Recent Advances in Cell Membrane Coated-Nanoparticles as Drug Delivery Systems for Tackling Urological Diseases
Abstract
:1. Introduction
2. Current State of Nanomedicine for Treating Urological Diseases
3. Synthesis and Surface Engineering of CMNPs
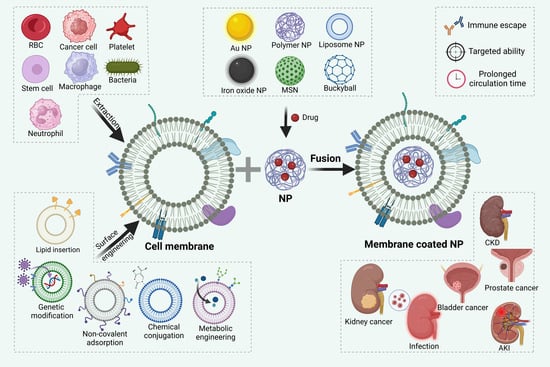
3.1. Extraction of Cell Membrane
3.2. Fusion of Cell Membranes with Core Nanoparticles
3.3. Surface Engineering
4. Application of CMNPs in Tackling Urological Diseases
4.1. Cancer Cell Membranes (CCMs)
4.2. Immune Cell Membranes (ICMs)
4.3. Stem Cell Membranes (SCMs)
4.4. Red Blood Cell Membranes (RBCMs)
4.5. Extracellular Vesicles (EVs)
4.6. Other Membranes
5. Prospects and Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414–422d. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, A.; Tonelli, M.; Bonventre, J.; Coresh, J.; Donner, J.A.; Fogo, A.B.; Fox, C.S.; Gansevoort, R.T.; Heerspink, H.J.L.; Jardine, M.; et al. Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 2017, 390, 1888–1917. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Y.; Yang, S.; Zou, K.; Pei, R.; He, J.; Deng, Y.; Zhou, M.; Zhao, L.; Guo, H. Premature deaths caused by smoking in Sichuan, Southwest China, 2015–2030. Sci. Rep. 2021, 11, 171. [Google Scholar] [CrossRef]
- Wang, J.; Masehi-Lano, J.J.; Chung, E.J. Peptide and antibody ligands for renal targeting: Nanomedicine strategies for kidney disease. Biomater. Sci. 2017, 5, 1450–1459. [Google Scholar] [CrossRef] [Green Version]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release Off. J. Control. Release Soc. 2020, 321, 442–462. [Google Scholar] [CrossRef]
- Williams, R.M.; Jaimes, E.A.; Heller, D.A. Nanomedicines for kidney diseases. Kidney Int. 2016, 90, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Poste, G.; Bucana, C.; Raz, A.; Bugelski, P.; Kirsh, R.; Fidler, I.J. Analysis of the fate of systemically administered liposomes and implications for their use in drug delivery. Cancer Res. 1982, 42, 1412–1422. [Google Scholar]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Gao, W.; Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023, 20, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; He, R.; Xu, D.; Zang, J.; Weeranoppanant, N.; Dong, H.; Li, Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2020, 8, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Wei, Y.; Shen, K.; Xiao, L.; Miron, R.J.; Zhang, Y. Cell-Membrane-Display Nanotechnology. Adv. Healthc. Mater. 2021, 10, e2001014. [Google Scholar] [CrossRef]
- Lee, N.H.; You, S.; Taghizadeh, A.; Taghizadeh, M.; Kim, H.S. Cell Membrane-Cloaked Nanotherapeutics for Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2223. [Google Scholar] [CrossRef]
- Zou, S.; Wang, B.; Wang, C.; Wang, Q.; Zhang, L. Cell membrane-coated nanoparticles: Research advances. Nanomedicine 2020, 15, 625–641. [Google Scholar] [CrossRef]
- Atala, A. What’s new in urology. J. Am. Coll. Surg. 2004, 199, 446–461. [Google Scholar] [CrossRef]
- Gupta, K.; Miller, J.D.; Li, J.Z.; Russell, M.W.; Charbonneau, C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): A literature review. Cancer Treat. Rev. 2008, 34, 193–205. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, B.; Hwang, E.C.; Hong, S.H.; Jeong, C.W.; Kwak, C.; Byun, S.S.; Chung, J. Retrospective Multicenter Long-Term Follow-up Analysis of Prognostic Risk Factors for Recurrence-Free, Metastasis-Free, Cancer-Specific, and Overall Survival after Curative Nephrectomy in Non-metastatic Renal Cell Carcinoma. Front. Oncol. 2019, 9, 859. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, P.; Wu, P.C.; Chancellor, M.; Yoshimura, N.; Huang, L. Recent advances in intravesical drug/gene delivery. Mol. Pharm. 2006, 3, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.P.; Hu, Y.; Lin, J.C.; Fu, H.L.; Lim, L.Y.; Yuan, Z.X. Targeting strategies for drug delivery to the kidney: From renal glomeruli to tubules. Med. Res. Rev. 2019, 39, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Yang, D.; He, W.; Zhu, X.; Yan, Y.; Liu, Z.; Liu, T.; Yang, J.; Tan, S.; Jiang, J.; et al. Ultrasound-mediated microbubbles cavitation enhanced chemotherapy of advanced prostate cancer by increasing the permeability of blood-prostate barrier. Transl. Oncol. 2021, 14, 101177. [Google Scholar] [CrossRef]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Zaro, J.; Shen, Y. Advances in Exosome-Based Drug Delivery and Tumor Targeting: From Tissue Distribution to Intracellular Fate. Int. J. Nanomed. 2020, 15, 9355–9371. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paluszkiewicz, P.; Martuszewski, A.; Zaręba, N.; Wala, K.; Banasik, M.; Kepinska, M. The Application of Nanoparticles in Diagnosis and Treatment of Kidney Diseases. Int. J. Mol. Sci. 2021, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, C.; Wang, J.; Chen, L.; Chen, J.; Chen, T.; Zeng, Q. Application of nanotechnology in the diagnosis and treatment of bladder cancer. J. Nanobiotechnol. 2021, 19, 393. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, G.; Zhang, D.; Lai, W.F. Mechanisms and strategies to enhance penetration during intravesical drug therapy for bladder cancer. J. Control. Release Off. J. Control. Release Soc. 2023, 354, 69–79. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Zheng, B.; Wang, H.; Qi, X.; Wang, S.; Liu, Z.; Sun, L.; Liu, Y.; Qin, X.; et al. Combined Self-Assembled Hendeca-Arginine Nanocarriers for Effective Targeted Gene Delivery to Bladder Cancer. Int. J. Nanomed. 2022, 17, 4433–4448. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ding, J.; He, C.; Cui, L.; Tang, C.; Yin, C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef]
- Miklavžin, A.; Cegnar, M.; Kerč, J.; Kristl, J. Effect of surface hydrophobicity of therapeutic protein loaded in polyelectrolyte nanoparticles on transepithelial permeability. Acta Pharm. 2018, 68, 275–293. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.J.; Garde, S. Efficient method to characterize the context-dependent hydrophobicity of proteins. J. Phys. Chem. B 2014, 118, 1564–1573. [Google Scholar] [CrossRef]
- Vaage, J.; Barberá-Guillem, E.; Abra, R.; Huang, A.; Working, P. Tissue distribution and therapeutic effect of intravenous free or encapsulated liposomal doxorubicin on human prostate carcinoma xenografts. Cancer 1994, 73, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Sousa, D.P.; Conniot, J.; Conde, J. Nanomedicine-based strategies to target and modulate the tumor microenvironment. Trends Cancer 2021, 7, 847–862. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Panté, N.; Kann, M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol. Biol. Cell 2002, 13, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Uertz, J.; Yohan, D.; Chithrani, B.D. Peptide modified gold nanoparticles for improved cellular uptake, nuclear transport, and intracellular retention. Nanoscale 2014, 6, 12026–12033. [Google Scholar] [CrossRef]
- Qu, Q.; Ma, X.; Zhao, Y. Targeted delivery of doxorubicin to mitochondria using mesoporous silica nanoparticle nanocarriers. Nanoscale 2015, 7, 16677–16686. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Silberman, P.C.; Rutkowski, M.R.; Chopra, S.; Perales-Puchalt, A.; Song, M.; Zhang, S.; Bettigole, S.E.; Gupta, D.; Holcomb, K.; et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015, 161, 1527–1538. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.Y.; Xing, L.; Cui, P.F.; Qiao, J.B.; He, Y.J.; Chang, X.; Zhou, T.J.; Jin, Q.R.; Jiang, H.L.; Xiao, Y. Regulating the Golgi apparatus by co-delivery of a COX-2 inhibitor and Brefeldin A for suppression of tumor metastasis. Biomater. Sci. 2018, 6, 2144–2155. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, e1901223. [Google Scholar] [CrossRef]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Su, J.; Ran, W.; Zhang, P.; Yin, Q.; Zhang, Z.; Yu, H.; Li, Y. Preparation and Application of Cell Membrane-Camouflaged Nanoparticles for Cancer Therapy. Theranostics 2017, 7, 2575–2592. [Google Scholar] [CrossRef]
- Copp, J.A.; Fang, R.H.; Luk, B.T.; Hu, C.M.; Gao, W.; Zhang, K.; Zhang, L. Clearance of pathological antibodies using biomimetic nanoparticles. Proc. Natl. Acad. Sci. USA 2014, 111, 13481–13486. [Google Scholar] [CrossRef]
- Gao, C.; Lin, Z.; Jurado-Sánchez, B.; Lin, X.; Wu, Z.; He, Q. Stem Cell Membrane-Coated Nanogels for Highly Efficient In Vivo Tumor Targeted Drug Delivery. Small 2016, 12, 4056–4062. [Google Scholar] [CrossRef]
- Meng, Q.F.; Rao, L.; Zan, M.; Chen, M.; Yu, G.T.; Wei, X.; Wu, Z.; Sun, Y.; Guo, S.S.; Zhao, X.Z.; et al. Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotechnology 2018, 29, 134004. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Wu, L.; Liu, Z.; Tian, R.; Yu, G.; Zhou, Z.; Yang, K.; Xiong, H.G.; Zhang, A.; Yu, G.T.; et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat. Commun. 2020, 11, 4909. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Park, W.; Park, S.B.; Rhim, W.K.; Han, D.K. Recent trends in cell membrane-cloaked nanoparticles for therapeutic applications. Methods 2020, 177, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, R.H.; Luk, B.T.; Hu, C.J.; Thamphiwatana, S.; Dehaini, D.; Angsantikul, P.; Kroll, A.V.; Pang, Z.; Gao, W.; et al. Nanoparticle-Based Antivirulence Vaccine for the Management of Methicillin-Resistant Staphylococcus aureus Skin Infection. Adv. Funct. Mater. 2016, 26, 1628–1635. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Zhang, G.; Ran, D.; Krishnan, N.; Fang, R.H.; Gao, W.; Spector, S.A.; Zhang, L. T-Cell-Mimicking Nanoparticles Can Neutralize HIV Infectivity. Adv. Mater. 2018, 30, e1802233. [Google Scholar] [CrossRef]
- Rao, L.; Cai, B.; Bu, L.L.; Liao, Q.Q.; Guo, S.S.; Zhao, X.Z.; Dong, W.F.; Liu, W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef]
- Molinaro, R.; Evangelopoulos, M.; Hoffman, J.R.; Corbo, C.; Taraballi, F.; Martinez, J.O.; Hartman, K.A.; Cosco, D.; Costa, G.; Romeo, I.; et al. Design and Development of Biomimetic Nanovesicles Using a Microfluidic Approach. Adv. Mater. 2018, 30, e1702749. [Google Scholar] [CrossRef]
- Jain, N.; Shahrukh, S.; Famta, P.; Shah, S.; Vambhurkar, G.; Khatri, D.K.; Singh, S.B.; Srivastava, S. Immune cell-camouflaged surface-engineered nanotherapeutics for cancer management. Acta Biomater. 2023, 155, 57–79. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Fang, R.H.; Dong, A.; Zhang, L. Synthesis of Nanogels via Cell Membrane-Templated Polymerization. Small 2015, 11, 4309–4313. [Google Scholar] [CrossRef] [Green Version]
- Chugh, V.; Vijaya Krishna, K.; Pandit, A. Cell Membrane-Coated Mimics: A Methodological Approach for Fabrication, Characterization for Therapeutic Applications, and Challenges for Clinical Translation. ACS Nano 2021, 15, 17080–17123. [Google Scholar] [CrossRef]
- Bu, Y.; Zhang, X.; Zhu, A.; Li, L.; Xie, X.; Wang, S. Inside-Out-Oriented Cell Membrane Biomimetic Magnetic Nanoparticles for High-Performance Drug Lead Discovery. Anal. Chem. 2021, 93, 7898–7907. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, Q.; Huang, K.; Zheng, T.; Cheng, L.; Zhang, Z.; Yu, Y.; Liao, G.; Wang, X.; Li, C. Oriented Assembly of Cell-Mimicking Nanoparticles via a Molecular Affinity Strategy for Targeted Drug Delivery. ACS Nano 2019, 13, 5268–5277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhen, X.; Yang, Y.; Feng, Q.; Yuan, W.; Xie, X. Precise assembly of inside-out cell membrane camouflaged nanoparticles via bioorthogonal reactions for improving drug leads capturing. Acta Pharm. Sin. B 2023, 13, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, L.; Liu, N.; Zhao, Y.; Zhang, T.; Xie, X. Inside-out extracellular vesicles-like biomimetic magnetic nanoparticles for efficient screening P-Glycoprotein inhibitors to overcome cancer multidrug resistance. Colloids Surf. B Biointerfaces 2023, 222, 113134. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Jiang, W.; Kim, B.Y.S.; Zhang, C.C.; Fu, Y.X.; Weissman, I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 568–586. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Luk, B.T.; Chen, K.N.; Carpenter, C.; Gao, W.; Zhang, K.; Zhang, L. ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale 2013, 5, 2664–2668. [Google Scholar] [CrossRef] [Green Version]
- Klaus, C.; Liao, H.; Allendorf, D.H.; Brown, G.C.; Neumann, H. Sialylation acts as a checkpoint for innate immune responses in the central nervous system. Glia 2021, 69, 1619–1636. [Google Scholar] [CrossRef]
- Liu, L.; Bai, X.; Martikainen, M.V.; Kårlund, A.; Roponen, M.; Xu, W.; Hu, G.; Tasciotti, E.; Lehto, V.P. Cell membrane coating integrity affects the internalization mechanism of biomimetic nanoparticles. Nat. Commun. 2021, 12, 5726. [Google Scholar] [CrossRef]
- Sun, D.; Chen, J.; Wang, Y.; Ji, H.; Peng, R.; Jin, L.; Wu, W. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics 2019, 9, 6885–6900. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, Z.; Wang, H.; Sun, L.; Lai, W.F.; Zhang, H.; Wang, J.; Liu, Y.; Qin, X.; Qi, X.; et al. R11 modified tumor cell membrane nanovesicle-camouflaged nanoparticles with enhanced targeting and mucus-penetrating efficiency for intravesical chemotherapy for bladder cancer. J. Control. Release Off. J. Control. Release Soc. 2022, 351, 834–846. [Google Scholar] [CrossRef]
- Guo, M.; Xia, C.; Wu, Y.; Zhou, N.; Chen, Z.; Li, W. Research Progress on Cell Membrane-Coated Biomimetic Delivery Systems. Front. Bioeng. Biotechnol. 2021, 9, 772522. [Google Scholar] [CrossRef]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.; Balogh, G.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog. Lipid Res. 2015, 59, 38–53. [Google Scholar] [CrossRef] [Green Version]
- Greene, M.K.; Richards, D.A.; Nogueira, J.C.F.; Campbell, K.; Smyth, P.; Fernández, M.; Scott, C.J.; Chudasama, V. Forming next-generation antibody-nanoparticle conjugates through the oriented installation of non-engineered antibody fragments. Chem. Sci. 2018, 9, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Luk, B.T.; Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release Off. J. Control. Release Soc. 2015, 220, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Guo, Q.; Chu, Y.; Li, C.; Zhang, Y.; Liu, P.; Zhao, Z.; Wang, Y.; Luo, Y.; Zhou, Z.; et al. Smart hypoxia-responsive transformable and charge-reversible nanoparticles for the deep penetration and tumor microenvironment modulation of pancreatic cancer. Biomaterials 2022, 287, 121599. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Aryal, S. Surface functionalization strategies of extracellular vesicles. J. Mater. Chem. B 2020, 8, 4552–4569. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface functionalization of exosomes using click chemistry. Bioconjugate Chem. 2014, 25, 1777–1784. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Jawaid, A.M.; Snee, P.T. Poly(ethylene glycol) carbodiimide coupling reagents for the biological and chemical functionalization of water-soluble nanoparticles. ACS Nano 2009, 3, 915–923. [Google Scholar] [CrossRef]
- Levy, O.; Zhao, W.; Mortensen, L.J.; Leblanc, S.; Tsang, K.; Fu, M.; Phillips, J.A.; Sagar, V.; Anandakumaran, P.; Ngai, J.; et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood 2013, 122, e23–e32. [Google Scholar] [CrossRef] [Green Version]
- Stephan, M.T.; Irvine, D.J. Enhancing Cell therapies from the Outside In: Cell Surface Engineering Using Synthetic Nanomaterials. Nano Today 2011, 6, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Perez, E.E.; Wang, J.; Miller, J.C.; Jouvenot, Y.; Kim, K.A.; Liu, O.; Wang, N.; Lee, G.; Bartsevich, V.V.; Lee, Y.L.; et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef]
- Kim, T.K.; Eberwine, J.H. Mammalian cell transfection: The present and the future. Anal. Bioanal. Chem. 2010, 397, 3173–3178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, P.; Liu, X.; Chen, X.; Liu, C.; Zhang, Y.; Chu, C.; Wang, J.; Wang, X.; Chen, X.; Liu, G. Genetically Engineered Cell Membrane Nanovesicles for Oncolytic Adenovirus Delivery: A Versatile Platform for Cancer Virotherapy. Nano Lett. 2019, 19, 2993–3001. [Google Scholar] [CrossRef]
- García-Granados, R.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Metabolic Engineering and Synthetic Biology: Synergies, Future, and Challenges. Front. Bioeng. Biotechnol. 2019, 7, 36. [Google Scholar] [CrossRef]
- Han, Y.; Pan, H.; Li, W.; Chen, Z.; Ma, A.; Yin, T.; Liang, R.; Chen, F.; Ma, Y.; Jin, Y.; et al. T Cell Membrane Mimicking Nanoparticles with Bioorthogonal Targeting and Immune Recognition for Enhanced Photothermal Therapy. Adv. Sci. 2019, 6, 1900251. [Google Scholar] [CrossRef] [PubMed]
- Biz, A.; Proulx, S.; Xu, Z.; Siddartha, K.; Mulet Indrayanti, A.; Mahadevan, R. Systems biology based metabolic engineering for non-natural chemicals. Biotechnol. Adv. 2019, 37, 107379. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhao, Y.; Li, Y.; Jiang, L.; Gu, Y.; Liu, J. Cell-derived biomimetic nanocarriers for targeted cancer therapy: Cell membranes and extracellular vesicles. Drug Deliv. 2021, 28, 1237–1255. [Google Scholar] [CrossRef]
- Harris, J.C.; Scully, M.A.; Day, E.S. Cancer Cell Membrane-Coated Nanoparticles for Cancer Management. Cancers 2019, 11, 1836. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Su, J.; Meng, Q.; Yin, Q.; Chen, L.; Gu, W.; Zhang, P.; Zhang, Z.; Yu, H.; Wang, S.; et al. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv. Mater. 2016, 28, 9581–9588. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, C.M.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Chen, G.B.; Chen, H.H.; Zhang, J.B.; Li, H.Z.; Sheng, M.X.; Weng, W.B.; Guo, S.M. Cancer cell membrane-cloaked mesoporous silica nanoparticles with a pH-sensitive gatekeeper for cancer treatment. Colloids Surf. B Biointerfaces 2019, 175, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Rasool, R.U.; Natesan, R.; Deng, Q.; Aras, S.; Lal, P.; Sander Effron, S.; Mitchell-Velasquez, E.; Posimo, J.M.; Carskadon, S.; Baca, S.C.; et al. CDK7 Inhibition Suppresses Castration-Resistant Prostate Cancer through MED1 Inactivation. Cancer Discov. 2019, 9, 1538–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.; Li, Z.; Hu, Q.; Sun, J.; Chen, M. CRPC Membrane-Camouflaged, Biomimetic Nanosystem for Overcoming Castration-Resistant Prostate Cancer by Cellular Vehicle-Aided Tumor Targeting. Int. J. Mol. Sci. 2022, 23, 3623. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Wu, J.; Lv, X.; Yang, N.; Wei, Q.; Wang, C.; Chen, J. Surgically Derived Cancer Cell Membrane-Coated R837-Loaded Poly(2-Oxazoline) Nanoparticles for Prostate Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2023, 15, 7878–7886. [Google Scholar] [CrossRef]
- Chen, D.; Cai, L.; Guo, Y.; Chen, J.; Gao, Q.; Yang, J.; Li, Y. Cancer Cell Membrane-Decorated Zeolitic-Imidazolate Frameworks Codelivering Cisplatin and Oleanolic Acid Induce Apoptosis and Reversed Multidrug Resistance on Bladder Carcinoma Cells. ACS Omega 2020, 5, 995–1002. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhong, Y.; Fan, W.; Xiang, J.; Wang, G.; Zhou, Q.; Wang, J.; Geng, Y.; Sun, R.; Zhang, Z.; et al. Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion-drug conjugates with cell-membrane affinity. Nat. Biomed. Eng. 2021, 5, 1019–1037. [Google Scholar] [CrossRef]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, e2006484. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Zhou, Z.; Bai, D.; Zhang, Q.; Ai, X.; Gao, W.; Zhang, L. White Blood Cell Membrane-Coated Nanoparticles: Recent Development and Medical Applications. Adv. Healthc. Mater. 2022, 11, e2101349. [Google Scholar] [CrossRef]
- Hansen, M.; Andersen, M.H. The role of dendritic cells in cancer. Semin. Immunopathol. 2017, 39, 307–316. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Yu, Y.; Fu, Y.; Jiang, H.; Lu, M.; Sun, Z.; Jiang, S.; Lu, L.; Wu, M.X. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 2020, 367, eaau0810. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef]
- Pei, W.; Li, X.; Bi, R.; Zhang, X.; Zhong, M.; Yang, H.; Zhang, Y.; Lv, K. Exosome membrane-modified M2 macrophages targeted nanomedicine: Treatment for allergic asthma. J. Control. Release Off. J. Control. Release Soc. 2021, 338, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Petri, B.; Phillipson, M.; Kubes, P. The physiology of leukocyte recruitment: An in vivo perspective. J. Immunol. 2008, 180, 6439–6446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Liu, X.; Yang, Q.; Yu, L.; Chang, Y.; Qu, M. Neutrophil membrane-enveloped nanoparticles for the amelioration of renal ischemia-reperfusion injury in mice. Acta Biomater. 2020, 104, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Lagarkova, M.A. Such Various Stem Cells. Biochem. Biokhimiia 2019, 84, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Madeddu, P. The role of chemokines, cytokines and adhesion molecules in stem cell trafficking and homing. Curr. Pharm. Des. 2011, 17, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Ackova, D.G.; Kanjevac, T.; Rimondini, L.; Bosnakovski, D. Perspectives in Engineered Mesenchymal Stem/Stromal Cells Based Anti- Cancer Drug Delivery Systems. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Gong, C.; Chen, Z.; Li, M.; Li, Y.; Gao, J. Tumor microenvironment-activated cancer cell membrane-liposome hybrid nanoparticle-mediated synergistic metabolic therapy and chemotherapy for non-small cell lung cancer. J. Nanobiotechnol. 2021, 19, 339. [Google Scholar] [CrossRef]
- Mu, X.; Li, J.; Yan, S.; Zhang, H.; Zhang, W.; Zhang, F.; Jiang, J. siRNA Delivery with Stem Cell Membrane-Coated Magnetic Nanoparticles for Imaging-Guided Photothermal Therapy and Gene Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3895–3905. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhang, M.; Wei, A.; Yin, F.; Wang, Y.; Hu, K.; Jiang, J. Doxorubicin and PD-L1 siRNA co-delivery with stem cell membrane-coated polydopamine nanoparticles for the targeted chemoimmunotherapy of PCa bone metastases. Nanoscale 2021, 13, 8998–9008. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Huang, K.; Su, T.; Li, Z.; Hu, S.; Dinh, P.U.; Wrona, E.A.; Shao, C.; Qiao, L.; Vandergriff, A.C.; et al. Mesenchymal Stem Cell/Red Blood Cell-Inspired Nanoparticle Therapy in Mice with Carbon Tetrachloride-Induced Acute Liver Failure. ACS Nano 2018, 12, 6536–6544. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, S.; Fan, Q.; Chen, G.; Archibong, E.; Dotti, G.; Liu, Z.; Gu, Z.; Wang, C. Red blood cell-derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci. Adv. 2019, 5, eaaw6870. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Yao, Q.; Yang, G.; Wang, H.; Liu, J.; Zheng, J.; Lv, B.; Yang, M.; Yang, Y.; Gao, C.; Guo, Y. Aging erythrocyte membranes as biomimetic nanometer carriers of liver-targeting chromium poisoning treatment. Drug Deliv. 2021, 28, 1455–1465. [Google Scholar] [CrossRef]
- Fritzenwanker, M.; Imirzalioglu, C.; Herold, S.; Wagenlehner, F.M.; Zimmer, K.P.; Chakraborty, T. Treatment Options for Carbapenem- Resistant Gram-Negative Infections. Dtsch. Arztebl. Int. 2018, 115, 345–352. [Google Scholar] [CrossRef]
- Su, J.; Zhang, R.; Lian, Y.; Kamal, Z.; Cheng, Z.; Qiu, Y.; Qiu, M. Preparation and Characterization of Erythrocyte Membrane-Camouflaged Berberine Hydrochloride-Loaded Gelatin Nanoparticles. Pharmaceutics 2019, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Zhang, Y.; Li, R.; Chen, W.; Li, Y.; Chen, G. Berberine regulated Gck, G6pc, Pck1 and Srebp-1c expression and activated AMP-activated protein kinase in primary rat hepatocytes. Int. J. Biol. Sci. 2011, 7, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, S.; Dumoga, S.; Sirohi, P.; Singh, N. Red Blood Cells-Derived Vesicles for Delivery of Lipophilic Drug Camptothecin. ACS Appl. Mater. Interfaces 2019, 11, 22141–22151. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, Z.; Lemons, P.K.; Cheng, H. A Facile Approach to Functionalize Cell Membrane-Coated Nanoparticles. Theranostics 2016, 6, 1012–1022. [Google Scholar] [CrossRef]
- Aaltomaa, S.; Lipponen, P.; Tammi, R.; Tammi, M.; Viitanen, J.; Kankkunen, J.P.; Kosma, V.M. Strong Stromal Hyaluronan Expression Is Associated with PSA Recurrence in Local Prostate Cancer. Urol. Int. 2002, 69, 266–272. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhou, X.; Zhang, H.; Yao, Q.; Liu, Y.; Dong, Z. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am. J. Physiol. Ren. Physiol. 2016, 311, F844–F851. [Google Scholar] [CrossRef] [Green Version]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Di Francesco, M.; Dolo, V. Extracellular Vesicles in Glioblastoma: Role in Biological Processes and in Therapeutic Applications. Curr. Cancer Drug Targets 2017, 17, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteom. 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Zhou, H.; Pisitkun, T.; Aponte, A.; Yuen, P.S.; Hoffert, J.D.; Yasuda, H.; Hu, X.; Chawla, L.; Shen, R.F.; Knepper, M.A.; et al. Exosomal Fetuin-A identified by proteomics: A novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006, 70, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Bie, N.; Yong, T.; Tang, K.; Shi, X.; Wei, Z.; Jia, H.; Zhang, X.; Zhao, H.; Huang, W.; et al. The softness of tumour-cell-derived microparticles regulates their drug-delivery efficiency. Nat. Biomed. Eng. 2019, 3, 729–740. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Brennan, M.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, W.; Ha, L.; Han, X.; Hao, S.; Wan, Y.; Wang, Z.; Dong, F.; Zou, X.; Mao, Y.; et al. Self-Assembly of Extracellular Vesicle-like Metal-Organic Framework Nanoparticles for Protection and Intracellular Delivery of Biofunctional Proteins. J. Am. Chem. Soc. 2018, 140, 7282–7291. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Li, Y.; Chang, J.; Tian, F.; Zhao, F.; Ma, Y.; Sun, J. Microfluidic Sonication To Assemble Exosome Membrane-Coated Nanoparticles for Immune Evasion-Mediated Targeting. Nano Lett. 2019, 19, 7836–7844. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, Y.; Huang, M.; Deng, Z.; Zhang, A.; Pei, L.; Wang, L.; Zhao, W.; Ma, L.; Zhang, Q.; et al. Urinary exosomes-based Engineered Nanovectors for Homologously Targeted Chemo-Chemodynamic Prostate Cancer Therapy via abrogating EGFR/AKT/NF-kB/IkB signaling. Biomaterials 2021, 275, 120946. [Google Scholar] [CrossRef]
- Huang, L.; Xu, C.; Xu, P.; Qin, Y.; Chen, M.; Feng, Q.; Pan, J.; Cheng, Q.; Liang, F.; Wen, X.; et al. Intelligent Photosensitive Mesenchymal Stem Cells and Cell-Derived Microvesicles for Photothermal Therapy of Prostate Cancer. Nanotheranostics 2019, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release Off. J. Control. Release Soc. 2015, 220, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Ding, W.; Qian, Z.; Zhu, Q.; Sun, C.; Yu, Q.; Tai, Z.; Xu, K. Immunotherapy Strategy Targeting Programmed Cell Death Ligand 1 and CD73 with Macrophage-Derived Mimetic Nanovesicles to Treat Bladder Cancer. Mol. Pharm. 2021, 18, 4015–4028. [Google Scholar] [CrossRef]
- Zhuang, J.; Gao, P.; Chen, H.; Fang, Z.; Zheng, J.; Zhu, D.; Hou, J. Extracellular vesicles from human urine-derived stem cells merged in hyaluronic acid ameliorate erectile dysfunction in type 2 diabetic rats by glans administration. Andrology 2022, 10, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.S.; Raza, K.; Cooper, M.S. Therapeutic glucocorticoids: Mechanisms of actions in rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, J.E. Role of the glucocorticoid receptor in glomerular disease. Am. J. Physiol. Ren. Physiol. 2019, 317, F133–F136. [Google Scholar] [CrossRef]
- Tang, T.T.; Lv, L.L.; Wang, B.; Cao, J.Y.; Feng, Y.; Li, Z.L.; Wu, M.; Wang, F.M.; Wen, Y.; Zhou, L.T.; et al. Employing Macrophage-Derived Microvesicle for Kidney-Targeted Delivery of Dexamethasone: An Efficient Therapeutic Strategy against Renal Inflammation and Fibrosis. Theranostics 2019, 9, 4740–4755. [Google Scholar] [CrossRef]
- Tang, T.T.; Wang, B.; Li, Z.L.; Wen, Y.; Feng, S.T.; Wu, M.; Liu, D.; Cao, J.Y.; Yin, Q.; Yin, D.; et al. Kim-1 Targeted Extracellular Vesicles: A New Therapeutic Platform for RNAi to Treat AKI. J. Am. Soc. Nephrol. JASN 2021, 32, 2467–2483. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Zhang, A.; Hassounah, F.; Seow, Y.; Wood, M.; Ma, F.; Klein, J.D.; Price, S.R.; Wang, X.H. Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Diao, Y.; Wang, G.; Zhu, B.; Li, Z.; Wang, S.; Yu, L.; Li, R.; Fan, W.; Zhang, Y.; Zhou, L.; et al. Loading of “cocktail siRNAs” into extracellular vesicles via TAT-DRBD peptide for the treatment of castration-resistant prostate cancer. Cancer Biol. Ther. 2022, 23, 163–172. [Google Scholar] [CrossRef]
- Kurniawati, I.; Liu, M.C.; Hsieh, C.L.; Do, A.D.; Sung, S.Y. Targeting Castration-Resistant Prostate Cancer Using Mesenchymal Stem Cell Exosomes for Therapeutic MicroRNA-let-7c Delivery. Front. Biosci. (Landmark Ed.) 2022, 27, 256. [Google Scholar] [CrossRef]
- Zhupanyn, P.; Ewe, A.; Büch, T.; Malek, A.; Rademacher, P.; Müller, C.; Reinert, A.; Jaimes, Y.; Aigner, A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J. Control. Release Off. J. Control. Release Soc. 2020, 319, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Jobin, C.; Panja, A.; Hellerbrand, C.; Iimuro, Y.; Didonato, J.; Brenner, D.A.; Sartor, R.B. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J. Immunol. 1998, 160, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, Y.; Mirzaaghasi, A.; Heo, J.; Kim, Y.N.; Shin, J.H.; Kim, S.; Kim, N.H.; Cho, E.S.; In Yook, J.; et al. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality. Sci. Adv. 2020, 6, eaaz6980. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem Cells Int. 2016, 2016, 1240301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Cheng, Y.; Gao, H.; Zhuang, J.; Zhang, W.; Bian, Q.; Wang, F.; Du, Y.; Li, Z.; Kong, D.; et al. In Vivo Tracking of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improving Mitochondrial Function in Renal Ischemia-Reperfusion Injury. ACS Nano 2020, 14, 4014–4026. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef]
- Dominguez, J.M., 2nd; Dominguez, J.H.; Xie, D.; Kelly, K.J. Human extracellular microvesicles from renal tubules reverse kidney ischemia-reperfusion injury in rats. PLoS ONE 2018, 13, e0202550. [Google Scholar] [CrossRef]
- Park, K.S.; Svennerholm, K.; Shelke, G.V.; Bandeira, E.; Lässer, C.; Jang, S.C.; Chandode, R.; Gribonika, I.; Lötvall, J. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res. Ther. 2019, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.L.; Qu, X.J.; Zhao, M.F.; Teng, Y.E.; Zhang, Y.; Hou, K.Z.; Jiang, Y.H.; Yang, X.H.; Liu, Y.P. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2009, 41, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.A.; Fliervoet, L.A.L.; van der Meel, R.; Fens, M.; Heijnen, H.F.G.; van Bergen En Henegouwen, P.M.P.; Vader, P.; Schiffelers, R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release Off. J. Control. Release Soc. 2016, 224, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Ma, X.; Cheng, K.; Liu, G.; Li, Y.; Yue, Y.; Liang, J.; Zhang, L.; Zhang, T.; Wang, X.; et al. Engineered Bacterial Outer Membrane Vesicles as Controllable Two-Way Adaptors to Activate Macrophage Phagocytosis for Improved Tumor Immunotherapy. Adv. Mater. 2022, 34, e2206200. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.M.; Zhang, L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Xu, L.; Yang, B.; Fan, F.; Yang, L. Kill the Real with the Fake: Eliminate Intracellular Staphylococcus aureus Using Nanoparticle Coated with Its Extracellular Vesicle Membrane as Active-Targeting Drug Carrier. ACS Infect. Dis. 2019, 5, 218–227. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef]
- Chen, H.Y.; Deng, J.; Wang, Y.; Wu, C.Q.; Li, X.; Dai, H.W. Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020, 112, 1–13. [Google Scholar] [CrossRef]
- Dehaini, D.; Wei, X.; Fang, R.H.; Masson, S.; Angsantikul, P.; Luk, B.T.; Zhang, Y.; Ying, M.; Jiang, Y.; Kroll, A.V.; et al. Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization. Adv. Mater. 2017, 29, 1606209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Liu, P.; Gao, F.; Gu, Y.; Xiao, Y.; Wu, P.; Chen, B.; Liu, W.; Liu, Q. Platelet-neutrophil hybrid membrane-coated gelatin nanoparticles for enhanced targeting ability and intelligent release in the treatment of non-alcoholic steatohepatitis. Nanomed. Nanotechnol. Biol. Med. 2022, 42, 102538. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Z.; Mu, C.; Song, D.; Wang, J.; Meng, X.; Li, Z.; Qing, H.; Dong, Y.; Xie, H.Y.; et al. Enhanced Proliferation of Visualizable Mesenchymal Stem Cell-Platelet Hybrid Cell for Versatile Intracerebral Hemorrhage Treatment. ACS Nano 2023, 17, 7352–7365. [Google Scholar] [CrossRef]
- Delaney, M.; Wendel, S.; Bercovitz, R.S.; Cid, J.; Cohn, C.; Dunbar, N.M.; Apelseth, T.O.; Popovsky, M.; Stanworth, S.J.; Tinmouth, A.; et al. Transfusion reactions: Prevention, diagnosis, and treatment. Lancet 2016, 388, 2825–2836. [Google Scholar] [CrossRef]
- Gao, J.; Chu, D.; Wang, Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J. Control. Release Off. J. Control. Release Soc. 2016, 224, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Fusion Methods | Process | Advantages | Limitations | References |
|---|---|---|---|---|
| Extrusion | NPs and the extracted purified cell membranes are co-extruded through a porous membrane using extruders | Simple and practical operation steps | Inefficient synthesis Low rate of synthesis | [46,51] |
| Ultrasound | The mixture formed by mixing cell membranes and NPs is sonicated at a certain frequency for a certain period of time | Reducing loss of raw materials for mass production | Affecting the size and stability of core NPs Potentially disrupting NPs | [52] |
| Microfluidic electroporation | Mix cell membranes and NPs in a microfluidic chip, then flow through the electroporation zone, inducing the formation of micropores on the cell membrane | Avoiding the destruction of NPs Less time-consuming and labor-intensive | Complex operating procedures | [53] |
| In situ polymerization | Using cell membranes to template inner core NPs, polymerize NPs by the action of initiators | Ensuring the integrity of the coating Easy control of the size and stiffness of CMNPs with no easy leakage of contents | Small application range High selectivity for core NPs | [57] |
| Method | Mechanism | Superiority | Deficiency | References |
|---|---|---|---|---|
| Lipid insertion | Ligands or therapeutic molecules are anchored to the cell membrane via lipid–lipid interactions | Maintaining the integrity of content while avoiding complex steps | Not suitable for large transmembrane protein receptors or ligands | [71] |
| Chemical conjugation | Adding functional moieties by strong covalent connection on the surface | High yield, wide in scope, and easy product separation | Affecting protein integrity and function potentially by the use of chemical reagents | [75] |
| Non-covalent adsorption | Non-covalent and weaker binding of functional moiety to CMNPs | Enhancing the surface function without affecting the orientation of cell membrane coating | Poor adhesion and unable to maintain long-term stability | [69] |
| Genetic modification | Presenting functional moieties on the cell membrane via gene transfection | Functionalizing cell membranes precisely in a non-invasive strategy | Not suitable for small molecule therapeutic factors or ligands and operation is challenging | [78] |
| Metabolic engineering | Manipulating cellular natural biosynthetic pathways to transport functional moieties onto the cell membrane | Allowing for straightforward cell membrane functionalization by endogenous processes in cells | Difficult to control splice site specificity and efficiency | [84] |
| Sources of Membrane | Cargoes/Nanoparticle | Diseases/Effect | Properties | References |
|---|---|---|---|---|
| LNCaP-AI cell | DOX/MSN | PCa | Adhesion to targeted tumor sites Proteins that mediate homologous binding Promotes tumor-specific immunity | [91] |
| DU145 cell | DTX/PLGA | CRPC | [93] | |
| Surgically derived cancer cell | Imiquimod/PMBEOx-COOH | Pca | [94] | |
| BCa cell | Cisplatin and oleanolic acid/Hybrid nanoparticle | BCa | [95] | |
| BCa cell | Gemcitabine/PLGA | BCa | [69] | |
| Neutrophil | Coenzyme Q10/PEG-PLA | Renal ischemia-reperfusion injury | Good biocompatibility Mitigates the inflammatory conditions and tumors specifically Produces toxic molecules to quickly eradicate the phagocytosed pathogen | [105] |
| RBC | BH/Gelatin | Achieve sustained release and reduce the nephrotoxicity of BH | Prolonged blood circulation CD47 expression Immune evasion | [117] |
| RBC | Ciprofloxacin/PLGA | Klebsiella pneumoniae-Induced sepsis | [116] | |
| RBC nanovesicles | Camptothecin/- | Reduce the accumulation of camptothecin in the kidneys | [119] | |
| RBC | -/PLGA | PCa | [121] | |
| Mesenchymal stem cell | siRNA/Fe3O4@PDA | PCa | Penetrates across the endothelium May target particular tumor Homing ability | [110] |
| Stem cell | DOX and PD-L1 siRNA/Polydopamine | PCa bone metastases | [111] | |
| MSC-Derived MVs | -/Gold nanostars | Photothermal Therapy of PCa | Possess functional intracellular components Inheritance of parent cell characteristics High deformability to cross physiological barriers Therapeutic biomolecules directly | [140] |
| Cancer cell-derived MVs and EXOs | Paclitaxel/- | PCa | [141] | |
| Urinary exosomes | DOX/Fe3O4 | PCa | [139] | |
| MSC-derived Nanovesicles | IL-10/- | Alleviate and treat sepsis-associated acute kidney injury | [159] | |
| Macrophage-Derived exosome-mimetic nanovesicles | Monoclonal antibody to PD-L1 and CD73 inhibitor | Immunotherapy strategy for bladder cancer | [142] | |
| Macrophage-derived MVs | Dexamethasone/- | Renal inflammation and fibrosis | [146] | |
| RBC-derived-EVs | Transcription factors P65 and snai1 siRNA | Acute kidney injury | [145] | |
| EXOs | miRNA-29 | Kidney Fibrosis | [147] | |
| EVs | siRNA | CRPC | [148] | |
| MSC-exosome | miRNA-lethal 7c | CRPC | [150] | |
| EVs | siRNA/Polyethylenimines | PCa | [151] | |
| EXOs | inhibitor of NF-κB | Sepsis and ischemia-injured kidneys | [153] | |
| Urine-derived stem cells EVs | HA | Erectile dysfunction | [143] | |
| Bacterium | Gold nanoparticles | Mitigate metastatic foci of infection in kidneys | Immune stimulation | [166] |
| Cell Membranes | Typical Biomarkers | Advantages | Limitations | References |
|---|---|---|---|---|
| CCMs | Selectins, Integrins, CD47, and TAG | Homologous targeting and culture conveniently in vitro | Potential causes of tumor metastasis | [87,88,89,90] |
| ICMs | CD45, CD47, TCRs, Co-stimulatory/inhibitory molecules | Immune escape and good biocompatibility | Complexity of extraction, immunogenicity | [97,98,99,100,101,102] |
| SCMs | CD74, CXCR, and Other chemokine | Particular tumor homing ability and inflammatory migratory | High cost of preparation and low specificity | [106,107,108,109,112] |
| RBCMs | CD47 | Long circulation time and simple for surface engineering | Lack of targeting capabilities | [46,68,114] |
| EVs | ESCRT protein and Accessory proteins | High deformability and inheritance of parent cell | Lack of immune evasion and may promote disease progression | [125,126,127,128,129,130,131,132,133,134,135,136] |
| OMVs | Virulence actors | Immune activation | Insecurity in vivo | [162,163,164,165] |
| Platelets | P-selectin, CD47, CD55 and CD59 | Tumor and inflammation targeting | Instability | [52,53,167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.; Zhang, D.; Wang, H.; Zhang, P. Recent Advances in Cell Membrane Coated-Nanoparticles as Drug Delivery Systems for Tackling Urological Diseases. Pharmaceutics 2023, 15, 1899. https://doi.org/10.3390/pharmaceutics15071899
Yao C, Zhang D, Wang H, Zhang P. Recent Advances in Cell Membrane Coated-Nanoparticles as Drug Delivery Systems for Tackling Urological Diseases. Pharmaceutics. 2023; 15(7):1899. https://doi.org/10.3390/pharmaceutics15071899
Chicago/Turabian StyleYao, Cenchao, Dahong Zhang, Heng Wang, and Pu Zhang. 2023. "Recent Advances in Cell Membrane Coated-Nanoparticles as Drug Delivery Systems for Tackling Urological Diseases" Pharmaceutics 15, no. 7: 1899. https://doi.org/10.3390/pharmaceutics15071899