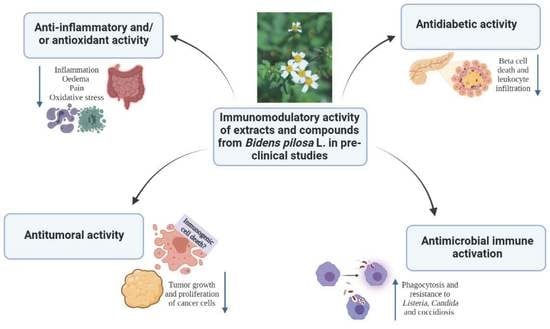
Immunomodulatory Properties of Natural Extracts and Compounds Derived from Bidens pilosa L.: Literature Review
Abstract
:1. Introduction

2. Materials and Methods
3. Results
3.1. B. pilosa Extracts Have Anti-Inflammatory Properties Associated with Antioxidant and/or Analgesic Effects
| Part of the Plant | Extract Type | Experimental Model Type | Biological Effect | Metabolites Isolated or Identified | Study Conclusions | Reference |
|---|---|---|---|---|---|---|
| Leaves | Ethyl acetate fraction | Chemical and thermal models of nociception in Mus musculus mice and Wistar rats In vivo | Anti-inflammatory and analgesic | Quercetin 3,3′—dimethyl ether 7-O-β-D-glucopyranoside Iso-okanin 7-O-β-D-(2″,4″,6″-triacetyl)- glycopyranoside | Neurogenic pain inhibition Pain relief through peripheral mechanisms Oedema inhibition | [25] |
| Not specified | Ecobidens® (Glycolic extract in a poloxamer-based liquid formulation) | 5-FU-induced intestinal mucositis in male Swiss mice In vivo | Anti-inflammatory, antioxidant, and intestinal protector | - | Restoration of proliferative activity of intestinal cells ↑ Ki-67 levels Modulation of the expression of Bax and p53 Regulation of lipid peroxidation and inflammatory infiltration ↓ MPO y MDA | [27] |
| Not specified | Ecobidens®) in mucoadhesive formulation with curcuminoids | 5-FU-induced intestinal mucositis in male Swiss mice In vivo | Anti-inflammatory, antioxidant, and intestinal protector | - | ↑ Ki-67 and Bcl-2 expression ↓ Pro-apoptotic regulator Bax, activity of MPO and MDA ↑ IL-10 levels | [28] |
| Not specified | FITOPROT (Formulation of glycolytic extract B. pilosa, poloxamer, and curcuminoids) | HaCaT cell line exposed to 5-FU In vitro | Anti-inflammatory and antioxidant | - | ↓ ROS, membrane potential, and cytochrome C release Maintenance of cell proliferative capacity ↓ Oxidative stress due to Nrf2 involvement Normal expression of TNF-R1 and NF-κB ↓ Production of TNF, IL1β, IL-6, and IL-8 | [29] |
| Whole except root | Aqueous (infusion) | Wistar rats treated with CCl4 In vivo | Anti-inflammatory | - | Protective effect on liver, intestine, and kidney injury ↓ Hepatic and intestinal inflammation Renal tubular regeneration ↓ Intestinal mucosa inflammation in exposed animals | [39] |
| Dry leaves | Aqueous extract | Dry eye model in healthy female Wistar rats In vivo | Anti-inflammatory | - | ↓ IL-1β, FasL and TNF-α. ↓ Leukocyte infiltration and inflammatory response in the lacrimal glands | [42] |
| Aerial | SCF | TNBS-induced intestinal inflammation in male Wistar rats and male Swiss mice In vivo | Anti-inflammatory and antioxidant | Fatty acids | ↑ IL-10 production ↓ Level of IL-1β, IL-6, and TNF-α ↓ Oxidative stress ↑ Mucin production ↓ Heparanase, Hsp70, MAPK3, and NF-κB signaling Blocking of MAPK signaling pathways | [43] |
| Whole | Methanolic extract | TNBS-induced colitis in Wistar rats In vivo | Anti-inflammatory and antioxidant | - | ↓ Leukocytes’ infiltration and TNF-α production Inhibition of oxidative stress and pro-inflammatory cytokines | [44] |
| Dry leaves | Methanolic extract | Human PBMCs, murine lymphocytes, and B10.ArSg SnJ mice with Zymosan-induced arthritis In vitro/vivo | Antiproliferative, and anti- inflammatory | Polyacetylene 2-O-β-D-glucosyltrideca-11E-en-3,5,7,9-tetrayn-1,2-diol | ↓ Proliferation of human lymphocytes (Extract) ↓ Peripheral node swelling at the site of injury induced by zymosan ↓ Murine lymphocyte proliferation (Isolated Compound) | [10] |
3.2. The Methanolic Extract, the Essential Oil, and the Cp Obtained from B. pilosa Modulate the Activation of the Immune System and Induce a Protective Effect against Infectious Diseases
| Part of the Plant | Extract Type | Experimental Model Type | Biological Effect | Metabolites Isolated or Identified | Study Conclusions | References |
|---|---|---|---|---|---|---|
| Whole | Methanolic extract | Listeria monocytogenes C57BL/6J mice infection In vivo | Antimicrobial Immunomodulator | Cp | Higher resistance to infection by intracellular pathogens ↓ CFU count and severity of lesions in infected mice | [47] |
| Whole | Methanolic extract | Candida parapsilosis infection in RAW264.7 cell line and BALB/c mice In vivo/vitro | Antimicrobial Immunomodulator | Cp | Enhanced PKC-dependent phagocytosis activity and intracellular death (RAW264.7) ↑ Resistance to infection in a macrophage-dependent manner (mice). Restriction of Candida dissemination (mice) Alleviation of liver and splenic lesions (mice) ↑ Phagolysosomal fusion, phagosomal acidification, and lysosomal enzymatic activity of macrophages. | [48] |
| Whole | Methanolic extract and fractionation | Eimeria tenella infection in Lohmann chickens MDKB cells In vivo/vitro | Anticoccidial and immunomodulator | (Cp) 2-β-D-glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne 2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne 3-β-D-glucopyranosyloxy-1-hydroxy-6(E)-tetradecene-8,10,12-triyne | Anticoccidial effects only evident with the extract and Cp ↓ Mortality in infected chickens (both) ↓ Invasion of sporozoites, interfering with the life cycle of the parasite (Both) ↓ Excretion of oocysts in fecal matter ↓ Intestinal injury (both) ↑ IFN-γ production in LT cells (Extract) | [52] |
| Flowers | Monofloral honeys from the nectar of B. pilosa | Bacteria growth inhibition by agar disk- diffusion method In vitro | Antibacterial | Polyphenols and flavonoids | Inhibition of the growth of S. aureus, S. intermedius B, S. xylosus, C. koseri, hemolytic E. coli, and S. cholearasuis | [54] |
| Leaves | Hexane Dichloromethane Ethyl acetate Acetone Methanol | MIC In vitro | Antibacterial | Group of phenolic compounds | Bacterial (Klebsiella pneumoniae, commercial probiotics, E. coli, Salmonella typhimurium, Shigella boydii, and Vibrio parahaemolyticus) growth inhibition by the dichloromethane fraction | [55] |
| Not specified | Essential oil | Human gingival fibroblasts In vitro | Antimicrobial and Pro-inflammatory | - | ↑ PGE-2 secretion in synergy with IL-1β No inhibition of IL-6 and IL-8 synthesis | [56,58] |
3.3. Extracts of B. pilosa Have Antiproliferative and Antitumor Effects in Different Experimental Models through the Induction of Apoptosis
3.4. Extracts of the Aerial Part of B. pilosa and Honey Produced from the Plant’s Flowers Have Antioxidant Properties Which Reduce the Harmful Effects Associated with Oxidative Stress, as Demonstrated in Cellular and Animal Models
| Part of the Plant | Extract Type | Experimental Model Type | Biological Effect | Metabolites Isolated or Identified | Study Conclusions | Reference |
|---|---|---|---|---|---|---|
| Aerial | HCE Chloroform fraction EtOAc Methanolic fraction | DPPH CCl4-induced hepatotoxicity in male BALB/c mice In vivo/vitro | Antioxidant | Quercetin 3,3′-dimethyl ether 7-O-β-D-glycopyranoside | ↓ Generation of hydroxyl radicals in vitro, especially EtOAc. ↓ Lipid peroxidation and protein carbonylation in the liver (EtOAc as HCE). Total recovery of FRAP (EtOAc). Prevention of GSH depletion (EtOAc as HCE). ↓ Serum AST, ALT, and LDH (EtOAc as HCE) Prevention of hepatocyte DNA fragmentation (EtOAc) | [92] |
| Flowers | Monofloral honeys from the nectar of B. pilosa | DPPH Measurement of antioxidant capacity In vitro | Antioxidant | Polyphenols and flavonoids | ↑ Radical scavenging activity ↓ Hydroxyl radical formation ↑ Reducing power | [54] |
| Leaves | Aqueous | Characterization of the chemical composition Measurement of antioxidant enzyme activity In vitro | Antioxidant | Phenolic compounds, carbohydrates, ascorbic acid, and carotenoids | High content of compounds during early vegetative growth stage ↑ Enzymatic activity of SOD, CAT, and POD during early vegetative growth stages Increasing FRAP in the developmental stages | [96] |
| Leaves | Hexane Dichloromethane Ethyl acetate Acetone Methanol | Antioxidant activity by DPPH C2C12 cell line In vitro | Antioxidant | Group of phenolic compounds | High antioxidant activity (ethyl acetate, acetone, and methanolic fractions) No cytotoxic effect | [55] |
| Leaves | Methanolic extract | Measurement of antioxidant/radical scavenger activity L929 cell line Caco-2 cells In vitro | Antioxidant | Phenolic compounds | ↑ Antioxidant activity ↑ Capture of radicals Protection of Caco-2 cells, LDL, and plasmid DNA against oxidative damage | [97] |
3.5. Whole Plant Extracts of B. pilosa Exert an Anti-Diabetic Effect by Modulating the Adaptive Immune Response in Murine Models of Type 1 Diabetes Mellitus
| Part of the Plant | Extract Type | Experimental Model Type | Biological Effect | Metabolites Isolated or Identified | Study Conclusions | Reference |
|---|---|---|---|---|---|---|
| Whole plant | Butanol fraction | NOD mice Human CD4+ Th0 cells Jurkat cells In vivo/vitro | Antidiabetic | Polyacetylenes: 2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne 3-β-D-glucopyranosyloxy-1-hydroxy-6(E)- tetradecene-8,10,12-triyne | ↓ Severity and development of autoimmune diabetes Maintenance of normal morphology of pancreatic islets ↓ Beta-cell death and leukocyte infiltration (mice) ↓ Th1 cytokine synthesis ↑ Th2 cytokine synthesis ↓ Th1 cell differentiation ↑ Th2 cell differentiation ↑ GATA-3 but not T-bet transcription ↑ Airway inflammation in OVA-challenged mice | [106] |
| Whole plant | Methanolic extract and fractionation with ethyl acetate | EL-4 Primary T cells Primary β cells NOD mice NOD-SCID mice In vivo/vitro | Antidiabetic Antiproliferative Immunomodulator | Cp: 2- β-D-glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne | Effective prevention of diabetes development ↓ Proliferation of CD4+ T cells ↓ Differentiation of Th0 to Th1 cells ↓ Serum IFN-γ ↑ Differentiation of Th0 to Th2 cells ↑ Serum IL-4 ↑ GATA-3 but not T-bet transcription ↑ FasL transcription in pancreatic islet cells ↑ Long-term phagocytic cells No effect of decreased T-cell proliferation on OVA response | [108] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Arthur, G.; Naidoo, K.; Coopoosamy, R. Bidens pilosa L.: Agricultural and pharmaceutical importance. J. Med. Plants Res. 2012, 6, 3282–3287. [Google Scholar] [CrossRef]
- Bartolome, A.P.; Villaseñor, I.M.; Yang, W.-C. Bidens pilosa L. (Asteraceae): Botanical Properties, Traditional Uses, Phytochemistry, and Pharmacology. Evid.-Based Complement. Altern. Med. ECAM 2013, 2013, 340215. [Google Scholar]
- Borges, C.C.; Matos, T.F.; Moreira, J.; Rossato, A.E.; Zanette, V.C.; Amaral, P.A. Bidens pilosa L. (Asteraceae): Traditional use in a community of southern Brazil. Rev. Bras. Plantas Med. 2013, 15, 34–40. [Google Scholar] [CrossRef]
- MU/Chipaca. Muysc Cubun—Lengua Muisca. Available online: http://muysca.cubun.org/MU/chipaca (accessed on 27 April 2021).
- Silva, F.L.; Fischer, D.C.H.; Tavares, J.F.; Silva, M.S.; de Athayde-Filho, P.F.; Barbosa-Filho, J.M. Compilation of secondary metabolites from Bidens pilosa L. Molecules 2011, 16, 1070–1102. [Google Scholar] [CrossRef]
- Alcaraz, M.; Jimenez, M. Flavonoids as anti-inflammatory agents. Fitoterapia 1988, 59, 25–38. [Google Scholar]
- Xuan, T.D.; Khanh, T.D. Chemistry and pharmacology of Bidens pilosa: An overview. J. Pharm. Investig. 2016, 46, 91–132. [Google Scholar] [CrossRef]
- Oliveira, F.Q.; Andrade-Neto, V.; Krettli, A.U.; Brandão, M.G.L. New evidences of antimalarial activity of Bidens pilosa roots extract correlated with polyacetylene and flavonoids. J. Ethnopharmacol. 2004, 93, 39–42. [Google Scholar] [CrossRef]
- Dimo, T.; Azay, J.; Tan, P.V.; Pellecuer, J.; Cros, G.; Bopelet, M.; Serrano, J.J. Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. J. Ethnopharmacol. 2001, 76, 215–221. [Google Scholar] [CrossRef]
- Pereira, R.L.; Ibrahim, T.; Lucchetti, L.; da Silva, A.J.; de Moraes, V.L.G. Immunosuppressive and anti-inflammatory effects of methanolic extract and the polyacetylene isolated from Bidens pilosa L. Immunopharmacology 1999, 43, 31–37. [Google Scholar] [CrossRef]
- Tan, P.V.; Dimo, T.; Dongo, E. Effects of methanol, cyclohexane and methylene chloride extracts of Bidens pilosa on various gastric ulcer models in rats. J. Ethnopharmacol. 2000, 73, 415–421. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Chuang, D.Y.; Wang, S.Y.; Kuo, Y.H.; Tsai, P.W.; Shyur, L.F. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J. Ethnopharmacol. 2004, 95, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Tewtrakul, S.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T.; Yoshinaga, T.; Fujiwara, T.; Supavita, T.; Yuenyongsawad, S.; et al. HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytother. Res. PTR 2003, 17, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, H.; Hua, X.; Shi, Z.; Zhang, L.; Chen, J. Clinical therapeutic effect of drug-separated moxibustion on chronic diarrhea and its immunologic mechanisms. J. Tradit. Chin. Med. Chung Tsa Chih Ying Wen Pan 1997, 17, 253–258. [Google Scholar]
- Carter, J.L.; Hege, K.; Yang, J.; Kalpage, H.A.; Su, Y.; Edwards, H.; Hüttemann, M.; Taub, J.W.; Ge, Y. Targeting multiple signaling pathways: The new approach to acute myeloid leukemia therapy. Signal Transduct. Target Ther. 2020, 18, 5. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7746731/ (accessed on 16 April 2021). [CrossRef]
- Solomon, D.H.; Glynn, R.J.; Karlson, E.W.; Lu, F.; Corrigan, C.; Colls, J.; Xu, C.; MacFadyen, J.; Barbhaiya, M.; Berliner, N.; et al. Adverse Effects of Low-Dose Methotrexate: A Randomized Trial. Ann. Intern Med. 2020, 172, 369–380. [Google Scholar] [CrossRef]
- Maetzel, A.; Wong, A.; Strand, V.; Tugwell, P.; Wells, G.; Bombardier, C. Meta-analysis of treatment termination rates among rheumatoid arthritis patients receiving disease-modifying anti-rheumatic drugs. Rheumatology 2000, 39, 975–981. [Google Scholar] [CrossRef]
- Lehners, N.; Goldschmidt, H.; Raab, M.S. Immunostimulating drugs and cytokines. Ther. Umsch. Rev Ther. 2011, 68, 655–658. [Google Scholar] [CrossRef]
- Osipov, A.; Saung, M.T.; Zheng, L.; Murphy, A.G. Small molecule immunomodulation: The tumor microenvironment and overcoming immune escape. J. Immunother. Cancer 2019, 7, 224. [Google Scholar] [CrossRef]
- Takx-Köhlen, B.C. Immunomodulators. Future prospects. Pharm. Weekbl. Sci. 1992, 14, 245–252. [Google Scholar]
- The New York Botanical Garden. Original Watercolor of Bidens pilosa by Frances W. Horne for Flora Borinqueña. Available online: https://sweetgum.nybg.org/science/vh/multimedia-details/?irn=114024 (accessed on 17 April 2023).
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Fotso, A.; Longo, F.; Djomeni, P.; Kouam, S.; Spiteller, M.; Dongmo, A.; Savineau, J.P. Analgesic and antiinflammatory activities of the ethyl acetate fraction of Bidens pilosa (Asteraceae). Inflammopharmacology 2014, 22, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- De Ávila, P.H.M.; de Ávila, R.I.; Dos Santos Filho, E.X.; Cunha Bastos, C.C.; Batista, A.C.; Mendonça, E.F.; Serpa, R.C.; Marreto, R.N.; da Cruz, A.F.; Lima, E.M.; et al. Mucoadhesive formulation of Bidens pilosa L. (Asteraceae) reduces intestinal injury from 5-fluorouracil-induced mucositis in mice. Toxicol. Rep. 2015, 2, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Bastos, C.C.C.; de Ávila, P.H.M.; Filho, E.X.D.S.; de Ávila, R.I.; Batista, A.C.; Fonseca, S.G.; Lima, E.M.; Marreto, R.N.; de Mendonça, E.F.; Valadares, M.C. Use of Bidens pilosa L. (Asteraceae) and Curcuma longa L. (Zingiberaceae) to treat intestinal mucositis in mice: Toxico-pharmacological evaluations. Toxicol. Rep. 2016, 3, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Filho, E.X.; da Silva, A.C.G.; de Ávila, R.I.; Batista, A.C.; Marreto, R.N.; Lima, E.M.; de Oliveira, C.M.A.; Mendonça, E.F.; Valadares, M.C. Chemopreventive effects of FITOPROT against 5-fluorouracil-induced toxicity in HaCaT cells. Life Sci. 2018, 193, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomed. Int. J. Phytother. Phytopharm. 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies—Methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Cobrin, G.M.; Abreu, M.T. Defects in mucosal immunity leading to Crohn’s disease. Immunol. Rev. 2005, 206, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Targan, S.R.; Karp, L.C. Defects in mucosal immunity leading to ulcerative colitis. Immunol. Rev. 2005, 206, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Andritoiu, C.V.; Ochiuz, L.; Andritoiu, V.; Popa, M. Effect of apitherapy formulations against carbon tetrachloride-induced toxicity in Wistar rats after three weeks of treatment. Molecules 2014, 19, 13374–13391. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Bokhari, J. Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 2469–2476. [Google Scholar] [CrossRef]
- Ganie, S.A.; Haq, E.; Hamid, A.; Qurishi, Y.; Mahmood, Z.; Zargar, B.A.; Masood, A.; Zargar, M.A. Carbon tetrachloride induced kidney and lung tissue damages and antioxidant activities of the aqueous rhizome extract of Podophyllum hexandrum. BMC Complement Altern. Med. 2011, 11, 17. [Google Scholar] [CrossRef]
- Pegoraro, C.M.R.; Nai, G.A.; Garcia, L.A.; Serra, F.D.M.; Alves, J.A.; Chagas, P.H.N.; de Oliveira, D.G.; Zocoler, M.A. Protective effects of Bidens pilosa on hepatoxicity and nephrotoxicity induced by carbon tetrachloride in rats. Drug Chem. Toxicol. 2021, 44, 64–74. [Google Scholar] [CrossRef]
- Smith, R.E. The tear film complex: Pathogenesis and emerging therapies for dry eyes. Cornea 2005, 24, 1–7. [Google Scholar] [CrossRef]
- Lee, W.B.; Hamilton, S.M.; Harris, J.P.; Schwab, I.R. Ocular complications of hypovitaminosis a after bariatric surgery. Ophthalmology 2005, 112, 1031–1034. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; Yang, Z.; Wang, Y.; Si, H. The Effect of the Aqueous Extract of Bidens pilosa L. on Androgen Deficiency Dry Eye in Rats. Cell Physiol. Biochem. Int. J. Exp. Cell Physiol. Biochem Pharmacol. 2016, 39, 266–277. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Cruz, V.M.; Almeida-Junior, L.D.; Costa, C.A.R.A.; Di Stasi, L.C. Bidens pilosa (Black Jack) Standardized Extract Ameliorates Acute TNBS-induced Intestinal Inflammation in Rats. Planta Med. 2020, 86, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, O.O.; Sosanya, A.S.; Nwadike, N.; Oshinloye, A.O. Beneficial effect of Bidens pilosa L. (Asteraceae) in a rat model of colitis. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Subramaniyam, G.; Shanmugam, N. Proinflammatory effects of malondialdehyde in lymphocytes. J. Leukoc. Biol. 2012, 92, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Fura, J.M.; Sarkar, S.; Pidgeon, S.E.; Pires, M.M. Combatting Bacterial Pathogens with Immunomodulation and Infection Tolerance Strategies. Curr. Top. Med. Chem. 2017, 17, 290–304. [Google Scholar] [CrossRef]
- Chung, C.Y.; Yang, W.C.; Liang, C.L.; Liu, H.Y.; Lai, S.K.; Chang, C.L.T. Data on the effect of Cytopiloyne against Listeria monocytogenes infection in mice. Data Brief 2016, 7, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Yang, W.C.; Liang, C.L.; Liu, H.Y.; Lai, S.K.; Chang, C.L.T. Cytopiloyne, a polyacetylenic glucoside from Bidens pilosa, acts as a novel anticandidal agent via regulation of macrophages. J. Ethnopharmacol. 2016, 184, 72–80. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Lillehoj, H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccines 2006, 5, 143–163. [Google Scholar] [CrossRef]
- Faber, T.A.; Dilger, R.N.; Hopkins, A.C.; Price, N.P.; Fahey, G.C. The effects of a galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex in broiler chicks challenged with Eimeria acervulina. Poult. Sci. 2012, 91, 1089–1096. [Google Scholar] [CrossRef]
- Williams, R.B. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int. J. Parasitol. 1999, 29, 1209–1229. [Google Scholar] [CrossRef]
- Yang, W.C.; Yang, C.Y.; Liang, Y.C.; Yang, C.W.; Li, W.Q.; Chung, C.Y.; Yang, M.-T.; Kuo, T.-F.; Lin, C.-F.; Liang, C.-L.; et al. Anti-coccidial properties and mechanisms of an edible herb, Bidens pilosa, and its active compounds for coccidiosis. Sci. Rep. 2019, 9, 2896. [Google Scholar] [CrossRef]
- Lillehoj, H.S.; Trout, J.M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996, 9, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Ye, Y.L.; Lin, T.Y.; Wang, Y.W.; Peng, C.C. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013, 139, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Shandukani, P.D.; Tshidino, S.C.; Masoko, P.; Moganedi, K.M. Antibacterial activity and in situ efficacy of Bidens pilosa Linn and Dichrostachys cinerea Wight et Arn extracts against common diarrhoea-causing waterborne bacteria. BMC Complement Altern. Med. 2018, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Ocheng, F.; Bwanga, F.; Joloba, M.; Softrata, A.; Azeem, M.; Pütsep, K.; Borg-Karlson, A.-K.; Obua, C.; Gustafsson, A. Essential Oils from Ugandan Aromatic Medicinal Plants: Chemical Composition and Growth Inhibitory Effects on Oral Pathogens. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 230832. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Ocheng, F.; Bwanga, F.; Almer Boström, E.; Joloba, M.; Borg-Karlson, A.K.; Yucel-Lindberg, T.; Obua, C.; Gustafsson, A. Essential Oils from Ugandan Medicinal Plants: In Vitro Cytotoxicity and Effects on IL-1β-Induced Proinflammatory Mediators by Human Gingival Fibroblasts. Evid.-Based Complement. Altern. Med. ECAM 2016, 2016, 5357689. [Google Scholar]
- Doyle, C.J.; Fitzsimmons, T.R.; Marchant, C.; Dharmapatni, A.A.S.S.K.; Hirsch, R.; Bartold, P.M. Azithromycin suppresses P. gingivalis LPS-induced pro-inflammatory cytokine and chemokine production by human gingival fibroblasts in vitro. Clin. Oral Investig. 2015, 19, 221–227. [Google Scholar] [CrossRef]
- Sreenivasan, P.K.; Gaffar, A. Antibacterials as anti-inflammatory agents: Dual action agents for oral health. Antonie Van Leeuwenhoek 2008, 93, 227–239. [Google Scholar] [CrossRef]
- Bai, H.W.; Zhu, B.T. Strong activation of cyclooxygenase I and II catalytic activity by dietary bioflavonoids. J. Lipid Res. 2008, 49, 2557–2570. [Google Scholar] [CrossRef]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; et al. Synergy-directed fractionation of botanical medicines: A case study with goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Britton, E.R.; Kellogg, J.J.; Kvalheim, O.M.; Cech, N.B. Biochemometrics to Identify Synergists and Additives from Botanical Medicines: A Case Study with Hydrastis canadensis (Goldenseal). J. Nat. Prod. 2018, 81, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Catteau, L.; Olson, J.; Van Bambeke, F.; Leclercq, J.; Nizet, V. Ursolic acid from shea butter tree (Vitellaria paradoxa) leaf extract synergizes with β-lactams against methicillin-resistant Staphylococcus aureus. FASEB J. 2017, 31, 1000.5. [Google Scholar]
- Ettefagh, K.A.; Burns, J.T.; Junio, H.A.; Kaatz, G.W.; Cech, N.B. Goldenseal (Hydrastis canadensis L.) extracts synergistically enhance the antibacterial activity of berberine via efflux pump inhibition. Planta Med. 2011, 77, 835–840. [Google Scholar] [CrossRef]
- Leyte-Lugo, M.; Britton, E.R.; Foil, D.H.; Brown, A.R.; Todd, D.A.; Rivera-Chávez, J.; Oberlies, N.H.; Cech, N.B. Secondary Metabolites from the Leaves of the Medicinal Plant Goldenseal (Hydrastis canadensis). Phytochem. Lett. 2017, 20, 54–60. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim. Biophys. Acta 2010, 1805, 53–71. [Google Scholar] [CrossRef]
- Tesniere, A.; Panaretakis, T.; Kepp, O.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008, 15, 3–12. [Google Scholar] [CrossRef]
- Alvarez, L.; Marquina, S.; Villarreal, M.L.; Alonso, D.; Aranda, E.; Delgado, G. Bioactive Polyacetylenes from Bidens pilosa. Planta Med. 1996, 62, 355–357. [Google Scholar] [CrossRef]
- Kviecinski, M.R.; Felipe, K.B.; Schoenfelder, T.; de Lemos Wiese, L.P.; Rossi, M.H.; Gonçalez, E.; Felicio, J.D.; Filho, D.W.; Pedrosa, R.C. Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J. Ethnopharmacol. 2008, 117, 69–75. [Google Scholar] [CrossRef]
- Lastra Valdés, H.A.; de León Rego, H.P. Bidens pilosa Linné. Rev. Cuba Plantas Med. 2001, 6, 28–33. [Google Scholar]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Kviecinski, M.R.; Benelli, P.; Felipe, K.B.; Correia, J.F.G.; Pich, C.T.; Ferreira, S.R.S.; Pedrosa, R. SFE from Bidens pilosa Linné to obtain extracts rich in cytotoxic polyacetylenes with antitumor activity. J. Supercrit. Fluids 2011, 56, 243–248. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, Z.; Shi, P.; Wang, G.; Wu, Y.; Li, S.; Zheng, Y.; Huang, L.; Lin, L.; Lin, X.; et al. Anticancer effect of petroleum ether extract from Bidens pilosa L. and its constituent’s analysis by GC-MS. J. Ethnopharmacol. 2018, 217, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J.Y.; Yin, S.Y.; Chang, S.L.; Hsu, Y.J.; Chen, K.W.; Kuo, T.F.; Feng, C.-S.; Yang, N.-S.; Shyur, L.-F.; Yang, W.-C.; et al. Mechanistic Study of the Phytocompound, 2-β-D-Glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne in Human T-Cell Acute Lymphocytic Leukemia Cells by Using Combined Differential Proteomics and Bioinformatics Approaches. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 475610. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5551541/ (accessed on 3 May 2021). [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. CB 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Kamp, D.W.; Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer: The role of the mitochondria. Oncology 2011, 25, 400–410, 413. [Google Scholar]
- Green, K.; Brand, M.D.; Murphy, M.P. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 2004, 53 (Suppl. S1), S110–S118. [Google Scholar] [CrossRef]
- Nishikawa, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2007, 9, 343–353. [Google Scholar] [CrossRef]
- Schrauwen, P.; Hesselink, M.K.C. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 2004, 53, 1412–1417. [Google Scholar] [CrossRef]
- Ballinger, S.W.; Patterson, C.; Knight-Lozano, C.A.; Burow, D.L.; Conklin, C.A.; Hu, Z.; Reuf, J.; Horaist, C.; Lebovitz, R.; Hunter, G.C.; et al. Mitochondrial integrity and function in atherogenesis. Circulation 2002, 106, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Trzyna, W.C.; McClintock, D.S.; Schumacker, P.T. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J. Immunol. 2000, 165, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.D.; Guzy, R.D.; Pan, Y.; Young, R.M.; Cash, T.P.; Schumacker, P.T.; Simon, M.C. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005, 1, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Malo, D.; Hekimi, S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/− mouse mutants. J. Immunol. 2010, 184, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, H.; Parthasarathi, K.; Quadri, S.; Issekutz, A.C.; Bhattacharya, J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J. Clin. Investig. 2003, 111, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef]
- Wang, C.; Schuller Levis, G.B.; Lee, E.B.; Levis, W.R.; Lee, D.W.; Kim, B.S.; Park, S.Y.; Park, E. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-α in activated RAW 264.7 cells. Int. Immunopharmacol. 2004, 4, 1039–1049. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, e7432797. [Google Scholar] [CrossRef]
- Kviecinski, M.R.; Felipe, K.B.; Correia, J.F.G.; Ferreira, E.A.; Rossi, M.H.; de Moura Gatti, F.; Filho, D.W.; Pedrosa, R.C. Brazilian Bidens pilosa Linné yields fraction containing quercetin-derived flavonoid with free radical scavenger activity and hepatoprotective effects. Libyan J. Med. 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Britton, G.; Liaen, J.; Pfander, H. Spectroscopy; Birkhauser: Basel, Switzerland, 1995; Volume 1B. [Google Scholar]
- Britton, G.; Liaen, J.; Pfander, H. Biosynthesis and Metabolism; Birkhauser: Basel, Switzerland, 1998; Volume 3. [Google Scholar]
- Telfer, A. Too much light? How β-carotene protects the photosystem II reaction centre. Photochem. Photobiol. Sci. 2005, 4, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Tesfay, S.Z.; Mathe, S.; Modi, A.T.; Mabhaudhi, T. A Comparative Study on Antioxidant Potential of Selected African and Exotic Leafy Vegetables. HortScience 2016, 51, 1529–1536. [Google Scholar] [CrossRef]
- Moyo, S.M.; Serem, J.C.; Bester, M.J.; Mavumengwana, V.; Kayitesi, E. Influence of boiling and subsequent phases of digestion on the phenolic content, bioaccessibility, and bioactivity of Bidens pilosa (Blackjack) leafy vegetable. Food Chem. 2020, 311, 126023. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Mehta, J.P.; Parmar, P.H.; Vadia, S.H.; Patel, M.K.; Tripathi, C.B. In-vitro antioxidant and in-vivo anti-inflammatory activities of aerial parts of Cassia species. Arab. J. Chem. 2017, 10, S1654–S1662. [Google Scholar] [CrossRef]
- Sheeja, K.; Shihab, P.K.; Kuttan, G. Antioxidant and Anti-Inflammatory Activities of the Plant Andrographis paniculata Nees. Immunopharmacol. Immunotoxicol. 2006, 28, 129–140. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Hull, C.M.; Peakman, M.; Tree, T.I.M. Regulatory T cell dysfunction in type 1 diabetes: What’s broken and how can we fix it? Diabetologia 2017, 60, 1839–1850. [Google Scholar] [CrossRef]
- Hassan, G.A.; Sliem, H.A.; Ellethy, A.T.; Salama, M.E.S. Role of immune system modulation in prevention of type 1 diabetes mellitus. Indian J. Endocrinol. Metab. 2012, 16, 904–909. [Google Scholar]
- Phillips, B.; Trucco, M.; Giannoukakis, N. Current state of type 1 diabetes immunotherapy: Incremental advances, huge leaps, or more of the same? Clin. Dev. Immunol. 2011, 2011, 432016. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.T.; Kuo, H.K.; Chang, S.L.; Chiang, Y.M.; Lee, T.H.; Wu, W.M.; Shyur, L.-F.; Yang, W.-C. The distinct effects of a butanol fraction of Bidens pilosa plant extract on the development of Th1-mediated diabetes and Th2-mediated airway inflammation in mice. J. Biomed. Sci. 2005, 12, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chakir, H.; Wang, H.; Lefebvre, D.E.; Webb, J.; Scott, F.W. T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine profile in mixed cell populations: Predominant role of GATA-3. J. Immunol. Methods 2003, 278, 157–169. [Google Scholar] [CrossRef]
- Chang, C.L.T.; Chang, S.L.; Lee, Y.M.; Chiang, Y.M.; Chuang, D.Y.; Kuo, H.K.; Yang, W.-C. Cytopiloyne, a polyacetylenic glucoside, prevents type 1 diabetes in nonobese diabetic mice. J. Immunol. 2007, 178, 6984–6993. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.L.; Lawrence-Miyasaki, L.S.; Garcia-Kennedy, R.; Lennette, E.T.; Martinez, O.M.; Krams, S.M.; Berquist, W.E.; So, S.K.; Esquivel, C.O. An increased incidence of Epstein-Barr virus infection and lymphoproliferative disorder in young children on FK506 after liver transplantation. Transplantation 1995, 59, 524–529. [Google Scholar] [CrossRef]
- Kirby, B.; Owen, C.M.; Blewitt, R.W.; Yates, V.M. Cutaneous T-cell lymphoma developing in a patient on cyclosporin therapy. J. Am. Acad. Dermatol. 2002, 47 (Suppl. S2), S165–S167. [Google Scholar] [CrossRef]
- Mohamed, S.I.A.; Jantan, I.; Haque, M.d.A. Naturally occurring immunomodulators with antitumor activity: An insight on their mechanisms of action. Int. Immunopharmacol. 2017, 50, 291–304. [Google Scholar] [CrossRef]
- Santos Filho, E.X.D.; Arantes, D.A.C.; Oton Leite, A.F.; Batista, A.C.; Mendonça, E.F.D.; Marreto, R.N.; Naves, L.N.; Lima, E.M.; Valadares, M.C. Randomized clinical trial of a mucoadhesive formulation containing curcuminoids (Zingiberaceae) and Bidens pilosa Linn (Asteraceae) extract (FITOPROT) for prevention and treatment of oral mucositis—Phase I study. Chem. Biol. Interact. 2018, 291, 228–236. [Google Scholar] [CrossRef]
- Arantes, D.A.C.; da Silva, A.C.G.; Freitas, N.M.A.; Lima, E.M.; de Oliveira, A.C.; Marreto, R.N.; Mendonça, E.F.; Valadares, M.C. Safety and efficacy of a mucoadhesive phytomedication containing curcuminoids and Bidens pilosa L. extract in the prevention and treatment of radiochemotherapy-induced oral mucositis: Triple-blind, randomized, placebo-controlled, clinical trial. Head Neck 2021, 43, 3922–3934. [Google Scholar] [CrossRef]
- Hassan, A.; Ibrahim, A.; Mbodji, K.; Coëffier, M.; Ziegler, F.; Bounoure, F.; Chardigny, J.M.; Skiba, M.; Savoye, G.; Déchelotte, P.; et al. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-κB in rats with TNBS-induced colitis. J. Nutr. 2010, 140, 1714–1721. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Montico, B.; Nigro, A.; Casolaro, V.; Dal Col, J. Immunogenic Apoptosis as a Novel Tool for Anticancer Vaccine Development. Int. J. Mol. Sci. 2018, 19, 594. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5855816/ (accessed on 27 April 2021). [CrossRef] [PubMed]
- Vandenabeele, P.; Vandecasteele, K.; Bachert, C.; Krysko, O.; Krysko, D.V. Immunogenic Apoptotic Cell Death and Anticancer Immunity. Adv. Exp. Med. Biol. 2016, 930, 133–149. [Google Scholar] [PubMed]
- Krysko, D.; Abhishek, G.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. Available online: https://pubmed.ncbi.nlm.nih.gov/23151605/ (accessed on 29 November 2021). [CrossRef] [PubMed]
- Wei, W.C.; Lin, S.Y.; Lan, C.W.; Huang, Y.C.; Lin, C.Y.; Hsiao, P.W.; Chen, Y.-R.; Yang, W.-C.; Yang, N.-S. Inhibiting MDSC differentiation from bone marrow with phytochemical polyacetylenes drastically impairs tumor metastasis. Sci. Rep. 2016, 6, 36663. [Google Scholar] [CrossRef]
- Mortezaee, K. Myeloid-derived suppressor cells in cancer immunotherapy-clinical perspectives. Life Sci. 2021, 277, 119627. [Google Scholar] [CrossRef]
- Toor, S.M.; Elkord, E. Therapeutic prospects of targeting myeloid-derived suppressor cells and immune checkpoints in cancer. Immunol. Cell Biol. 2018, 96, 888–897. [Google Scholar] [CrossRef]
- Zhou, J.; Donatelli, S.S.; Gilvary, D.L.; Tejera, M.M.; Eksioglu, E.A.; Chen, X.; Coppola, D.; Wei, S.; Djeu, J.Y. Therapeutic targeting of myeloid-derived suppressor cells involves a novel mechanism mediated by clusterin. Sci. Rep. 2016, 6, 29521. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Chang, C.L.T.; Chang, S.L.; Yang, W.C.; Shyur, L.F. Cytopiloyne, a novel polyacetylenic glucoside from Bidens pilosa, functions as a T helper cell modulator. J. Ethnopharmacol. 2007, 110, 532–538. [Google Scholar] [CrossRef]
- Chang, S.L.; Chiang, Y.M.; Chang, C.L.T.; Yeh, H.H.; Shyur, L.F.; Kuo, Y.H.; Wu, T.K.; Yang, W.C. Flavonoids, centaurein and centaureidin, from Bidens pilosa, stimulate IFN-gamma expression. J. Ethnopharmacol. 2007, 112, 232–236. [Google Scholar] [CrossRef]
- Chang, S.L.; Yeh, H.H.; Lin, Y.S.; Chiang, Y.M.; Wu, T.K.; Yang, W.C. The effect of centaurein on interferon-gamma expression and Listeria infection in mice. Toxicol. Appl. Pharmacol. 2007, 219, 54–61. [Google Scholar] [CrossRef] [PubMed]

| Item 1 | Item 2 | Item 3 |
|---|---|---|
| Bidens pilosa OR * bidens | AND * | Immunomodulation |
| Inflammation | ||
| Anti-inflammatory agents | ||
| Antitumor agents | ||
| Antioxidant effects | ||
| Infections |
| Part of the Plant | Extract Type | Experimental Model Type | Biological Effect | Metabolites Isolated or Identified | Study Conclusions | Reference |
|---|---|---|---|---|---|---|
| Aerial | Ethanol extract: water (9:1) fractionated SCF | MCF-7 cell line EAC in BALB/c mice In vitro/vivo | Antitumor and Antiproliferative | Polyacetylenes | Cytotoxic activity in a concentration-dependent manner ↓ Body weight, ascites fluid volume, and tumor cells ↑ Non-viable/viable tumor cells ratio ↑ Inhibition of tumor growth ↑ Mean survival time and life expectancy of mice | [74] |
| Aerial | Petroleum ether | Cell lines: HepG2 A549 CNE-2 B16 A549-xenograft murine model. In vitro/In vivo | Antitumor and Antiproliferative | Triterpenes Aliphatic hydrocarbon | Antiproliferative activity on tumor cell lines Tumor growth (A549) inhibition in mice ↓ Bcl-2 protein expression ↑ Bax and caspase-3 expression | [75] |
| Whole plant | Ethanolic crude extract fractionated with ethyl acetate and n-butanol | Jurkat cells (clon E6-1, TIB 152) In vitro | Antitumoral | Cp 2-β-D-glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne | ↑ Expression of the GDIR2, GSTO1, HBB, PDIA3, ADA, SERA, PARK7, IDHC, LTOR3, LMNB1, GSTP1, and VDAC2 proteins ↓ RM39, MCCB, TXNL1, C3orf60, BID, PRDX3, PPID, GLRX3, and NDUA5 protein expression | [76] |
| Flowers | Monofloral honeys from the nectar of B. pilosa | WiDr cell line MTT In vitro | No anti-inflammatory effect No cytotoxic effect | Polyphenols and flavonoids | No cytotoxic effect No inhibition of IL-8 synthesis | [54] |
| Compound | Type | Chemical Structure | Immunomodulatory Property |
|---|---|---|---|
| 2-β-D-Glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne | Polyyne |  | Antimicrobial Antidiabetic Antitumor |
| 2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne | Polyyne |  | Antimicrobial Antidiabetic |
| 3-β-D-glucopyranosyloxy-1-hydroxy-6(E)-tetradecene-8,10,12-triyne | Polyyne |  | Antimicrobial Antidiabetic |
| 2-O-D-glucosyltrideca-11E-en-3,5,7,9-tetrayn-1,2-diol | Polyyne |  | Anti-inflammatory |
| Quercetin 3,3′-dimethyl ether 7-O-β-D-glucopyranoside | Flavonoid |  | Anti-inflammatory Antioxidant |
| Iso-okanin 7-O-β-D-(2″,4″,6″-triacetyl)- glycopyranoside | Flavonoid |  | Anti-inflammatory |
| Quercetin-3-O-robinobioside | Flavonoid |  | Antioxidant |
| Rutin | Flavonoid |  | Antioxidant |
| Quercetin-3-O-glucoside | Flavonoid |  | Antioxidant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Mesa, X.M.; Contreras Bolaños, L.A.; Mejía, A.; Pombo, L.M.; Modesti Costa, G.; Santander González, S.P. Immunomodulatory Properties of Natural Extracts and Compounds Derived from Bidens pilosa L.: Literature Review. Pharmaceutics 2023, 15, 1491. https://doi.org/10.3390/pharmaceutics15051491
Rodríguez-Mesa XM, Contreras Bolaños LA, Mejía A, Pombo LM, Modesti Costa G, Santander González SP. Immunomodulatory Properties of Natural Extracts and Compounds Derived from Bidens pilosa L.: Literature Review. Pharmaceutics. 2023; 15(5):1491. https://doi.org/10.3390/pharmaceutics15051491
Chicago/Turabian StyleRodríguez-Mesa, Xandy Melissa, Leonardo Andres Contreras Bolaños, Antonio Mejía, Luis Miguel Pombo, Geison Modesti Costa, and Sandra Paola Santander González. 2023. "Immunomodulatory Properties of Natural Extracts and Compounds Derived from Bidens pilosa L.: Literature Review" Pharmaceutics 15, no. 5: 1491. https://doi.org/10.3390/pharmaceutics15051491