Release Kinetics of Monomers from Dental Composites Containing Fluoride-Doped Calcium Phosphates
Abstract
:1. Introduction
2. Materials and Methods
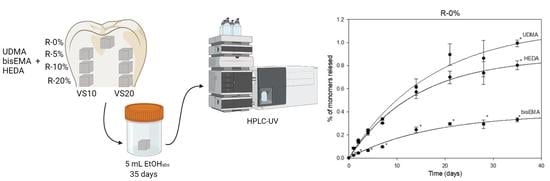
2.1. Specimen Preparation
2.2. HPLC Analysis
2.3. Release Studies
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dionysopoulos, D.; Gerasimidou, O. Wear of Contemporary Dental Composite Resin Restorations: A Literature Review. Restor. Dent. Endod. 2021, 46, e18. [Google Scholar] [CrossRef]
- Hickel, R.; Paschos, E.; Buerkle, V.; Garcia-Godoy, F.; Kaaden, C.; García-Godoy, F.; Manhart, J. Longevity of Occlusally-Stressed Restorations in Posterior Primary Teeth. Longev. Restor. 2005, 18, 198–211. [Google Scholar]
- Downer, M.C.; Azli, N.A.; Bedi, R.; Moles, D.R.; Setchell, D.J. How Long Do Routine Dental Restorations Last? A Systematic Review. Br. Dent. J. 1999, 187, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Deligeorgi, V.; Mjör, I.A.; Wilson, N.H. An Overview of Reasons for the Placement and Replacement of Restorations. Prim. Dent. Care 2001, 8, 5–11. [Google Scholar] [CrossRef]
- Marks, L.A.M.; Weerheijm, K.L.; Van Amerongen, W.E.; Groen, H.J.; Martens, L.C. Dyract versus Tytin Class II Restorations in Primary Molars: 36 Months Evaluation. Caries Res. 1999, 33, 387–392. [Google Scholar] [CrossRef]
- Krämer, N.; Schmidt, M.; Lücker, S.; Domann, E.; Frankenberger, R. Glass Ionomer Cement Inhibits Secondary Caries in an in Vitro Biofilm Model. Clin. Oral Investig. 2018, 22, 1019–1031. [Google Scholar] [CrossRef]
- Alenezi, A.; Alkhudhayri, O.; Altowaijri, F.; Aloufi, L.; Alharbi, F.; Alrasheed, M.; Almutairi, H.; Alanazi, A.; Yehya, M.; Al Asmari, D. Secondary Caries in Fixed Dental Prostheses: Long-Term Clinical Evaluation. Clin. Exp. Dent. Res. 2023, 9, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.K.; Condon, J.R.; Ferracane, J.L. The Effects of Adhesive Thickness on Polymerization Contraction Stress of Composite. J. Dent. Res. 2000, 79, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.; Brunner, T.J.; Vollenweider, M.; Stark, W.J.; Zehnder, M. Antimicrobial Effect of Nanometric Bioactive Glass 45S5. J. Dent. Res. 2007, 86, 754–757. [Google Scholar] [CrossRef]
- Zehnder, M.; Söderling, E.; Salonen, J.; Waltimo, T. Preliminary Evaluation of Bioactive Glass S53P4 as an Endodontic Medication In Vitro. J. Endod. 2004, 30, 220–224. [Google Scholar] [CrossRef]
- Abuna, G.; Feitosa, V.P.; Correr, A.B.; Cama, G.; Giannini, M.; Sinhoreti, M.A.; Pashley, D.H.; Sauro, S. Bonding Performance of Experimental Bioactive/Biomimetic Self-Etch Adhesives Doped with Calcium-Phosphate Fillers and Biomimetic Analogs of Phosphoproteins. J. Dent. 2016, 52, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Rifane, T.O.; Cordeiro, K.E.M.; Silvestre, F.A.; Souza, M.T.; Zanotto, E.D.; Araújo-Neto, V.G.; Giannini, M.; Sauro, S.; de Paula, D.M.; Feitosa, V.P. Impact of Silanization of Different Bioactive Glasses in Simplified Adhesives on Degree of Conversion, Dentin Bonding and Collagen Remineralization. Dent. Mater. 2023, 39, 217–226. [Google Scholar] [CrossRef]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive Glass Fillers Reduce Bacterial Penetration into Marginal Gaps for Composite Restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Gubler, M.; Brunner, T.J.; Zehnder, M.; Waltimo, T.; Sener, B.; Stark, W.J. Do Bioactive Glasses Convey a Disinfecting Mechanism beyond a Mere Increase in PH? Int. Endod. J. 2008, 41, 670–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial Activity of Particulate Bioglass® against Supra- and Subgingival Bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Khvostenko, D.; Mitchell, J.C.; Hilton, T.J.; Ferracane, J.L.; Kruzic, J.J. Mechanical Performance of Novel Bioactive Glass Containing Dental Restorative Composites. Dent. Mater. 2013, 29, 1139–1148. [Google Scholar] [CrossRef] [Green Version]
- Raszewski, Z.; Kulbacka, J.; Nowakowska-Toporowska, A. Mechanical Properties, Cytotoxicity, and Fluoride Ion Release Capacity of Bioactive Glass-Modified Methacrylate Resin Used in Three-Dimensional Printing Technology. Materials 2022, 15, 1133. [Google Scholar] [CrossRef]
- Tauböck, T.T.; Zehnder, M.; Schweizer, T.; Stark, W.J.; Attin, T.; Mohn, D. Functionalizing a Dentin Bonding Resin to Become Bioactive. Dent. Mater. 2014, 30, 868–875. [Google Scholar] [CrossRef]
- Hume, W.R.; Gerzina, T.M. Bioavailability of Components of Resin-Based Materials Which are Applied to Teeth. Crit. Rev. Oral Biol. Med. 1996, 7, 172–179. [Google Scholar] [CrossRef]
- Gerzina, T.M.; Hume, W.R. Diffusion of Monomers from Bonding Resin-Resin Composite Combinations through Dentine in Vitro*. J. Dent. 1996, 24, 125–128. [Google Scholar] [CrossRef]
- Gupta, S.; Saxena, P.; Pant, V.; Pant, A. Release and Toxicity of Dental Resin Composite. Toxicol. Int. 2012, 19, 225–234. [Google Scholar] [PubMed] [Green Version]
- Sauro, S.; Spagnuolo, G.; Del Giudice, C.; Neto, D.M.A.; Fechine, P.B.A.; Chen, X.; Rengo, S.; Chen, X.; Feitosa, V.P. Chemical, Structural and Cytotoxicity Characterisation of Experimental Fluoride-Doped Calcium Phosphates as Promising Remineralising Materials for Dental Applications. Dent. Mater. 2023, 39, 391–401. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular Toxicology of Substances Released from Resin-Based Dental Restorative Materials. Int. J. Mol. Sci. 2009, 10, 3861–3899. [Google Scholar] [CrossRef] [Green Version]
- Putzeys, E.; De Nys, S.; Cokic, S.M.; Duca, R.C.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Long-Term Elution of Monomers from Resin-Based Dental Composites. Dent. Mater. 2019, 35, 477–485. [Google Scholar] [CrossRef]
- Łagocka, R.; Mazurek-Mochol, M.; Jakubowska, K.; Bendyk-Szeffer, M.; Chlubek, D.; Buczkowska-Radlińska, J. Analysis of Base Monomer Elution from 3 Flowable Bulk-Fill Composite Resins Using High Performance Liquid Chromatography (HPLC). Med. Sci. Monit. 2018, 24, 4679–4690. [Google Scholar] [CrossRef]
- Cebe, M.A.; Cebe, F.; Cengiz, M.F.; Cetin, A.R.; Arpag, O.F.; Ozturk, B. Elution of Monomer from Different Bulk Fill Dental Composite Resins. Dent. Mater. 2015, 31, e141–e149. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Higuchi, T.; York, N. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Student. The Probable Error of a Mean. Biometrika 1908, 6, 1–25. [CrossRef]
- Lee, S.Y.; Greener, E.H.; Menis, D.L. Detection of Leached Moieties from Dental Composites in Fluids Simulating Food and Saliva. Dent. Mater. 1995, 11, 348–353. [Google Scholar] [CrossRef]
- Geurtsen, W. Substances Released from Dental Resin Composites and Glass Ionomer Cements. Eur. J. Oral Sci. 1998, 106, 687–695. [Google Scholar] [CrossRef]
- Munksgaard, E.C.; Peutzfeldt, A.; Asmussen, E. Elution of TEGDMA and BisGMA from a Resin and a Resin Composite Cured with Halogen or Plasma Light. Eur. J. Oral Sci. 2000, 108, 341–345. [Google Scholar] [CrossRef]
- Geurtsen, W.; Lehmann, F.; Spahl, W.; Leyhausen, G. Cytotoxicity of 35 Dental Resin Composite Monomers/Additives in Permanent 3T3 and Three Human Primary Fibroblast Cultures. J. Biomed. Mater. Res. 1998, 41, 474–480. [Google Scholar] [CrossRef]
- Polydorou, O.; Trittler, R.; Hellwig, E.; Kümmerer, K. Elution of Monomers from Two Conventional Dental Composite Materials. Dent. Mater. 2007, 23, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Kleinsasser, N.H.; Schmid, K.; Sassen, A.W.; Harréus, U.A.; Staudenmaier, R.; Folwaczny, M.; Glas, J.; Reichl, F.X. Cytotoxic and Genotoxic Effects of Resin Monomers in Human Salivary Gland Tissue and Lymphocytes as Assessed by the Single Cell Microgel Electrophoresis (Comet) Assay. Biomaterials 2006, 27, 1762–1770. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhao, C.; Bu, W.; Zhang, Y.; Na, H. Synthesis, Characterization and Evaluation of a Fluorinated Resin Monomer with Low Water Sorption. J. Mech. Behav. Biomed. Mater. 2018, 77, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.M.; de Almeida Neves, A.; Makeeva, I.M.; Schwendicke, F.; Faus-Matoses, V.; Yoshihara, K.; Banerjee, A.; Sauro, S. Contemporary Restorative Ion-Releasing Materials: Current Status, Interfacial Properties and Operative Approaches. Br. Dent. J. 2020, 229, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U. New Developments of Polymeric Dental Composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Ferracane, J.L. Elution of Leachable Components from Composites. J. Oral Rehabil. 1994, 21, 441–452. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Rosenbauer, E.U. Residual Methyl Methacrylate Monomer, Water Sorption, and Water Solubility of Hypoallergenic Denture Base Materials. J. Prosthet. Dent. 2004, 92, 72–78. [Google Scholar] [CrossRef]
- Delikan, E.; Erturk-Avunduk, A.T.; Karatas, O.; Saçmacı, Ş. Effect of Topical Fluoride Applications on Residual Monomer Release from Resin-Based Restorative Materials. BMC Oral Health 2023, 23, 1. [Google Scholar] [CrossRef]
- Putzeys, E.; Vercruyssen, C.; Duca, R.C.; Saha, P.S.; Godderis, L.; Vanoirbeek, J.; Peumans, M.; Van Meerbeek, B.; Van Landuyt, K.L. Monomer Release from Direct and Indirect Adhesive Restorations: A Comparative In Vitro Study. Dent. Mater. 2020, 36, 1275–1281. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Viana, Í.E.L.; Lopes, R.M.; Silva, F.R.O.; Lima, N.B.; Aranha, A.C.C.; Feitosa, S.; Scaramucci, T. Novel Fluoride and Stannous -Functionalized β-Tricalcium Phosphate Nanoparticles for the Management of Dental Erosion. J. Dent. 2020, 92, 103263. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium Orthophosphates (CaPO4): Occurrence and Properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tredwin, C.J.; Young, A.M.; Georgiou, G.; Shin, S.H.; Kim, H.W.; Knowles, J.C. Hydroxyapatite, Fluor-Hydroxyapatite and Fluorapatite Produced via the Sol-Gel Method. Optimisation, Characterisation and Rheology. Dent. Mater. 2013, 29, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Neel, E.A.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–Remineralization Dynamics in Teeth and Bone. Int. J. Nanomed. 2016, 11, 4743. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynolds, E.C. New Approaches to Enhanced Remineralization of Tooth Enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef]
- Fernando, J.R.; Shen, P.; Walker, G.D.; Yuan, Y.; Stanton, D.P.; Reynolds, C.; Reynolds, E.C. Acceleration of Enamel Subsurface Lesion Remineralisation by Intralesion PH Modulation. Caries Res. 2021, 55, 130–136. [Google Scholar] [CrossRef]
- Zitz, A.; Gedalia, I.; Grajower, R. Addition of Fluoride Compounds to Acrylic Resin Plates: Bending Strength and Fluoride Release. J. Oral Rehabil. 1981, 8, 37–41. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Antonucci, J.M. Dimethacrylate Monomers with Varied Fluorine Contents and Distributions. Dent. Mater. 1999, 15, 166–173. [Google Scholar] [CrossRef]
- Papagiannoulis, T.; Tzoutzas, J.; Eliades, G. Effect of Topical Fluoride Agents on the Morphologic Characteristics and Composition of Resin Composite Restorative Materials. J. Prosthet. Dent. 1997, 77, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Plueddemann, E.P. Adhesion Through Silane Coupling Agents. J. Adhes. 2008, 2, 184–201. [Google Scholar] [CrossRef]
- Waclawczyk, A.; Postek-Stefanska, L.; Pietraszewska, D.; Birkner, E.; Zalejska-Fiolka, J.; Wysoczanska-Jankowicz, I. TEGDMA and UDMA Monomers Released from Composite Dental Material Polymerized with Diode and Halogen Lamps. Adv. Clin. Exp. Med. 2018, 27, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Rueggeberg, F.A.; Lapp, C.A.; Lewis, J.B.; Lockwood, P.E.; Ergle, J.W.; Mettenburg, D.J. In Vitro Cytotoxicity of Resin-Containing Restorative Materials after Aging in Artificial Saliva. Clin. Oral Investig. 1999, 3, 144–149. [Google Scholar] [CrossRef]
- Germain, H.S.; Swartz, M.L.; Phillips, R.W.; Moore, B.K.; Roberts, T.A. Properties of Microfilled Composite Resins as Influenced by Filler Content. J. Dent. Res. 1985, 64, 155–160. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, H.C.; Lee, Y. Der The Effects of Filler Content and Size on the Properties of PTFE/SiO2 Composites. J. Polym. Res. 2003, 10, 247–258. [Google Scholar] [CrossRef]
- Leinfelder, K.F. Posterior Composite Resins: The Materials and Their Clinical Performance. J. Am. Dent. Assoc. 1995, 126, 663–676. [Google Scholar] [CrossRef]




| 0 | 1 | P | H | |
|---|---|---|---|---|
| UDMA | 0.9084 | 0.9604 | 0.9493 | 0.9449 |
| HEDA | 0.9047 | 0.9756 | 0.9616 | 0.9610 |
| Bis-EMA | 0.8978 | 0.9639 | 0.9404 | 0.9370 |
| M∞ | k | R2 | |
|---|---|---|---|
| UDMA R0% (VS-free) | 1.14 ± 0.05 A | 0.058 ± 0.006 | 0.9604 |
| VS10-UDMA-R5% | 0.83 ± 0.04 B | 0.050 ± 0.005 | 0.9743 |
| VS20-UDMA-R5% | 1.13 ± 0.06 A | 0.078 ± 0.010 | 0.9269 |
| VS10-UDMA-R10% | 0.90 ± 0.02 C | 0.063 ± 0.003 | 0.9897 |
| VS20-UDMA-R10% | 0.97 ± 0.04 D | 0.084 ± 0.009 | 0.945 |
| VS10-UDMA-R20% | 0.97 ± 0.03 C | 0.062 ± 0.004 | 0.9808 |
| VS20-UDMA-R20% | 1.47 ± 0.08 E | 0.071 ± 0.009 | 0.9367 |
| HEDA R0% (VS-free) | 0.87 ± 0.03 A | 0.071 ± 0.005 | 0.9756 |
| VS10-HEDA-R5% | 0.59 ± 0.03 B | 0.053 ± 0.006 | 0.9661 |
| VS20-HEDA-R5% | 0.71 ± 0.06 D | 0.076 ± 0.016 | 0.8375 |
| VS10-HEDA-R10% | 0.67 ± 0.02 C | 0.067 ± 0.005 | 0.9793 |
| VS20-HEDA-R10% | 0.59 ± 0.04 E | 0.074 ± 0.013 | 0.8830 |
| VS10-HEDA-R20% | 0.64 ± 0.01 C | 0.063 ± 0.003 | 0.9887 |
| VS20-HEDA-R20% | 0.92 ± 0.11 A | 0.078 ± 0.024 | 0.7084 |
| bisEMA R0%(VS-free) | 0.38 ± 0.02 A | 0.058 ± 0.006 | 0.9639 |
| VS10-bis-EMA-R5% | 0.24 ± 0.01 B | 0.047 ± 0.006 | 0.9639 |
| VS20-bis-EMA-R5% | 0.51 ± 0.03 D | 0.099 ± 0.019 | 0.8196 |
| VS10-bis-EMA-R10% | 0.30 ± 0.02 C | 0.060 ± 0.008 | 0.9489 |
| VS20-bis-EMA-R10% | 0.33 ± 0.01 E | 0.15 ± 0.02 | 0.8441 |
| VS10-bis-EMA-R20% | 0.23 ± 0.01 B | 0.053 ± 0.004 | 0.9802 |
| VS20-bis-EMA-R20% | 0.71 ± 0.06 F | 0.093 ± 0.023 | 0.7385 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alambiaga-Caravaca, A.M.; López-Castellano, A.; Chou, Y.F.; Luzi, A.; Núñez, J.M.; Banerjee, A.; Jovani Sancho, M.d.M.; Sauro, S. Release Kinetics of Monomers from Dental Composites Containing Fluoride-Doped Calcium Phosphates. Pharmaceutics 2023, 15, 1948. https://doi.org/10.3390/pharmaceutics15071948
Alambiaga-Caravaca AM, López-Castellano A, Chou YF, Luzi A, Núñez JM, Banerjee A, Jovani Sancho MdM, Sauro S. Release Kinetics of Monomers from Dental Composites Containing Fluoride-Doped Calcium Phosphates. Pharmaceutics. 2023; 15(7):1948. https://doi.org/10.3390/pharmaceutics15071948
Chicago/Turabian StyleAlambiaga-Caravaca, Adrián M., Alicia López-Castellano, Yu Fu Chou, Arlinda Luzi, Juan Manuel Núñez, Avijit Banerjee, María del Mar Jovani Sancho, and Salvatore Sauro. 2023. "Release Kinetics of Monomers from Dental Composites Containing Fluoride-Doped Calcium Phosphates" Pharmaceutics 15, no. 7: 1948. https://doi.org/10.3390/pharmaceutics15071948