Chitosan Scaffolds as Microcarriers for Dynamic Culture of Human Neural Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microcarrier Fabrication
2.2. FTIR and 1H NMR Spectroscopy
2.3. Microstructure Characterization
2.4. Mechanical Property
2.5. Degradation Test
2.6. Cell Culture
2.7. Cell Proliferation in a Rotational Bioreactor as a Dynamic Culture
2.8. Flow Cytometry
2.9. Immunocytochemistry for NSC Marker Proteins
2.10. Statistical Analysis
3. Results and Discussion
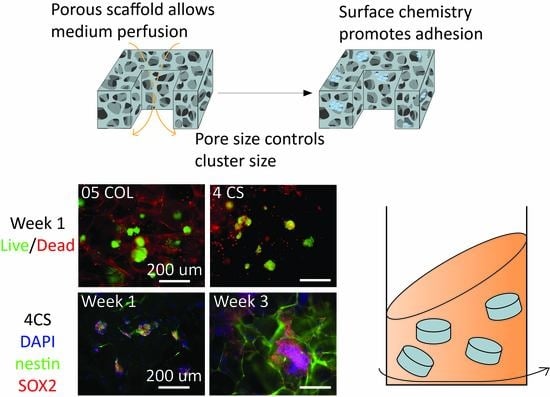
3.1. Development of Porous Microcarriers for hNSC Expansion in a Defined Environment Condition
3.2. Scaffold Microstructure Analysis
3.3. Mechanical Properties of the Scaffolds
3.4. Stability and Degradation in Culture Medium
3.5. Maintenance of hNSCs in Dynamic Cultures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Yu, P.; Cheng, L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017, 8, e3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Moore, D.L. Neural stem cells: Developmental mechanisms and disease modeling. Cell Tissue Res. 2018, 371, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévesque, M.F.; Neuman, T.; Rezak, M. Therapeutic Microinjection of Autologous Adult Human Neural Stem Cells and Differentiated Neurons for Parkinson’s Disease: Five-Year Post-Operative Outcome. Open Stem. Cell J. 2009, 1, 10–19. [Google Scholar]
- Mazzini, L.; Gelati, M.; Profico, D.C.; Sgaravizzi, G.; Projetti Pensi, M.; Muzi, G.; Ricciolini, C.; Rota Nodari, L.; Carletti, S.; Giorgi, C.; et al. Human neural stem cell transplantation in ALS: Initial results from a phase I trial. J. Transl. Med. 2015, 13, 17. [Google Scholar] [CrossRef]
- Liu, N.; Zang, R.; Yang, S.-T.; Li, Y. Stem cell engineering in bioreactors for large-scale bioprocessing. Eng. Life Sci. 2014, 14, 4–15. [Google Scholar] [CrossRef]
- Greiner, A.M.; Richter, B.; Bastmeyer, M. Micro-Engineered 3D Scaffolds for Cell Culture Studies. Macromol. Biosci. 2012, 12, 1301–1314. [Google Scholar] [CrossRef]
- Amato, L.; Heiskanen, A.; Caviglia, C.; Shah, F.; Zór, K.; Skolimowski, M.; Madou, M.J.; Gammelgaard, L.; Hansen, R.; Seiz, E.G.; et al. Pyrolysed 3D-Carbon Scaffolds Induce Spontaneous Differentiation of Human Neural Stem Cells and Facilitate Real-Time Dopamine Detection. Adv. Funct. Mater. 2014, 24, 7042–7052. [Google Scholar] [CrossRef]
- Li, H.; Wijekoon, A.; Leipzig, N.D. 3D Differentiation of Neural Stem Cells in Macroporous Photopolymerizable Hydrogel Scaffolds. PLoS ONE 2012, 7, e48824. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.-Y.; Li, Y.; Liu, J. Progress in clinical trials of stem cell therapy for cerebral palsy. Neural Regen. Res. 2021, 16, 1377–1382. [Google Scholar] [CrossRef]
- Borys, B.S.; Dang, T.; So, T.; Rohani, L.; Revay, T.; Walsh, T.; Thompson, M.; Argiropoulos, B.; Rancourt, D.E.; Jung, S.; et al. Overcoming bioprocess bottlenecks in the large-scale expansion of high-quality hiPSC aggregates in vertical-wheel stirred suspension bioreactors. Stem Cell Res. Ther. 2021, 12, 55. [Google Scholar] [CrossRef]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galderisi, U.; Peluso, G.; di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells Are Exponentially Increasing: Where Are We in Recent Years? Stem. Cell Rev. Rep. 2022, 18, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Karvelas, N.; Bennett, S.; Politis, G.; Kouris, N.-I.; Kole, C. Advances in stem cell therapy in Alzheimer’s disease: A comprehensive clinical trial review. Stem Cell Investig. 2022, 9, 2. [Google Scholar] [CrossRef]
- Razeghian-Jahromi, I.; Matta, A.G.; Canitrot, R.; Zibaeenezhad, M.J.; Razmkhah, M.; Safari, A.; Nader, V.; Roncalli, J. Surfing the clinical trials of mesenchymal stem cell therapy in ischemic cardiomyopathy. Stem Cell Res. Ther. 2021, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Kretzmer, G. Influence of Stress on Adherent Cells. In Influence of Stress on Cell Growth and Product Formation; Springer: Berlin/Heidelberg, Germany, 2000; pp. 123–137. [Google Scholar]
- King, J.A.; Miller, W.M. Bioreactor development for stem cell expansion and controlled differentiation. Curr. Opin. Chem. Biol. 2007, 11, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Jeon, K.J.; Park, S.H.; Shin, J.W.; Kang, Y.G.; Hyun, J.-S.; Oh, M.J.; Kim, S.Y.; Shin, J.-W. Combined effects of flow-induced shear stress and micropatterned surface morphology on neuronal differentiation of human mesenchymal stem cells. J. Biosci. Bioeng. 2014, 117, 242–247. [Google Scholar] [CrossRef]
- Lu, J. A novel hypothesis of blood-brain barrier (BBB) development and in vitro BBB model: Neural stem cell is the driver of BBB formation and maintenance. J. Exp. Integr. Med. 2012, 2, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Olmer, R.; Lange, A.; Selzer, S.; Kasper, C.; Haverich, A.; Martin, U.; Zweigerdt, R. Suspension Culture of Human Pluripotent Stem Cells in Controlled, Stirred Bioreactors. Tissue Eng. Part C Methods 2012, 18, 772–784. [Google Scholar] [CrossRef] [Green Version]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef]
- Stevens, K.R.; Pabon, L.; Muskheli, V.; Murry, C.E. Scaffold-Free Human Cardiac Tissue Patch Created from Embryonic Stem Cells. Tissue Eng. Part A 2009, 15, 1211–1222. [Google Scholar] [CrossRef]
- Sen, A.; Kallos, M.S.; Behie, L.A. Effects of Hydrodynamics on Cultures of Mammalian Neural Stem Cell Aggregates in Suspension Bioreactors. Ind. Eng. Chem. Res. 2001, 40, 5350–5357. [Google Scholar] [CrossRef]
- Baghbaderani, B.A.; Mukhida, K.; Sen, A.; Hong, M.; Mendez, I.; Behie, L.A. Expansion of Human Neural Precursor Cells in Large-Scale Bioreactors for the Treatment of Neurodegenerative Disorders. Biotechnol. Prog. 2008, 24, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Song, L.; Tsai, A.-C.; Ma, T.; Li, Y. Generation of Neural Progenitor Spheres from Human Pluripotent Stem Cells in a Suspension Bioreactor. In Bioreactors in Stem Cell Biology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 119–128. [Google Scholar] [CrossRef]
- Simão, D.; Arez, F.; Terasso, A.P.; Pinto, C.; Sousa, M.F.Q.; Brito, C.; Alves, P.M. Perfusion Stirred-Tank Bioreactors for 3D Differentiation of Human Neural Stem Cells. In Bioreactors in Stem Cell Biology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 129–142. [Google Scholar] [CrossRef]
- Nemati, S.; Abbasalizadeh, S.; Baharvand, H. Scalable Expansion of Human Pluripotent Stem Cell-Derived Neural Progenitors in Stirred Suspension Bioreactor Under Xeno-free Condition. In Bioreactors in Stem Cell Biology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 143–158. [Google Scholar] [CrossRef]
- Rodrigues, C.A.V.; Diogo, M.M.; da Silva, C.L.; Cabral, J.M.S. Microcarrier expansion of mouse embryonic stem cell-derived neural stem cells in stirred bioreactors. Biotechnol. Appl. Biochem. 2011, 58, 231–242. [Google Scholar] [CrossRef]
- Dos Santos, F.F.; Andrade, P.Z.; da Silva, C.L.; Cabral, J.M.S. Bioreactor Design for Clinical-grade Expansion of Stem Cells. Biotechnol. J. 2013, 8, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Skop, N.B.; Calderon, F.; Levison, S.W.; Gandhi, C.D.; Cho, C.H. Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomater. 2013, 9, 6834–6843. [Google Scholar] [CrossRef]
- Tsai, A.-C.; Liu, Y.; Ma, T. Expansion of human mesenchymal stem cells in fibrous bed bioreactor. Biochem. Eng. J. 2016, 108, 51–57. [Google Scholar] [CrossRef]
- Jeske, R.; Lewis, S.; Tsai, A.-C.; Sanders, K.; Liu, C.; Yuan, X.; Li, Y. Agitation in a microcarrier-based spinner flask bioreactor modulates homeostasis of human mesenchymal stem cells. Biochem. Eng. J. 2021, 168, 107947. [Google Scholar] [CrossRef]
- Rogers, R.E.; Haskell, A.; White, B.P.; Dalal, S.; Lopez, M.; Tahan, D.; Pan, S.; Kaur, G.; Kim, H.; Barreda, H.; et al. A Scalable System for Generation of Mesenchymal Stem Cells Derived from Induced Pluripotent Cells Employing Bioreactors and Degradable Microcarriers. Stem. Cells Transl. Med. 2021, 10, 1650–1665. [Google Scholar] [CrossRef]
- Hammel, E.C.; Ighodaro, O.-R.; Okoli, O. Processing and properties of advanced porous ceramics: An application based review. Ceram. Int. 2014, 40, 15351–15370. [Google Scholar] [CrossRef]
- Li, H.; Koenig, A.M.; Sloan, P.; Leipzig, N.D. In vivo assessment of guided neural stem cell differentiation in growth factor immobilized chitosan-based hydrogel scaffolds. Biomaterials 2014, 35, 9049–9057. [Google Scholar] [CrossRef]
- Moore, K.M.; Graham-Gurysh, E.G.; Bomba, H.N.; Murthy, A.B.; Bachelder, E.M.; Hingtgen, S.D.; Ainslie, K.M. Impact of composite scaffold degradation rate on neural stem cell persistence in the glioblastoma surgical resection cavity. Mater. Sci. Eng. C 2020, 111, 110846. [Google Scholar] [CrossRef]
- Guo, R.; Li, J.; Chen, C.; Xiao, M.; Liao, M.; Hu, Y.; Liu, Y.; Li, D.; Zou, J.; Sun, D.; et al. Biomimetic 3D bacterial cellulose-graphene foam hybrid scaffold regulates neural stem cell proliferation and differentiation. Colloids Surf. B Biointerfaces 2021, 200, 111590. [Google Scholar] [CrossRef]
- Lee, Y.-B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.-S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010, 223, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.E.; Rooney, G.E.; Gross, L.; Nesbitt, J.J.; Galvin, K.E.; Knight, A.; Chen, B.; Yaszemski, M.J.; Windebank, A.J. Neural Stem Cell– and Schwann Cell–Loaded Biodegradable Polymer Scaffolds Support Axonal Regeneration in the Transected Spinal Cord. Tissue Eng. Part A 2009, 15, 1797–1805. [Google Scholar] [CrossRef] [Green Version]
- Sheets, K.T.; Ewend, M.G.; Mohiti-Asli, M.; Tuin, S.A.; Loboa, E.G.; Aboody, K.S.; Hingtgen, S.D. Developing Implantable Scaffolds to Enhance Neural Stem Cell Therapy for Post-Operative Glioblastoma. Mol. Ther. 2020, 28, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.K.; Singh, A.; Kipke, D.R. Alginate Composition Effects on a Neural Stem Cell–Seeded Scaffold. Tissue Eng. Part C Methods 2009, 15, 541–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevanato, L.; Sinden, J.D. The effects of microRNAs on human neural stem cell differentiation in two- and three-dimensional cultures. Stem Cell Res. Ther. 2014, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ikram, S. Chitosan: Derivatives, Composites and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 1119364809. [Google Scholar]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozłowska, J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef]
- Han, H.-W.; Hsu, S.-H. Chitosan derived co-spheroids of neural stem cells and mesenchymal stem cells for neural regeneration. Colloids Surf. B Biointerfaces 2017, 158, 527–538. [Google Scholar] [CrossRef]
- Neubrech, F.; Sauerbier, M.; Moll, W.; Seegmüller, J.; Heider, S.; Harhaus, L.; Bickert, B.; Kneser, U.; Kremer, T. Enhancing the Outcome of Traumatic Sensory Nerve Lesions of the Hand by Additional Use of a Chitosan Nerve Tube in Primary Nerve Repair: A Randomized Controlled Bicentric Trial. Plast. Reconstr. Surg. 2018, 142, 415–424. [Google Scholar] [CrossRef]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef] [Green Version]
- Dietzmeyer, N.; Förthmann, M.; Grothe, C.; Haastert-Talini, K. Modification of tubular chitosan-based peripheral nerve implants: Applications for simple or more complex approaches. Neural Regen. Res. 2020, 15, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 12. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Cheng, S.; Han, S.; Shu, M.; Chen, B.; Chen, X.; Tang, F.; Wang, N.; Tu, Y.; et al. Cetuximab modified collagen scaffold directs neurogenesis of injury-activated endogenous neural stem cells for acute spinal cord injury repair. Biomaterials 2017, 137, 73–86. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, D.; Shen, H.; Zhao, Y.; Xu, B.; Fan, Y.; Sun, Z.; Chen, B.; Xue, W.; Shi, Y.; et al. Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomater. Sci. 2020, 8, 5145–5156. [Google Scholar] [CrossRef]
- Moore, L.; Skop, N.B.; Rothbard, D.E.; Corrubia, L.R.; Levison, S.W. Tethered growth factors on biocompatible scaffolds improve stemness of cultured rat and human neural stem cells and growth of oligodendrocyte progenitors. Methods 2018, 133, 54–64. [Google Scholar] [CrossRef]
- Zheng, K.; Feng, G.; Zhang, J.; Xing, J.; Huang, D.; Lian, M.; Zhang, W.; Wu, W.; Hu, Y.; Lu, X.; et al. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous chitosan scaffolds to neural differentiation. Int. J. Neurosci. 2021, 131, 625–633. [Google Scholar] [CrossRef] [PubMed]
- O’rourke, C.; Day, A.G.E.; Murray-Dunning, C.; Thanabalasundaram, L.; Cowan, J.; Stevanato, L.; Grace, N.; Cameron, G.; Drake, R.A.L.; Sinden, J.; et al. An allogeneic ‘off the shelf’ therapeutic strategy for peripheral nerve tissue engineering using clinical grade human neural stem cells. Sci. Rep. 2018, 8, 2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.-H.; Chao, H.-M.; Chern, E.; Hsu, S. Chitosan 3D cell culture system promotes naïve-like features of human induced pluripotent stem cells: A novel tool to sustain pluripotency and facilitate differentiation. Biomaterials 2021, 268, 120575. [Google Scholar] [CrossRef]
- Sood, D.; Cairns, D.M.; Dabbi, J.M.; Ramakrishnan, C.; Deisseroth, K.; Black, L.D.; Santaniello, S.; Kaplan, D.L. Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Sci. Rep. 2019, 9, 17874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indrani, D.J.; Lukitowati, F.; Yulizar, Y. Preparation of Chitosan/Collagen Blend Membranes for Wound Dressing: A Study on FTIR Spectroscopy and Mechanical Properties. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Xiamen, China, 20–22 October 2017; IOP Publishing: Tokyo, Japan, 2017; Volume 202, p. 012020. [Google Scholar]
- Ren, L.; Xu, J.; Zhang, Y.; Zhou, J.; Chen, D.; Chang, Z. Preparation and characterization of porous chitosan microspheres and adsorption performance for hexavalent chromium. Int. J. Biol. Macromol. 2019, 135, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.B.; Muniz, E.C.; Hsieh, Y.-L. 1H NMR and 1H–13C HSQC Surface Characterization of Chitosan–Chitin Sheath-Core Nanowhiskers. Carbohydr. Polym. 2015, 123, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Megia, E.; Novoa-Carballal, R.; Quiñoá, E.; Riguera, R. Optimal routine conditions for the determination of the degree of acetylation of chitosan by 1H-NMR. Carbohydr. Polym. 2005, 61, 155–161. [Google Scholar] [CrossRef]
- Gonzalez Badillo, F.; Zisi Tegou, F.; Masina, R.; Wright, S.; Scully, M.; Harwell, L.; Lupp, M.; Postigo-Fernandez, J.; Creusot, R.J.; Tomei, A.A. Tissue-Engineered Stromal Reticula to Study Lymph Node Fibroblastic Reticular Cells in Type I Diabetes. Cell. Mol. Bioeng. 2020, 13, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Lin, X.; Pan, Y.; Zheng, X.; Shi, L.; Zhang, Y.; Ma, L.; Gao, C.; Zhang, S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019, 92, 160–171. [Google Scholar] [CrossRef]
- Laiva, A.L.; O’brien, F.J.; Keogh, M.B. SDF-1α Gene-Activated Collagen Scaffold Restores Pro-Angiogenic Wound Healing Features in Human Diabetic Adipose-Derived Stem Cells. Biomedicines 2021, 9, 160. [Google Scholar] [CrossRef]
- Ryan, A.J.; O’Brien, F.J. Insoluble elastin reduces collagen scaffold stiffness, improves viscoelastic properties, and induces a contractile phenotype in smooth muscle cells. Biomaterials 2015, 73, 296–307. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Trung, T.S.; Thein-Han, W.W.; Qui, N.T.; Ng, C.-H.; Stevens, W.F. Functional characteristics of shrimp chitosan and its membranes as affected by the degree of deacetylation. Bioresour. Technol. 2006, 97, 659–663. [Google Scholar] [CrossRef]
- Chang, F.; Zhou, Y.; James, M.M.; Zareie, H.M.; Ando, Y.; Yang, J.; Zhang, M. Effect of Degree of Deacetylation of Chitosan/Chitin on Human Neural Stem Cell Culture. Macromol. Biosci. 2022, 23, 200389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chang, S.J.; Jing, Y.; Wang, L.; Chen, C.-J.; Liu, J.-T. Application of chitosan with different molecular weights in cartilage tissue engineering. Carbohydr. Polym. 2023, 314, 120890. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, M.; Nomura, T.; Suetsugu, T.; Matsuzaki, F.; Kojima, S.; Kosodo, Y. Comparative Analysis of Brain Stiffness Among Amniotes Using Glyoxal Fixation and Atomic Force Microscopy. Front. Cell Dev. Biol. 2020, 8, 574619. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.M.; Schwarb, H.D.; McGarry, M.D.J.; Johnson, C.L.; Cohen, N.J. Magnetic Resonance Elastography of Human Hippocampal Subfields: CA3-Dentate Gyrus Viscoelasticity Predicts Relational Memory Accuracy. J. Cogn. Neurosci. 2020, 32, 1704–1713. [Google Scholar] [CrossRef]
- Donzelli, E.; Salvadè, A.; Mimo, P.; Viganò, M.; Morrone, M.; Papagna, R.; Carini, F.; Zaopo, A.; Miloso, M.; Baldoni, M.; et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch. Oral Biol. 2007, 52, 64–73. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [Green Version]
- Schneider, G.B.; English, A.; Abraham, M.; Zaharias, R.; Stanford, C.; Keller, J. The effect of hydrogel charge density on cell attachment. Biomaterials 2004, 25, 3023–3028. [Google Scholar] [CrossRef]
- Pottmeier, P.; Doszyn, O.; Peuckert, C.; Jazin, E. Increased Expression of Y-Encoded Demethylases During Differentiation of Human Male Neural Stem Cells. Stem Cells Dev. 2020, 29, 1497–1509. [Google Scholar] [CrossRef]
- Liu, D.; Pavathuparambil Abdul Manaph, N.; Al-Hawwas, M.; Bobrovskaya, L.; Xiong, L.-L.; Zhou, X.-F. Coating Materials for Neural Stem/Progenitor Cell Culture and Differentiation. Stem Cells Dev. 2020, 29, 463–474. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, Y.; Chang, F.-C.; James, M.; Zhou, Y.; Zhang, M. Chitosan Scaffolds as Microcarriers for Dynamic Culture of Human Neural Stem Cells. Pharmaceutics 2023, 15, 1957. https://doi.org/10.3390/pharmaceutics15071957
Ando Y, Chang F-C, James M, Zhou Y, Zhang M. Chitosan Scaffolds as Microcarriers for Dynamic Culture of Human Neural Stem Cells. Pharmaceutics. 2023; 15(7):1957. https://doi.org/10.3390/pharmaceutics15071957
Chicago/Turabian StyleAndo, Yoshiki, Fei-Chien Chang, Matthew James, Yang Zhou, and Miqin Zhang. 2023. "Chitosan Scaffolds as Microcarriers for Dynamic Culture of Human Neural Stem Cells" Pharmaceutics 15, no. 7: 1957. https://doi.org/10.3390/pharmaceutics15071957