Complexes on Mesoporous Silica to Enhance Cytotoxicity and Apoptosis against HeLa Cells" Pharmaceutics 16, no. 1: 92. https://doi.org/10.3390/pharmaceutics16010092