Development and Pharmacokinetic Evaluation of Novasomes for the Trans-nasal Delivery of Fluvoxamine Using Arachidonic Acid-Carboxymethyl Chitosan Conjugate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
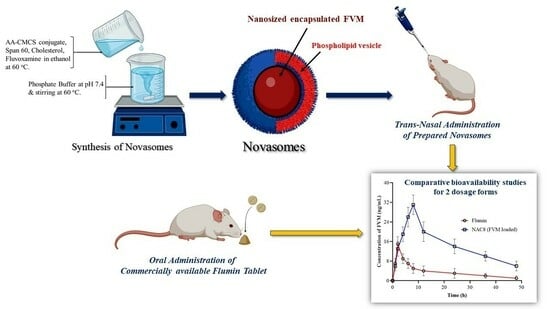
2.2. Synthesis of Arachidonic Acid-Carboxymethyl Chitosan (AA-CMCS) Conjugate
2.3. Experimental Design
2.4. Preparation of FVM-Loaded Novasomes
2.5. %Entrapment Efficiency (%EE) of FVM
2.6. Analytical Characterization
2.7. In Vitro Release FVM from Novasomes
2.8. FVM Release Kinetics
2.9. Permeation Studies
2.10. Mucoadhesion Studies
2.11. Cell Viability Assay
2.12. Anti-Depressant Activity
2.12.1. Animals
2.12.2. Preparation of Test Samples
2.12.3. Forced Swimming Test
2.12.4. Tail Suspension Test
2.13. HPLC Method for FVM Analysis
2.14. Pharmacokinetics of FVM
2.15. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of AA-CMCS Conjugate
3.2. Preparation of Novasomes and Experimental Design
3.3. % Entrapment Efficiency (%EE)
3.4. Particle Size and Zeta Potential Analysis
3.5. Fourier Transforms Infrared Spectroscopy (FTIR)
3.6. Differential Scanning Calorimetry (DSC)
3.7. Thermogravimetric Analysis (TGA)
3.8. Release of FVM from Novasomes
3.9. Release Kinetics
3.10. Permeation Studies
3.11. Mucoadhesion Study
3.12. Cell Viability
3.13. Antidepressant Activity
3.14. Pharmacokinetics of FVM from Novasomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosallam, S.; Ragaie, M.H.; Moftah, N.H.; Elshafeey, A.H.; Abdelbary, A.A. Use of novasomes as a vesicular carrier for improving the topical delivery of terconazole: In vitro characterization, in vivo assessment and exploratory clinical experimentation. Int. J. Nanomed. 2021, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Devraj, R.; Williams, H.D.; Warren, D.B.; Mullertz, A.; Porter, C.J.; Pouton, C.W. In vitro digestion testing of lipid-based delivery systems: Calcium ions combine with fatty acids liberated from triglyceride rich lipid solutions to form soaps and reduce the solubilization capacity of colloidal digestion products. Int. J. Pharm. 2013, 441, 323–333. [Google Scholar] [CrossRef]
- Benfield, P.; Ward, A. Fluvoxamine. Drugs 1986, 32, 313–334. [Google Scholar] [CrossRef] [PubMed]
- Van Harten, J. Over view of the pharmacokinetics of fluvoxamine. Clin. Pharmacokinet. 1995, 29, 1–9. [Google Scholar] [CrossRef]
- Srinivas, M. Design and in vitro Evaluation of Fluvoxamine Nanosuspension using PVA as Stabilizing Agent. Asian J. Pharm. 2021, 15, 205. [Google Scholar]
- Tyagi, Y.; Madhav, N.S. In built novel bio retardant feature of biopolymer isolated from cucumis sativa for designing drug loaded bionanosuspension. J. Drug Assess. 2020, 9, 72–81. [Google Scholar] [CrossRef]
- Kumar, A.; Mancy, S.; Manjunath, K.; Kulkarni, S.V.; Jagadeesh, R. Formulation and Evaluation of Fluvoxamine Maleate Loaded Lipid Nanoparticle. Int. J. Pharm. Sci. Nanotechnol. 2019, 12, 4593–4600. [Google Scholar]
- Kalliola, S.; Repo, E.; Srivastava, V.; Heiskanen, J.P.; Sirviö, J.A.; Liimatainen, H.; Sillanpää, M. The pH sensitive properties of carboxymethyl chitosan nanoparticles cross-linked with calcium ions. Colloids Surf. B Biointerfaces 2017, 153, 229–236. [Google Scholar] [CrossRef]
- Prabaharan, M.; Reis, R.; Mano, J. Carboxymethyl chitosan-graft-phosphatidyl ethanolamine: Amphiphilic matrices for controlled drug delivery. React. Funct. Polym. 2007, 67, 43–52. [Google Scholar] [CrossRef]
- Prabaharan, M.; Gong, S. Novel thiolated carboxymethyl chitosan-g-β-cyclodextrin as mucoadhesive hydrophobic drug delivery carriers. Carbohydr. Polym. 2008, 73, 117–125. [Google Scholar] [CrossRef]
- Singh, A.; Yadagiri, G.; Negi, M.; Kushwaha, A.K.; Singh, O.P.; Sundar, S.; Mudavath, S.L. Carboxymethyl chitosan modified lipid nano formulations as a highly efficacious and biocompatible oral anti-leishmanial drug carrier system. Int. J. Biol. Macromol. 2022, 204, 373–385. [Google Scholar] [CrossRef]
- Miyake, M.; Minami, T.; Yamazaki, H.; Emoto, C.; Mukai, T.; Toguchi, H. Arachidonic acid with taurine enhances pulmonary absorption of macromolecules without any serious histopathological damages. Eur. J. Pharm. Biopharm. 2017, 114, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sirvi, A.; Kaur, S.; Samal, S.K.; Roy, S.; Sangamwar, A.T. Polymeric micelles based on amphiphilic oleic acid modified carboxymethyl chitosan for oral drug delivery of bcs class iv compound: Intestinal permeability and pharmacokinetic evaluation. Eur. J. Pharm. Sci. 2020, 153, 105466. [Google Scholar] [CrossRef]
- Agirre, A.; Aguirre, M.; Leiza, J.R. Characterization of grafting properties of ABS latexes: ATR-FTIR vs. NMR spectroscopy. Polymer 2022, 253, 124997. [Google Scholar] [CrossRef]
- Johari, R.; Li, H.; Liskovich, I.; Weintraub, G.Y. Experimental design in two-sided platforms: An analysis of bias. Manag. Sci. 2022, 68, 7069–7089. [Google Scholar] [CrossRef]
- Abd-Elal, R.M.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef] [PubMed]
- Swamy, N.; Abbas, Z. Preparation and in vitro characterization of mucoadhesive hydroxypropyl guar microspheres containing amlodipine besylate for nasal administration. Indian J. Pharm. Sci. 2011, 73, 608. [Google Scholar] [CrossRef]
- Saibabu, C.; Malyadri, T. Formulation and In-vitro Characterization of Fluvoxamine Loaded Nanoparticles. Int. J. Health Care Biol. Sci. 2021, 2, 43–52. [Google Scholar]
- Shankar, V.K.; Wang, M.; Ajjarapu, S.; Kolimi, P.; Avula, B.; Murthy, R.; Khan, I.; Murthy, S.N. Analysis of docosanol using GC/MS: Method development, validation, and application to ex vivo human skin permeation studies. J. Pharm. Anal. 2022, 12, 287–292. [Google Scholar] [CrossRef]
- Khalid, S.; Abbas, G.; Hanif, M.; Shah, S.; Shah, S.N.H.; Jalil, A.; Yaqoob, M.; Ameer, N.; Anum, A. Thiolated sodium alginate conjugates for mucoadhesive and controlled release behavior of metformin microspheres. Int. J. Biol. Macromol. 2020, 164, 2691–2700. [Google Scholar] [CrossRef]
- Zirak, N.; Maadani, A.; Salahinejad, E.; Abbasnezhad, N.; Shirinbayan, M. Fabrication, drug delivery kinetics and cell viability assay of PLGA-coated vancomycin-loaded silicate porous microspheres. Ceram. Int. 2022, 48, 48–54. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar]
- Butterweck, V.; Nishibe, S.; Sasaki, T.; Uchida, M. Antidepressant effects of Apocynum venetum leaves in a forced swimmingtest. Biol. Pharm. Bull. 2001, 24, 848–851. [Google Scholar] [CrossRef] [PubMed]
- García-Durán, L.; Flores-Burgess, A.; Cantero-García, N.; Puigcerver-Martinez, A.; Narváez, J.Á.; Fuxe, K.; Santín, L.; Millón, C.; Díaz-Cabiale, Z. Galanin (1-15) enhanced the antidepressant-like effects of escitalopram in the olfactory bulb ectomy rats in the forced swimming test through 5-HT1 Areceptors. RiUMA 2022, 1, 1–25. [Google Scholar]
- Nakagawasai, O.; Takahashi, K.; Ambo, A.; Onuma, K.; Takahashi, N.; Nemoto, W.; Tan-No, K. Antidepressant Effect of Intracerebro ventricularly Administered Deltorphin Analogs in the Mouse Tail Suspension Test. Biol. Pharm. Bull. 2022, 45, 538–541. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, B.; Qin, X.; Li, Y.; Yuan, H.; Zhang, L.; Liu, X.; Zhang, Y.; Lu, J. Using Amphiphilic Polymer Micelles as the Templates of Antisolvent Crystallization to Produce Drug Nanocrystals. ACS Omega 2022, 7, 21000–21013. [Google Scholar] [CrossRef] [PubMed]
- Bukzem, A.L.; Signini, R.; DosSantos, D.M.; Lião, L.M.; Ascheri, D.P.R. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.I.; Tsiafoulis, C.G.; Gerothanassis, I.P. High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef]
- Aboud, H.M.; Hussein, A.K.; Zayan, A.Z.; Makram, T.S.; Sarhan, M.O.; El-Sharawy, D.M. Tailoring of Selenium-Plated Novasomes for Fine-Tuning Pharmacokinetic and Tumor Uptake of Quercetin: In Vitro Optimization and In Vivo Radiobiodistribution Assessment in Ehrlich Tumor-Bearing Mice. Pharmaceutics 2022, 14, 875. [Google Scholar] [CrossRef]
- Elkomy, M.H.; ElMenshawe, S.F.; Kharshoum, R.M.; Abdeltwab, A.M.; Hussein, R.R.; Hamad, D.S.; Alsalahat, I.; Aboud, H.M. Innovative pulmonary targeting of terbutaline sulfate-loaded novasomes fornon-invasive tackling of asthma: Statistical optimization and comparative in vitro/in vivo evaluation. Drug Deliv. 2022, 29, 2058–2071. [Google Scholar] [CrossRef]
- El-Dakroury, W.A.; Zewail, M.B.; Amin, M.M. Design, optimization, and in-vivo performance of glipizide-loaded O-carboxymethyl chitosan nanoparticles in insulin resistant/type2diabetic rat model. J. Drug Deliv. Sci. Technol. 2023, 79, 104040. [Google Scholar] [CrossRef]
- Basak, M.; Rahman, M.L.; Ahmed, M.F.; Biswas, B.; Sharmin, N. The use of X-ray diffraction peak profile analysis to determine the structural parameters of cobalt ferrite nanoparticles using Debye-Scherrer, Williamson-Hall, Halder-Wagner andSize-strain plot: Different precipitating agent approach. J. Alloys Compd. 2022, 895, 162694. [Google Scholar] [CrossRef]
- Ganorkar, S.B.; Vander Heyden, Y. Recent trends in pharmaceutical analysis to foster modern drug discovery by comparative in-silico profiling of drugs and related substances. TrAC Trends Anal. Chem. 2022, 157, 116747. [Google Scholar] [CrossRef]
- Eddarai, E.; ElMouzahim, M.; Boussen, R.; Bellaouchou, A.; Guenbour, A.; Zarrouk, A. Chitosan-kaolinite clay composite as durable coating material for slow release NPK fertilizer. Int. J. Biol. Macromol. 2022, 195, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Korma, S.A.; Li, L.; Wei, W.; Liu, P.; Zhang, X.; Bakry, I.A.; An, P.; Abdrabo, K.A.; Manzoor, M.F.; Umair, M. A Comparative Study of Milk Fat Extracted from the Milk of Different Goat Breeds in China: FattyAcids, Triacylglycerols and Thermaland Spectroscopic Characterization. Biomolecules 2022, 12, 730. [Google Scholar] [CrossRef]
- Han, X.; Zheng, Z.; Yu, C.; Deng, Y.; Ye, Q.; Niu, F.; Chen, Q.; Pan, W.; Wang, Y. Preparation, characterization and antibacterial activity of new ionized chitosan. Carbohydr. Polym. 2022, 290, 119490. [Google Scholar] [CrossRef] [PubMed]
- Janiaczyk, M.; Jelińska, A.; Woźniak-Braszak, A.; Bilski, P.; Popielarz-Brzezińska, M.; Wachowiak, M.; Baranowski, M.; Tomczak, S.; Ogrodowczyk, M. Electron Beam Radiation as a Safe Method for the Sterilization of Aceclofenac and Diclofenac—The Usefulness of EPR and 1H-NMR Methods in Determination of Molecular Structure and Dynamics. Pharmaceutics 2022, 14, 1331. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Abd-Allah, H. Span lastic nanovesicles for enhanced ocular delivery of vanillic acid: Design, in vitro characterization, and in vivo anti-inflammatory evaluation. Int. J. Pharm. 2022, 625, 122068. [Google Scholar] [CrossRef] [PubMed]
- Dow, G.-S.; Smith, B.-L. A Phase II, double blind, placebo-controlled, randomized evaluation of the safety and efficacy of tafenoquine in patients with mild-moderate COVID-19 disease. New Microbes New Infect. 2022, 47, 100986. [Google Scholar] [CrossRef]
- Ijaz, H.; Tulain, U.R.; Minhas, M.U.; Mahmood, A.; Sarfraz, R.M.; Erum, A.; Danish, Z. Design and in vitro evaluation of pH-sensitive crosslinked chitosan-grafted acrylic acid copolymer (CS-co-AA) for targeted drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 336–348. [Google Scholar] [CrossRef]
- Yuan, N.Y.; Maung, R.; Xu, Z.; Han, X.; Kaul, M. Arachidonic Acid Cascade and Eicosanoid Production Are Elevated While LTC4 Synthase Modulates the Lipidomics Profile in the Brain of the HIVgp120-Transgenic Mouse Model of Neuro HIV. Cells 2022, 11, 2123. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Amin, M.M.; El-Korany, S.M.; Sayed, S. Corneal targeted fenticonazole nitrate-loaded novasomes for the management of ocular candidiasis: Preparation, in vitro characterization, ex vivo and in vivo assessments. Drug Deliv. 2022, 29, 2428–2441. [Google Scholar] [CrossRef]
- Fatima, I.; Rasul, A.; Shah, S.; Saadullah, M.; Islam, N.; Khames, A.; Salawi, A.; Ahmed, M.M.; Almoshari, Y.; Abbas, G. Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections. Molecules 2022, 27, 2936. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Mi, L.; Li, F.; Li, Y.; Zhou, Y.; Chen, F.; Liu, L.; Chai, Y.; Yang, W.; Zhang, J. Fluvoxamine Confers Neuro protection via Inhibiting In filtration of Peripheral Leukocytes and M1 Polarization of Microglia/Macrophages in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2022, 39, 1240–1261. [Google Scholar] [CrossRef]
- Mahdi, M.; Hermán, L.; Réthelyi, J.M.; Bálint, B.L. Potential Role of the Antidepressants Fluoxetine and Fluvoxamine in the Treatment of COVID-19. Int. J. Mol. Sci. 2022, 23, 3812. [Google Scholar] [CrossRef] [PubMed]
- Labellarte, M.; Biederman, J.; Emslie, G.; Ferguson, J.; Khan, A.; Ruckle, J.; Sallee, R.; Riddle, M. Multiple-dose pharmacokinetics of fluvoxamine in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 1497–1505. [Google Scholar] [CrossRef]





| Run | AA-CMCS Conjugate (mg) | Span 60 (mg) | Stirring Speed (rpm) | Particle Size (nm) | Zeta Potential (mV) | PDI | %EE |
|---|---|---|---|---|---|---|---|
| NAC1 | 1.5 | 2.0 | 500 | 340 ± 5.9 | −12 ± 2.1 | 0.543 ± 0.002 | 59.56 ± 1.453 |
| NAC2 | 1.5 | 1.5 | 750 | 278 ± 5.1 | −19 ± 2.5 | 0.342 ± 0.004 | 67.34 ± 1.089 |
| NAC3 | 1.0 | 2.0 | 750 | 289 ± 4.2 | −17 ± 3.1 | 0.378 ± 0.005 | 71.67 ± 2.981 |
| NAC4 | 1.5 | 2.0 | 1000 | 108 ± 4.1 | −27 ± 2.1 | 0.305 ± 0.008 | 56.45 ± 1.652 |
| NAC5 | 1.5 | 1.5 | 750 | 256 ± 3.9 | −21 ± 1.6 | 0.299 ± 0.006 | 78.40 ± 1.789 |
| NAC6 | 2.0 | 1.5 | 1000 | 102 ± 3.6 | −28 ± 1.5 | 0.213 ± 0.014 | 81.34 ± 1.021 |
| NAC7 | 1.5 | 1.0 | 500 | 356 ± 6.1 | −13 ± 1.9 | 0.421 ± 0.005 | 49.81 ± 2.098 |
| NAC8 | 1.5 | 1.0 | 1000 | 101 ± 3.1 | −35 ± 2.6 | 0.263 ± 0.001 | 90.92 ± 1.567 |
| NAC9 | 1.0 | 1.0 | 750 | 226 ± 4.8 | −20 ± 3.2 | 0.341 ± 0.013 | 82.45 ± 1.043 |
| NAC10 | 1.5 | 1.5 | 750 | 209 ± 3.8 | −19 ± 2.7 | 0.305 ± 0.005 | 80.90 ± 1.498 |
| NAC11 | 1.0 | 1.5 | 1000 | 106 ± 5.2 | −33 ± 2.0 | 0.293 ± 0.004 | 67.92 ± 1.619 |
| NAC12 | 1.5 | 1.5 | 750 | 291 ± 3.8 | −15 ± 2.1 | 0.376 ± 0.008 | 75.39 ± 2.923 |
| NAC13 | 2.0 | 2.0 | 750 | 243 ± 4.6 | −17 ± 3.1 | 0.313 ± 0.003 | 63.34 ± 3.678 |
| NAC14 | 2.0 | 1.5 | 500 | 339 ± 4.6 | −16 ± 2.5 | 0.409 ± 0.015 | 53.21 ± 2.937 |
| NAC15 | 1.0 | 1.5 | 500 | 348 ± 5.3 | −15 ± 2.4 | 0.412 ± 0.006 | 59.82 ± 3.452 |
| NAC16 | 1.5 | 1.5 | 750 | 227 ± 4.6 | −20 ± 1.5 | 0.301 ± 0.004 | 78.45 ± 3.765 |
| NAC17 | 2.0 | 1.0 | 750 | 271 ± 4.1 | −21 ± 2.3 | 0.410 ± 0.007 | 80.67 ± 3.591 |
| Material | Dose | Duration of Immobility (Seconds) |
|---|---|---|
| Forced swimming test | ||
| Control | 1 mL/kg | 176 ± 2.88 |
| Pure Drug (FVM) | 1 mg/kg | 151 ± 3.89 |
| FVM-loaded novasomes (NAC8) | 1 mg/kg | 120 ± 5.64 |
| Tail suspension test | ||
| Control | 1 mL/kg | 126 ± 5.44 |
| Pure Drug (FVM) | 1 mg/kg | 109 ± 4.65 |
| FVM-loaded novasomes (NAC8) | 1 mg/kg | 86 ± 3.23 |
| Parameters | Units | FVM Suspension | Flumin® | NAC8 FVM-Loaded Novasomes |
|---|---|---|---|---|
| Cmax | ng/mL | 12.75 ± 2.78 | 11.46 ± 2.09 | 25.63 ± 3.21 |
| Tmax | h | 2.01 ± 0.93 | 2.23 ± 0.70 | 8.28 ± 1.56 |
| t1/2 | h | 7.72 ± 1.34 | 5.08 ± 1.20 | 15.39 ± 2.02 |
| AUC0−t | ng × h/mL | 349.31 ± 4.21 | 113.52 ± 4.23 | 707.26 ± 5.43 |
| AUC0−ꝏ | ng × h/mL | 415.39 ± 3.49 | 113.71 ± 2.10 | 821.66 ± 6.32 |
| AUMC | ng × h/mL | 1053.49 ± 6.09 | 940.99 ± 6.91 | 21,402.99 ± 8.21 |
| MRT | h | 12.87 ± 1.23 | 8.27 ± 1.09 | 26.048 ± 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulshan, S.; Shah, S.; Shah, P.A.; Irfan, M.; Saadullah, M.; Abbas, G.; Hanif, M.; Rasul, A.; Ahmad, N.; Mahmood, A.; et al. Development and Pharmacokinetic Evaluation of Novasomes for the Trans-nasal Delivery of Fluvoxamine Using Arachidonic Acid-Carboxymethyl Chitosan Conjugate. Pharmaceutics 2023, 15, 2259. https://doi.org/10.3390/pharmaceutics15092259
Gulshan S, Shah S, Shah PA, Irfan M, Saadullah M, Abbas G, Hanif M, Rasul A, Ahmad N, Mahmood A, et al. Development and Pharmacokinetic Evaluation of Novasomes for the Trans-nasal Delivery of Fluvoxamine Using Arachidonic Acid-Carboxymethyl Chitosan Conjugate. Pharmaceutics. 2023; 15(9):2259. https://doi.org/10.3390/pharmaceutics15092259
Chicago/Turabian StyleGulshan, Saima, Shahid Shah, Pervaiz Akhtar Shah, Muhammad Irfan, Malik Saadullah, Ghulam Abbas, Muhammad Hanif, Akhtar Rasul, Nabeel Ahmad, Abid Mahmood, and et al. 2023. "Development and Pharmacokinetic Evaluation of Novasomes for the Trans-nasal Delivery of Fluvoxamine Using Arachidonic Acid-Carboxymethyl Chitosan Conjugate" Pharmaceutics 15, no. 9: 2259. https://doi.org/10.3390/pharmaceutics15092259