E. coli Cell Lysis Induced by Lys394 Enzyme Assisted by Magnetic Nanoparticles Exposed to Non-Heating Low-Frequency Magnetic Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Expression and Purification
2.2. Cultivation of E. coli Cells as a Substrate for Lys394
2.3. Endolysin Activity Assay
2.4. Synthesis and Characterization of Rod-Like MNPs Functionalized by Dopamine
2.5. Lys394-Induced Lysis of E. coli Cells in the Presence of MNPs
2.6. Effect of LF MF on the Cell Lysis of E. coli
2.7. Effect of LF MF on the Cell Lysis of E. coli in the Presence of MNPs
2.8. Effect of LF MF on the Cell Lysis of E. coli Induced by Lys394 in the Presence of MNPs
2.9. Study of the Outer Membrane Permeability of JM 109 E. coli Strain Exposed to LF MF
2.9.1. Evaluation of the Release of β-Lactamase from E. coli Cell Periplasm
2.9.2. Nile Red Dye Fluorescence
3. Results
3.1. Characterization of Magnetic Nanorods
3.2. Recombinant Endolysin Lys394 Isolation and Purification
3.3. Enzymatic Activity of Endolysin Lys394
3.4. The Effect of MNPs on the Cell Lysis Induced by Lys394 Endolysin
3.5. Combined Effect of LF MF and MNPs on Spontaneous E. coli Cell Lysis
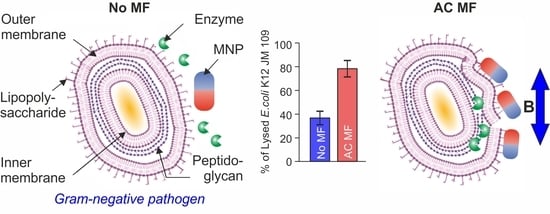
3.6. E. coli Cell Lysis Induced by Lys394 Recombinant Enzyme Mediated by MNPs in LF MF
3.7. The Study of the Outer Membrane Permeability of E. coli
3.7.1. The Detection of Periplasmic β-Lactamase Leakage
3.7.2. Application of Hydrophobic Nile Red Dye
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LF MF | Low-frequency magnetic field |
| MNPs | Magnetic nanoparticles |
| NR | Nile Red |
| NTA | Nanoparticle tracking analysis |
| TEM | Transmission electron microscopy |
| E. coli | Escherichia coli |
| RT | Room temperature |
References
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Disease-a-Month 2020, 66, 100971. [Google Scholar] [CrossRef]
- Maraia, Z.; Mazzoni, T.; Turtora, M.P.; Tempera, A.; Spinosi, M.; Vagnoni, A.; Mazzoni, I. Epidemiological Impact on Use of Antibiotics in Patients Hospitalized for COVID-19: A Retrospective Cohort Study in Italy. Antibiotics 2023, 12, 912. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hu, X.; Wang, Y. Alternatives to Conventional Antibiotic Therapy: Potential Therapeutic Strategies of Combating Antimicrobial-Resistance and Biofilm-Related Infections. Mol. Biotechnol. 2021, 63, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Bellisario, D.; Santo, L.; Quadrini, F.; Hassiba, M.; Bader, N.; Chowdhury, S.H.; Hassan, M.K.; Zughaier, S.M. Cytotoxicity and Antibiofilm Activity of Silver-Polypropylene Nanocomposites. Antibiotics 2023, 12, 924. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582–779. [Google Scholar] [CrossRef]
- Kokot, M.; Novak, D.; Zdovc, I.; Anderluh, M.; Hrast, M.; Minovski, N. Exploring Alternative Pathways to Target Bacterial Type II Topoisomerases Using NBTI Antibacterials: Beyond Halogen-Bonding Interactions. Antibiotics 2023, 12, 930. [Google Scholar] [CrossRef]
- Shi, S.; Shen, T.; Liu, Y.; Chen, L.; Wang, C.; Liao, C. Porcine Myeloid Antimicrobial Peptides: A Review of the Activity and Latest Advances. Front. Vet. Sci. 2021, 8, 664139. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage Endolysins: A Novel Anti-Infective to Control Gram-Positive Pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Danis-wlodarczyk, K.M.; Wozniak, D.J.; Abedon, S.T. Treating Bacterial Infections with Bacteriophage-Based Enzybiotics: In Vitro, In Vivo and Clinical Application. Antibiotics 2021, 10, 1497. [Google Scholar] [CrossRef]
- Ho, M.K.Y.; Zhang, P.; Chen, X.; Xia, J.; Leung, S.S.Y. Bacteriophage endolysins against gram-positive bacteria, an overview on the clinical development and recent advances on the delivery and formulation strategies. Crit. Rev. Microbiol. 2022, 48, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Mehmood Khan, F.; Singh Gondil, V.; Li, C.; Jiang, M.; Li, J.; Yu, J.; Wei, H.; Yang, H. A Novel Acinetobacter baumannii Bacteriophage Endolysin LysAB54 with High Antibacterial Activity Against Multiple Gram-Negative Microbes. Front. Cell. Infect. Microbiol. 2021, 11, 637313. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Volckaert, G.; Cornelissen, A.; Lagaert, S.; Michiels, C.W.; Hertveldt, K.; Lavigne, R. Muralytic Activity and Modular Structure of the Endolysins of Pseudomonas Aeruginosa Bacteriophages ΦKZ and EL. Mol. Microbiol. 2007, 65, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, I.; Chaudhry, W.N.; Akhtar, M.N.; Andleeb, S.; Qadri, I. Bacteriophages and Their Implications on Future Biotechnology: A Review. Virol. J. 2012, 9, 9. Available online: http://www.virologyj.com/content/9/1/9 (accessed on 10 January 2012). [CrossRef] [Green Version]
- Loganathan, A.; Manohar, P.; Eniyan, K.; VinodKumar, C.S.; Leptihn, S.; Nachimuthu, R. Phage Therapy as a Revolutionary Medicine against Gram-Positive Bacterial Infections. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 49. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wang, Y.; Wang, J.; Zhao, Y.; Zhong, Q.; Li, G.; Fu, Z.; Lu, S. Phage Endolysin LysP108 Showed Promising Antibacterial Potential Against Methicillin-resistant Staphylococcus aureus. Front Cell Infect Microbiol. 2021, 11, 668430. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of Gram-Negative Cell Walls and Their Derived Membrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [Green Version]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [Green Version]
- Egan, A.J.F. Bacterial outer membrane constriction. Mol. Microbiol. 2018, 107, 676–687. [Google Scholar] [CrossRef] [Green Version]
- Gilleland, H.E.; Murray, R.G.E. Ultrastructural Study of Polymyxin Resistant Isolates of Pseudomonas aeruginosa. J. Bacteriol. 1976, 125, 267–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The Expanding Scope of Antimicrobial Peptide Structures and Their Modes of Action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Agents That Increase the Permeability of the Outer Membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Kotrange, H.; Najda, A.; Bains, A.; Gruszecki, R.; Chawla, P.; Tosif, M.M. Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics. Int. J. Mol. Sci. 2021, 22, 9596. [Google Scholar] [CrossRef] [PubMed]
- Golovin, Y.I.; Klyachko, N.L.; Gribanovskii, S.L.; Golovin, D.Y.; Samodurov, A.A.; Majouga, A.G.; Sokolsky-Papkov, M.; Kabanov, A.V. Nanomechanical Control of Properties of Biological Membranes Achieved by Rodlike Magnetic Nanoparticles in a Superlow-Frequency Magnetic Field. Tech. Phys. Lett. 2015, 41, 455–457. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Vlasova, K.Y.; Kutsenok, E.O.; Usvaliev, A.D.; Efremova, M.V.; Zhigachev, A.O.; Rudakovskaya, P.G.; Golovin, D.Y.; Gribanovsky, S.L.; Kudryashova, E.V.; et al. Magnetic nanorods for remote disruption of lipid membranes by non-heating low frequency magnetic field. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102065. [Google Scholar] [CrossRef]
- Golovin, Y.I.; Klyachko, N.L.; Sokolsky-Papkov, M.; Kabanov, A.V. Single-Domain Magnetic Nanoparticles as Force Generators for the Nanomechanical Control of Biochemical Reactions by Low-Frequency Magnetic Fields. Bull. Russ. Acad. Sci. Phys. 2013, 77, 1350–1359. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Makridis, A.; Maniotis, N.; Karypidou, N.; Myrovali, E.; Samaras, T.; Angelakeris, M.; Chlichlia, K.; Kalogirou, O. Effect of low frequency magnetic fields on the growth of MNP-treated HT29 colon cancer cells. Nanotechnology 2018, 29, 175101. [Google Scholar] [CrossRef]
- Veselov, M.M.; Uporov, I.V.; Efremova, M.V.; Le-Deygen, I.M.; Prusov, A.N.; Shchetinin, I.V.; Savchenko, A.G.; Golovin, Y.I.; Kabanov, A.V.; Klyachko, N.L. Modulation of α-Chymotrypsin Conjugated to Magnetic Nanoparticles by the Non-Heating Low-Frequency Magnetic Field: Molecular Dynamics, Reaction Kinetics, and Spectroscopy Analysis. ACS Omega 2022, 7, 20644–20655. [Google Scholar] [CrossRef]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Nanoscale Iron Oxide Nanorods as High-Performance Magnetic Resonance Imaging Contrast Agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef] [Green Version]
- Shebl, R.I.; Farouk, F.; Azzazy, H.M.E.-S. Effect of Surface Charge and Hydrophobicity Modulation on the Antibacterial and Antibiofilm Potential of Magnetic Iron Nanoparticles. J. Nanomater. 2017, 20, 9095–9332. [Google Scholar] [CrossRef] [Green Version]
- Yanisch-Perron, C.; Vieira, J.; Messing, J. Improved Ml3 phage cloning vectors and host strains: Nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 1985, 33, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.M.; Caput, D.; Williams, S.R.; Martin, D.W., Jr. Cloning of human purine-nucleoside phosphorylase cDNA sequences by complementation in Escherichia coli. Proc. Natl. Acad. Sci. USA 1983, 80, 4281–4285. [Google Scholar] [CrossRef] [PubMed]
- Legotsky, S.A.; Vlasova, K.Y.; Priyma, A.D.; Shneider, M.M.; Pugachev, V.G.; Totmenina, O.D.; Kabanov, A.V.; Miroshnikov, K.A.; Klyachko, N.L. Peptidoglycan Degrading Activity of the Broad-Range Salmonella Bacteriophage S-394 Recombinant Endolysin. Biochimie 2014, 107, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Lavigne, R.; Volckaert, G.; Hertveldt, K. A Standardized Approach for Accurate Quantification of Murein Hydrolase Activity in High-Throughput Assays. J. Biochem. Biophys. Methods 2007, 70, 531–533. [Google Scholar] [CrossRef]
- Milosevic, I.; Jouni, H.; David, C.; Warmont, F.; Bonnin, D.; Motte, L. Facile Microwave Process in Water for the Fabrication of Magnetic Nanorods. J. Phys. Chem. C 2011, 115, 18999–19004. [Google Scholar] [CrossRef]
- Orza, A.; Wu, H.; Xu, Y.; Lu, Q.; Mao, H. One-Step Facile Synthesis of Highly Magnetic and Surface Functionalized Iron Oxide Nanorods for Biomarker-Targeted Applications. ACS Appl. Mater. Interfaces 2017, 9, 20719–20727. [Google Scholar] [CrossRef]
- Fujimoto, T.; Ohsaki, Y.; Suzuki, M.; Cheng, J. Imaging Lipid Droplets by Electron Microscopy. Methods Cell Biol. 2013, 116, 227–251. [Google Scholar] [CrossRef]
- Jones, R.N.; Wilson, H.W.; Novick, W.J.; Barry, A.L.; Thornsberry, C. In Vitro Evaluation of CENTA, a New Beta-Lactamase-Susceptible Chromogenic Cephalosporin Reagent. J. Clin. Microbiol. 1982, 15, 954–958. [Google Scholar] [CrossRef] [Green Version]
- Bebrone, C.; Moali, C.; Mahy, F.; Rival, S.; Docquier, J.D.; Rossolini, G.M.; Fastrez, J.; Pratt, R.F.; Frère, J.M.; Galleni, M. CENTA as a Chromogenic Substrate for Studying β-Lactamases. Antimicrob. Agents Chemother. 2001, 45, 1868–1871. [Google Scholar] [CrossRef] [Green Version]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrero, C.A.; García, K.E.; Morales, A.L.; Kodjikian, S.; Greneche, J.M. New Analysis of the Mössbauer Spectra of Akaganeite. J. Phys. Condens. Matter 2006, 18, 6827–6840. [Google Scholar] [CrossRef]
- García, K.E.; Barrero, C.A.; Morales, A.L.; Greneche, J.M. Magnetic Structure of Synthetic Akaganeite: A Review of Mössbauer Data. Rev. Fac. Ing. Univ. Antioq. 2009, 49, 185–191. [Google Scholar]
- Alibeigi, S.; Vaezi, M.R. Phase Transformation of Iron Oxide Nanoparticles by Varying the Molar Ratio of Fe2+:Fe3+. Chem. Eng. Technol. 2008, 31, 1591–1596. [Google Scholar] [CrossRef]
- Da Costa, A.C.S.; De Souza, I.G.; Batista, M.A.; Da Silva, K.L.; Bellini, J.V.; Paesano, A. Structural, Magnetic and Hyperfine Characterization of Zinc-Substituted Magnetites. Hyperfine Interact. 2007, 175, 103–111. [Google Scholar] [CrossRef]
- Winsett, J.; Moilanen, A.; Paudel, K.; Kamali, S.; Ding, K.; Cribb, W.; Seifu, D.; Neupane, S. Quantitative Determination of Magnetite and Maghemite in Iron Oxide Nanoparticles Using Mössbauer Spectroscopy. SN Appl. Sci. 2019, 1, 1636. [Google Scholar] [CrossRef] [Green Version]
- Coduri, M.; Masala, P.; Del Bianco, L.; Spizzo, F.; Ceresoli, D.; Castellano, C.; Cappelli, S.; Oliva, C.; Checchia, S.; Allieta, M.; et al. Local Structure and Magnetism of Fe2O3 Maghemite Nanocrystals: The Role of Crystal Dimension. Nanomaterials 2020, 10, 867. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between Particle Size/Domain Structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Golovin, Y.I.; Klyachko, N.L.; Majouga, A.G.; Sokolsky, M.; Kabanov, A.V. Theranostic Multimodal Potential of Magnetic Nanoparticles Actuated by Non-Heating Low Frequency Magnetic Field in the New-Generation Nanomedicine. J. Nanopart. Res. 2017, 19, 63. [Google Scholar] [CrossRef]
- Golovin, Y.I.; Gribanovsky, S.L.; Golovin, D.Y.; Klyachko, N.L.; Majouga, A.G.; Master, A.M.; Sokolsky, M.; Kabanov, A.V. Towards nanomedicines of the future: Remote magneto-mechanical actuation of nanomedicines by alternating magnetic fields. J. Control. Release 2015, 219, 43–60. [Google Scholar] [CrossRef] [Green Version]


















| Peak No. | Is, mm/s | Qs, mm/s | S Ref., % |
|---|---|---|---|
| D-1 | 0.37 | 0.56 | 68 |
| D-2 | 0.36 | 0.99 | 32 |
| Peak No. | Is, mm/s | Qs, mm/s | H, kOe | S Ref., % | G, mm/s |
|---|---|---|---|---|---|
| S-1 S-2 S-3 D-1 | 0.22 0.58 0.33 0.37 | 0.30 0.11 −0.02 0.60 | 482 450 495 | 6 13 7 55 | 0.77 0.78 0.56 0.38 |
| D-2 | 0.36 | 1.00 | 19 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usvaliev, A.D.; Belogurova, N.G.; Pokholok, K.V.; Finko, A.V.; Prusov, A.N.; Golovin, D.Y.; Miroshnikov, K.A.; Golovin, Y.I.; Klyachko, N.L. E. coli Cell Lysis Induced by Lys394 Enzyme Assisted by Magnetic Nanoparticles Exposed to Non-Heating Low-Frequency Magnetic Field. Pharmaceutics 2023, 15, 1871. https://doi.org/10.3390/pharmaceutics15071871
Usvaliev AD, Belogurova NG, Pokholok KV, Finko AV, Prusov AN, Golovin DY, Miroshnikov KA, Golovin YI, Klyachko NL. E. coli Cell Lysis Induced by Lys394 Enzyme Assisted by Magnetic Nanoparticles Exposed to Non-Heating Low-Frequency Magnetic Field. Pharmaceutics. 2023; 15(7):1871. https://doi.org/10.3390/pharmaceutics15071871
Chicago/Turabian StyleUsvaliev, Azizbek D., Natalia G. Belogurova, Konstantin V. Pokholok, Alexander V. Finko, Andrey N. Prusov, Dmitry Yu. Golovin, Konstantin A. Miroshnikov, Yuri I. Golovin, and Natalia L. Klyachko. 2023. "E. coli Cell Lysis Induced by Lys394 Enzyme Assisted by Magnetic Nanoparticles Exposed to Non-Heating Low-Frequency Magnetic Field" Pharmaceutics 15, no. 7: 1871. https://doi.org/10.3390/pharmaceutics15071871