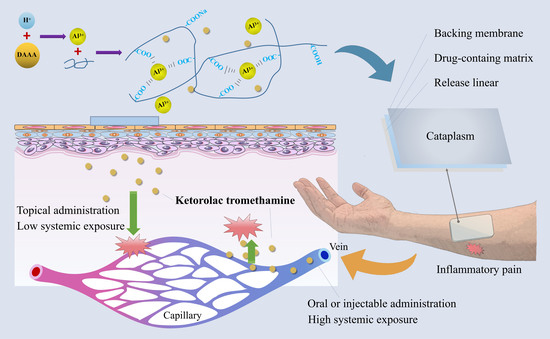
1. Introduction
Inflammatory pain, which is initiated by trauma, infection, chemical stimulation, and surgery, is a type of common disease. Osteoarthritis is one inflammatory pain disease with a very high incidence. It is estimated that more than 240 million people suffer from osteoarthritis worldwide, and the number of people in the United States alone exceeds 54 million [
1,
2]. The clinical characteristics of osteoarthritis can be persistent or intermittent, which brings great distress to patients [
2,
3]. Therefore, choosing an appropriate treatment for pain relief is of paramount importance.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used as a first-line treatment for osteoarthritis. As one of them, ketorolac tromethamine can reduce the production of prostaglandins (PG) by inhibiting the activity of cyclooxygenase (COX), thereby producing anti-inflammatory and analgesic effects. More importantly, ketorolac tromethamine can achieve an analgesic effect similar to that of opioids but without addiction [
2,
4,
5,
6]. Although effective, current dosage forms (e.g., injections and tablets) of ketorolac tromethamine result in systemic drug exposure, especially at noninflammatory sites, which may lead to adverse effects, such as gastric ulcers [
7,
8].
Therefore, it is greatly important to reduce the occurrence of adverse effects of ketorolac tromethamine while preserving the anti-inflammatory and analgesic efficacy. The development of ketorolac tromethamine eye drops (ACUVAIL
®) was based on this concept and are indicated for the treatment of pain and inflammation following cataract surgery [
9,
10,
11]. Considering the characteristics of inflammatory pain [
12], the delivery of ketorolac tromethamine through the skin to achieve local drug enrichment in the pain site is expected to accomplish this clinical need. Yang et al. [
13] and Moussaoui et al. [
14] have developed gels loaded with ketorolac tromethamine for periodontal pain and the postoperative treatment of acromegaly, respectively. Furthermore, the gel systems based on liposomal and nanodelivery technologies have shown more effective transdermal drug penetration and therapeutic effects [
15,
16]. However, when applied to the skin, the gel form has its own drawbacks, such as easy erasability, short duration of efficacy, and low patient compliance.
Here, based on the cross-linking reaction between dihydroxyaluminum aminoacetate (DAAA) and sodium polyacrylate without organic solvents, a three-dimensional reticulated skeleton of cataplasm was successfully constructed to deliver ketorolac tromethamine topically for the first time. Its matrix is believed to achieve a longer and constant rate of sustained drug release [
17], which can be particularly useful for providing pain relief over an extended period. Furthermore, cataplasm can also be used to provide a cooling effect to the applied area, which can help to reduce pain, inflammation, and swelling. The constructed cataplasm has the advantages of low skin irritation, controllable preparation process, and good reproducibility, and is suitable for industrial production. In this work, the cross-linking and molding mechanisms of the cataplasm are systematically characterized and discussed in detail, along with the adhesion, rheological, in vitro release, and permeation characteristics. The cataplasm developed herein has demonstrated excellent efficacy and safety in animal pharmacodynamic and human pharmacokinetic studies and holds promise for commercial transfer, hopefully providing a new and effective option for patients in the future.
2. Materials and Methods
2.1. Animals
The SD rats (200 ± 20 g) were purchased from Tianqin Biology (Changsha, China), whose quality certificate number is 430726211100339273 and license number is SYXK (Xiang) 2020-0015. To exclude the influence of animal gender, half of the purchased SD rats were male and half were female. The acquisition of and experiments on the SD rats were approved by the ethics committee of Xiangya School of Pharmaceutical Science, Central South University.
2.2. Materials
Ketorolac tromethamine was purchased from Renan Pharmaceutical (Chengdu, China). Commercially available ketorolac tromethamine tablets were manufactured by Mylan Pharmaceutical (Pittsburgh, PA, USA). Isopropyl myristate, 1,2-propanediol, and tartaric acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). Polyglycerol-3 oleate (Plurol® Oleique CC497) and diethylene glycol monoethyl ether (Transcutol®) were gifted by Gattefossé (Saint-Priest, Rhone, France). Partially neutralized polyacrylate (ViscomateTM, NP700) was obtained from Showa Denko KK (Kawasaki, Kanagawa, Japan). DAAA was purchased from Xiyue Pharmaceutical (Weinan, China). λ-carrageenan was received from Ika Bio (Shanghai, China). The artificial membrane (With 0.45 μm aperture, polyethersulfone material) was obtained from Keelong Laboratory Equipment (Tianjin, China). The isolated skin of Bama minipigs was purchased from Jingde Agricultural Products (Xingtai, China).
2.3. Preparation of Ketorolac Tromethamine Cataplasm
Briefly, dibutyl hydroxytoluene (BHT), DAAA, kaolin, disodium edetate (EDTA-2Na), titanium dioxide, partially neutralized polyacrylate, and povidone K90 were dispersed uniformly in polyethylene glycol (PEG) 400 with gentle mechanical stirring, which was named phase A. Sorbitol, tartaric acid, and 20% polyacrylic acid solution were sequentially dissolved in purified water with mechanical stirring, which was called phase B. Ketorolac tromethamine was completely dissolved in purified water with mechanical stirring, which was called phase C. Menthol, methylparaben, and propylparaben were added to 1,2-propanediol and mechanically stirred until they dissolved, which was called phase D.
Phase D was added to phase A with mechanical stirring for 30 min. Then, phase C was added to the mixture of phase A and phase D. Phase B was poured into a stirring kneader (Shenglong Chemical Machinery, Laizhou, China), and then the mixture of phase A, phase C, and phase D was added while kneading. After kneading evenly, the material was coated, laminated, and cut with a cataplasm coating machine (Xinyi Huida Electromechanical Equipment, HD-SIII type, Beijing, China). The cut samples were cross-linked at room temperature for seven days to form the cataplasm. The obtained ketorolac tromethamine cataplasm was sealed and stored at room temperature.
2.4. Microstructure Observation
2.4.1. Polarized Light Microscopy Characterization
Ketorolac tromethamine was uniformly dispersed in light liquid paraffin and observed using a polarizing microscope (Motic China Group, Panthera TEC-POL, Xiamen, China). A small amount of paste on the prepared cataplasm was scraped off and observed using the above-mentioned microscope.
2.4.2. ESEM Characterization
The test was carried out using an environmental vacuum scanning electron microscope (Thermo Scientific, Quattro, Waltham, MA, USA). A small amount of cataplasm paste was scraped and placed in purified water to swell for 6 min and then frozen using liquid nitrogen. The sample was placed on the cold table, and the surface morphology of the sample was observed. Test conditions: low-vacuum mode was selected, voltage was 7 kV, signal source was GSED, test pressure was maintained in the range of 50 to 700 Pa, cold table temperature was in gradient heating mode, temperature range was −20 °C to 10 °C, and heating rate was 10 °C/min.
2.5. Tack Test
There were two methods to detect the tack of ketorolac tromethamine cataplasm.
The first method was called the rolling-ball tack test [
18], and the tack was measured using a SUS304 inclined ball tack test device (Sumspring Co., Ltd., Jinan, China). First, the cataplasm was cut into a rectangular shape of 5.0 cm * 2.5 cm. Second, the backing membrane side of the cataplasm was fixed on a flat plate with an inclination angle of 15°, and the release liner was removed to expose the paste to the air. The rolling length of the ball on the cataplasm was 5.0 cm, and the distance between the initial position of the ball and the upper end of the test sample was 1.0 cm. Finally, the ball fell freely along the inclined plane and the largest ball that could stick to the cataplasm had its number recorded. Parallel operations were performed three times, and the largest ball number among the three results was recorded.
The second method was called the loop tack test [
19]. The cataplasm was cut into a rectangular shape of 2.4 cm * 10.0 cm, then the short sides were bent and glued together to form a ring. Next, the sample was clamped to the loop tack test device (Saicheng Electronic Technology Co., Ltd., Jinan, China) so that the annular bottom of the sample was 30 mm from the top of the test plate. Then, the sample was slowly lowered 45 mm so that the paste side of the annular sample was in contact with the standard steel test plate. After 1 min contact time, the sample was pulled up at a rate of 100 mm/min, and the maximum force to separate the sample from the standard steel test plate was recorded. The average value of three parallel experiments was taken.
2.6. Peel-Strength Test
PSTC 1 standard test method evaluated peel strength at a peel angle of 180° using an electronic peel-strength test device (Sumspring Co., Ltd., Jinan, China) [
20]. The backing membrane side of the cataplasm was fixed on a stainless-steel plate (length: 125 mm, width: 50 mm, and thickness: 2 mm). Then, the release liner was removed, and a polyester film covered the paste. After that, a pressing roller weighing 2 kg was rolled back and forth on the polyester film three times, and the sample was placed at room temperature for 30 min to ensure that the polyester film and the paste were fully contacted and bonded. The polyester film was driven with the clamp to move at a constant speed of 300 mm/min, and the average force required to peel the polyester film from the paste was recorded. The average value of three parallel experiments was taken.
2.7. Rheological Test
The rheological properties of cataplasm (dose of 1.0%) were studied using a rheometer (TA Instrument, DHR-1, New Castle, DE, USA) and tests were performed using a 25 mm flat plate certificate. Due to the high elasticity of the cataplasm, an axial-force-adjustment step was added before each test (axial force: 1 N; sensitivity: 0.1 N). Amplitude scans and frequency scans of the samples were performed in oscillation mode. Creep-recovery tests were performed in step (transient)-creep mode. Viscosity profile scans were performed in flow-ramp mode.
2.7.1. Oscillation Amplitude
The sample was exposed to increasing stresses from 10 to 3000 Pa at 32 °C with a constant 1 Hz frequency. Then, the storage modulus (G′), loss modulus (G″), and loss tangent (tan δ) values were plotted with a logarithmic scale. This oscillation test reflected the linear viscoelastic region (LVR) of the sample, as well as the trend of its viscoelasticity with increasing stress.
2.7.2. Oscillation Frequency
The change in viscoelastic modulus of the sample over a range of frequencies was measured by subjecting the sample to constant stress (50 Pa) at 32 °C [
17].
2.7.3. Creep-Recovery Test
The sample was held for 300 s at 32 °C with stress intensity set to 50 Pa. The stress was then immediately relieved, and the recovery was tracked by monitoring the strain over time for 300 s.
2.7.4. Viscosity Curve
The process duration was set to 60 s and the shear rate ranged from 100.0/s to 0.01/s. The variation in sample viscosity and stress with the shear rate was recorded.
2.8. In Vitro Release Study
In vitro release behavior of different doses of cataplasm (0.5%, 1.0%, and 1.5%) was studied using the vertical Franz diffusion cell (Yuyan Scientific Instruments, TK-12D, Shanghai, China). Conditions to be met in experiments with the Franz diffusion cell included a 12.0 mL receptor cell, a 1.54 cm
2 effective diffusion area, 12.0 mL of degassed 0.01 mol/L phosphate buffer receptor liquid (pH 7.4), a diffusion cell temperature of 32 °C, and a 600 rpm stirring speed. The cataplasm was tightly attached to the upper side of the artificial membrane, and 12.0 mL of sample solution was collected from the receptor cell at 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 10, and 12 h, respectively, and an equal volume of the receptor liquid was added. Samples were filtered through a filter with a pore size of 0.22 μm and analyzed via high-performance liquid chromatography (HPLC). The number of experiments for each dose of cataplasm was six times, and the cumulative release (
Q, μg/cm
2) of ketorolac tromethamine was calculated using Equation (1).
where
Ci is the drug concentration at each time point,
Vi is the sampling volume at each point, and
A is the effective diffusion area.
The experimental data before reaching the plateau (0–6 h) were fitted with different mathematical models (including zero-order, first-order, and Higuchi), and the correlation coefficient (r2) was used as an indicator to determine the best-fitting model.
2.9. Selection of the Type of Skin-Penetration Enhancers
To investigate the difference in the rate and extent of diffusion of ketorolac tromethamine in the skin among different types of transdermal enhancers, an in vitro penetration experiment was performed. Transcutol
®, 1,2-propanediol, Plurol
® Oleique CC497, and isopropyl myristate were used as transdermal penetration enhancers. Referring to other transdermal studies [
14], the isolated skin of a 1-month-old Bama minipig was chosen as the permeation barrier because it is similar in structure to human skin.
The skin was thawed at room temperature in 0.9% NaCl solution before application, and residual liquid on the skin’s surface was removed with absorbent paper. Then, clean skin was cut into a circle with a diameter of 3.0 cm and placed on the Franz diffusion cell with a diffusion area of 1.54 cm2. The stratum corneum of the skin faced the donor compartment and the dermis was in contact with the receptor fluid (degassed 0.9% NaCl solution) with a volume of 12.0 mL of the receiving cell at 600 rpm in a 32 °C diffusion cell. The cataplasm (cut into circles with a diameter of 1.4 cm), containing different penetration enhancers, adhered to the stratum corneum side of the skin. Samples were collected with a pipette in a volume of 500 μL and supplemented with an equal volume of 0.9% NaCl solution at 1, 2, 4, 8, 12, and 24 h. Samples were analyzed with the HPLC method to calculate the cumulative drug penetration amount per unit area of skin. After sampling, the cataplasm was removed, and the skin was washed with 50% methanol. The skin was then ground into a powder with a freezer grinder (Jingxin Technology, Shanghai, China). Then, methanol was added as a skin extract, sonicated for 10 min, and centrifuged. The supernatant was collected for HPLC analysis to calculate the skin retention of ketorolac tromethamine.
2.10. In Vitro Skin Penetration Study
The purpose of this experiment was to study the degree and rate of penetration of ketorolac tromethamine through isolated skin with different doses of cataplasm (0.5%, 1.0%, and 1.5%). The experimental method operated according to item 2.9. The ratio of cumulative-permeation-to-dose at 24 h in each group was calculated. In addition, a linear regression was performed on the cumulative permeation-time curve. The slope of the straight line with a linear correlation coefficient greater than 0.99 was defined as the steady-state transdermal permeation rate per unit area of the drug (J), and the intercept of the extrapolation of the regression curve and the time axis was defined as the lag time (Tlag).
2.11. Histological Evaluation
2.11.1. H and E Staining
Referring to the method in the literature [
21], the isolated skin samples of 1-month-old Bama minipigs, untreated and treated with the cataplasm (doses of 0.5%, 1.0%, and 1.5%, respectively) for 24 h, were repeatedly washed with normal saline, followed by the formulation of 4–7 μm tissue slices. After that, they were stained with hematoxylin and eosin and were observed using a microscope (ZEISS, AxioVert.A1, Oberkochen, Germany).
2.11.2. SEM Characterization
According to the method from previous studies [
22,
23], the isolated skin of 1-month-old Bama minipigs, untreated and treated with the cataplasm (dose of 1.0%) for 24 h, was fixed with a 2.5% glutaraldehyde solution at 4 °C for 24 h, and the fixative was poured out. After that, the samples were rinsed three times with 0.1 M phosphate buffer (pH 7.0) for 15 min, and the skin was fixed with 1% osmic acid solution for 1–2 h. Then, the osmic acid solution was removed, and the samples were rinsed three times with 0.1 M phosphate buffer (pH 7.0) for 15 min. Finally, the samples were dehydrated, dried, and coated, followed by observation using a scanning electron microscope (Hitachi, SU8010, Tokyo, Japan).
2.12. In Vivo Pharmacodynamic Study in SD Rats
2.12.1. Grouping and Administrating
SD rats (10 rats per group) were randomly assigned to the control group, oral group, 0.5% cataplasm group, 1.0% cataplasm group, and 1.5% cataplasm group. In the control group, rats were treated with a cataplasm vehicle. In the oral group, rats were administered using gavage (0.9 mg/kg). In the cataplasm group, rats were treated with cataplasm with different drug contents. After cataplasm administration for 3 h at the right hindfoot or oral administration, rats in all groups were injected subcutaneously with 1% λ-carrageenan (0.1 mL) at the right hindfoot pad.
2.12.2. Carrageenan-Induced Paw Edema
The toe volume was measured using a toe volume meter (Yiyan Technology, YLS-7C, Jinan, China) before (0 h) and 0.5, 1, 2, 4, and 6 h after the administration of carrageenan. The degree of paw swelling was calculated as:
where,
Vt is the volume of the carrageenan-treated paw, and
V is that of the paw before carrageenan treatment.
2.12.3. Carrageenan-Induced Paw Pain
The pain threshold (PT, gf) of the right hindfoot was measured before (0 h) and 0.5, 1, 2, 4, and 6 h after carrageenan administration, using an electronic Von Frey (Ugo Basile Bioresearch Instruments Inc., e-VF, Gemonio, Italy). The difference in the pain threshold before and after drug administration was calculated. The PT change rate was calculated as follows:
where
PTt is the pain threshold of the carrageenan-treated paw, and
PT is that of the paw before carrageenan treatment.
2.13. In Vivo Pharmacokinetic Study in Humans
2.13.1. Subjects
This trial’s subjects were selected from a healthy Chinese male population. After signing the informed consent form, participants were examined, and screening examinations were completed within 14 days of the study’s start date. Physical examinations, vital signs examinations, 12-lead electrocardiograms, and standard laboratory evaluations (including blood routine, urine, and blood biochemistry) were conducted to screen individuals to ensure that they met the clinical trial requirements. The ethics committee of Xiangya School of Pharmaceutical Science, Central South University, approved the human pharmacokinetic study.
2.13.2. Trial Design
This study was a single-center, open-label, parallel, two-period, two-treatment, fasting, and single-dose clinical trial. Subjects sequentially applied the ketorolac tromethamine cataplasm (one patch at a dose of 1.0%, i.e., 100 mg) to the dorsal skin versus a commercial ketorolac tromethamine oral product tablet (one tablet, 10 mg) during two periods. In the first period (cataplasm-treatment group), about 3 mL of venous blood was collected from subjects before (0 h) and 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 24, 28, 32, 36, 48, 60, and 72 h after administration in a tube containing sodium heparin. In the second period (tablet-treatment group), blood was collected from subjects before (0 h) and 0.17, 0.25, 0.33, 0.5, 0.75, 1, 1.25, 1.5, 2, 4, 6, 8, 10, 12, 16, 24, and 36 h after administration. The washout period was seven days between the two periods to ensure that the residual drug had been eluted from the subjects’ bodies before the oral administration of the tablets.
2.13.3. Safety and Tolerability Assessments
The investigators constantly examined the physical status of the recruited subjects throughout the study period.
2.13.4. Determination of Drug Concentrations in Human Plasma
Within 60 min of collection, blood samples were placed into a low-temperature centrifuge (GL12, Changsha Yingtai Instruments Co., Ltd., Changsha, China) and centrifuged for 10 min at 2480 g and 4 °C. The samples were placed into an ultra-low-temperature refrigerator at −80 °C for storage and subsequent analysis after centrifugation. The concentration of ketorolac tromethamine in plasma was measured via LC-MS/MS, using agomelatine as an internal standard and plasma from healthy subjects as a biological matrix. The experiment was performed with HPLC (Shimadzu, LC-30AD, Chukyo, Kyoto, Japan) with a C18 column (2.1 mm × 50 mm, 1.7 μm) at a flow rate of 0.40 mL/min and a column temperature of 40 °C. The mobile phase was in gradient elution mode. Mobile phase A was a 0.1% formic acid aqueous solution, and mobile phase B was acetonitrile. The elution procedures were 0–1.2 min, A:B = 70:30; 1.2–2.0 min, A:B = 10:90; and 2.0–3.5 min, A:B = 70:30. The detection and quantification were performed with a mass spectrometer (SCIEX, API 6500, Framingham, MA, USA), using the positive ion electrospray ionization method. The multiple reaction monitoring transitions for ketorolac tromethamine was m/z 256.1→105.0, and the internal standard (agomelatine) was 244.1→185.1. A standard curve was established for ketorolac tromethamine in the concentration range of 0.5–5000 ng/mL. The linearity was evaluated using weighted least-squares regression analysis, and the linearity was good in this concentration range (r2 > 0.995). The precision and accuracy of the assay met the experimental requirements.
2.13.5. Calculation of Pharmacokinetic Parameters
Noncompartmental analysis of pharmacokinetic parameters in humans was performed using Phoenix WinNonlin 8.3 pharmacokinetic software (Certara, Princeton, NJ, USA). Tmax and Cmax were read from the blood concentration-time curve, and AUC0-t was calculated using the linear trapezoidal rule. AUC0-∞ was the sum of AUC0-t and Ct/ke, where Ct is the last measurable blood concentration, ke is the rate of decrease in blood concentration per unit time, and t1/2 is the time it takes for the blood concentration to drop by 50%.
2.14. Statistical Analysis
The software used for the statistics of this work was SPSS (IBM, Armonk, NY, USA), and the measures were expressed as the standard deviation of means (mean ± SD). If there was no statistical significance (p > 0.05), statistical analysis was performed using the one-way analysis of variance (ANOVA). If the ANOVA was statistically significant (p ≤ 0.05), a comparative analysis was performed using the LSD test (parametric method). If the variance was not equal (p ≤ 0.05), the Kruskal-Wallis test was performed. If the Kruskal-Wallis test was statistically significant (p ≤ 0.05), the comparative analysis was performed using Dunnett’s test (nonparametric method). Statistical results were tested at α = 0.05, where p ≤ 0.05 was considered statistically significant and p ≤ 0.01 indicated that the difference tested was highly significant.
4. Conclusions
In our study, we prepared a cataplasm of ketorolac tromethamine and characterized its microstructure, mechanical properties, and rheological properties, which showed that the matrix is a viscous elastomer formed by a cross-linked mesh structure. In addition, the results of in vitro release tests of different doses of cataplasm showed that the release of the drug within the paste satisfied the Higuchi model and exhibited a diffusion behavior. Through in vitro permeation tests, 1,2-propanediol with an optimal effect was selected as the permeation enhancer. According to the results of pharmacodynamic experiments in rats, cataplasm in 1.0% and 1.5% doses provided anti-inflammatory and analgesic effects similar to those of oral administration, and there was no significant difference in therapeutic effects between the two doses of cataplasm. We also verified the safety of the formulation in the human pharmacokinetic study, wherein subjects using cataplasm presented lower plasma drug concentrations and fewer reported adverse events. Results from trials in animals and humans suggested that the 1.0% dose of cataplasm achieves a reduction in the risk of adverse events without loss of efficacy and that our cataplasm is a potential formulation for the treatment of inflammatory pain.