Anti-Oxidant Multi-Functionalized Materials: Strontium-Substituted Monetite and Brushite as Delivery Systems for Curcumin
Abstract
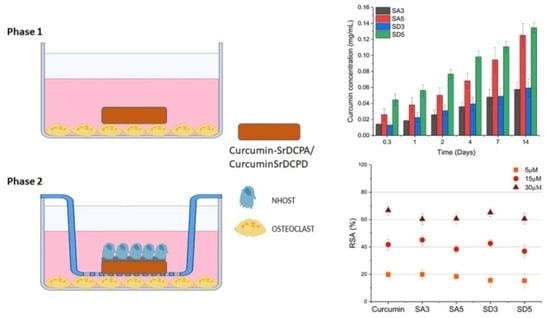
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Physico-Chemical Characterization of Materials
2.2.1. Preliminary Tests
2.2.2. Complete Tests
2.3. Biological Assays
2.3.1. Cell Cultures
2.3.2. Cell Viability
2.3.3. OC Differentiation and Morphology
2.3.4. Scaffold Colonization
2.3.5. Gene Expression Analysis
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. Materials Synthesis and Characterization
3.2. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirillo, M.; Martelli, G.; Boanini, E.; Rubini, K.; Di Filippo, M.; Torricelli, P.; Pagani, S.; Fini, M.; Bigi, A.; Giacomini, D. Strontium substituted hydroxyapatite with β-lactam integrin agonists to enhance mesenchymal cells adhesion and to promote bone regeneration. Colloids Surf. B Biointerfaces 2021, 200, 111580. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Boanini, E. Functionalized biomimetic calcium phosphates for bone tissue repair. J. Appl. Biomater. Funct. Mater. 2017, 15, e313–e325. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Sarkar, N.; Banerjee, D. Natural medicine delivery from biomedical devices to treat bone disorders: A review. Acta Biomater. 2021, 126, 63–91. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Strontium ranelate: A novel mode of action optimizing bone formation and resorption. Osteoporos. Int. 2005, 16, S7–S10. [Google Scholar] [CrossRef]
- Schumacher, M.; Hens, A.; Rohnke, M.; Gelinski, M. A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Giavaresi, G.; Contartese, D.; Bigi, A.; Boanini, E.; Parrilli, A.; Lolli, R.; Gasbarrini, A.; Barbanti Brodano, G.; Fini, M. Effect of strontium substituted ß-TCP associated to mesenchymal stem cells from bone marrow and adipose tissue on spinal fusion in healthy and ovariectomized rat. J. Cell. Physiol. 2019, 234, 20046–20056. [Google Scholar] [CrossRef]
- Boanini, E.; Pagani, S.; Tschon, M.; Rubini, K.; Fini, M.; Bigi, A. Monetite vs. Brushite: Different influences on bone cell response modulated by strontium functionalization. J. Funct. Biomater. 2022, 13, 65. [Google Scholar] [CrossRef]
- Lampe, V.; Milobedzka, J. Studien über Curcumin. Ber. Dtsch. Chem. Ges. 1913, 46, 2235–2240. [Google Scholar] [CrossRef]
- Zheng, K.M.; Zhang, J.; Zhang, C.L.; Zhang, Y.W.; Chen, X.C. Curcumin inhibits appoptosin-induced apoptosis via upregulating heme oxygenase-1 expression in SH-SY5Y cells. Acta Pharmacol. Sin. 2015, 36, 544–552. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Shedge, A.A.; Pansare, S.V.; Khairkar, S.R.; Chhatre, S.Y.; Chakrabarti, S.; Nagarkar, A.A.; Pansare, A.V.; Patil, V.R. Nanocomposite of functional silver metal containing curcumin biomolecule model systems: Protein BSA bioavailability. J. Inorg. Biochem. 2020, 212, 111210. [Google Scholar] [CrossRef] [PubMed]
- Llano, S.; Gòmez, S.; Londoño, J.; Restrepo, A. Antioxidant activity of curcuminoids. Phys. Chem. Chem. Phys. 2019, 21, 3752–3760. [Google Scholar] [CrossRef] [PubMed]
- Daya, R.; Xu, C.; Thi Nguyen, N.Y.; Liu, H.H. Angiogenic hyaluronic acid hydrogels with curcumin-coated magnetic nanoparticles for tissue repair. ACS Appl. Mater. Interfaces 2022, 14, 11051–11067. [Google Scholar] [CrossRef]
- Rubini, K.; Boanini, E.; Parmeggiani, S.; Bigi, A. Curcumin-functionalized gelatin films: Antioxidant materials with modulated physico-chemical properties. Polymers 2021, 13, 1824. [Google Scholar] [CrossRef]
- Forte, L.; Torricelli, P.; Boanini, E.; Rubini, K.; Fini, M.; Bigi, A. Quercetin and alendronate multi-functionalized materials as tools to hinder oxidative stress damage. J. Biomed. Mater. Res. A 2017, 105, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium Orthophosphates (CaPO4): Occurrence and Properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef]
- Gourgas, O.; Khan, K.; Schwertani, A.; Cerruti, M. Differences in mineral composition and morphology between men and women in aortic valve calcification. Acta Biomater. 2020, 106, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Nihouannen, D.L.; Eimar, H.; Sheikh, Z.; Komarova, S.; Barralet, J. The effect of autoclaving on the physical and biological properties of dicalcium phosphate dehydrate bioceramics: Brushite vs. monetite. Acta Biomater. 2012, 8, 3161–3169. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A. Dicalcium phosphate anhydrous: An appropriate bioceramic in regeneration of critical-sized radial bone defects in rats. Calcif. Tissue Int. 2017, 101, 530–544. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, C.C.; Xu, L.; Wang, Q. Preparation of dicalcium phosphate anhydrous (monetite) biological coating on titanium by spray-drying method. Adv. Mater. Sci. Eng. 2017, 2017, 8281523. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound–Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef]
- Gambardella, A.; Marchiori, G.; Maglio, M.; Russo, A.; Rossi, C.; Visani, A.; Fini, M. Determination of the spatial anisotropy of the surface microstructures of different implant materials: An atomic force microscopy study. Materials 2021, 14, 4803. [Google Scholar] [CrossRef] [PubMed]
- Polo, L.; Diaz de Greñu, B.; Della Bella, E.; Pagani, S.; Torricelli, P.; Vivancos, J.L.; Ruiz-Rico, M.; Barat, J.M.; Aznar, E.; Martínez-Máñez, R.; et al. Antimicrobial activity of commercial calcium phosphate based materials functionalized with vanillin. Acta Biomater. 2018, 81, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Rubini, K.; Boanini, E.; Bigi, A. Role of aspartic and polyaspartic acid on the synthesis and hydrolysis of brushite. J. Funct. Biomater. 2019, 10, 11. [Google Scholar] [CrossRef]
- Catti, M.; Ferraris, G.; Filhol, A. Hydrogen bonding in the crystalline state. CaHPO4 (Monetite), P-1 or PI? A novel neutron diffraction study. Acta Crystallogr. 1977, 33, 1223–1229. [Google Scholar] [CrossRef]
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and hydrolysis of brushite (DCPD): The role of ionic substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Rubini, K.; Mazzeo, P.P.; Bigi, A. Structural interplay between strontium and calcium in α-CaHPO4 and β-SrHPO4. Ceram. Int. 2021, 47, 24412–24420. [Google Scholar] [CrossRef]
- Matlinska, M.A.; Wasylishen, R.E.; Bernard, G.M.; Terskikh, V.V.; Brinkmann, A.; Michaelis, V.K. Capturing elusive polymorphs of curcumin: A structural characterization and computational study. Cryst. Growth Des. 2018, 18, 5556–5563. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Berlier, G.; Gastaldi, L.; Ugazio, E.; Miletto, I.; Iliade, P.; Sapino, S. Stabilization of quercetin flavonoid in MCM-41 mesoporous silica: Positive effect of surface functionalization. J. Colloid. Interface Sci. 2013, 393, 109–118. [Google Scholar] [CrossRef]
- Mohan, R.; Birari, R.; Karmase, A.; Jagtap, S.; Bhutani, K.K. Antioxidant activity of a new phenolic glycoside from Lagenaria siceraria Stand. fruits. Food Chem. 2012, 132, 244–251. [Google Scholar] [CrossRef]
- Bontempi, M.; Salamanna, F.; Capozza, R.; Visani, A.; Fini, M.; Gambardella, A. Nanomechanical mapping of hard tissues by atomic force microscopy: An application to cortical bone. Materials 2022, 15, 7512. [Google Scholar] [CrossRef]
- Ajaxon, I.; Persson, C. Mechanical properties of brushite calcium phosphate cements. Front. Nanobiomed. Res. 2017, 9, 285–300. [Google Scholar]
- Notoya, M.; Nishimura, H.; Woo, J.T.; Nagai, K.; Ishihara, Y.; Hagiwara, H. Curcumin inhibits the proliferation and mineralization of cultured osteoblasts. Eur. J. Pharmacol. 2006, 534, 55–62. [Google Scholar] [CrossRef]
- Son, H.-E.; Kim, E.-J.; Jang, W.-G. Curcumin induces osteoblast differentiation through mild-endoplasmic reticulum stress-mediated such as BMP2 on osteoblast cells. Life Sci. 2018, 193, 34–39. [Google Scholar] [CrossRef]
- Gu, Q.; Cai, Y.; Huang, C.; Shi, Q.; Yang, H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn. Mag. 2012, 8, 202–208. [Google Scholar] [CrossRef]
- Xidaki, D.; Agrafioti, P.; Diomatari, D.; Kaminari, A.; Tsalavoutas-Psarras, E.; Alexiou, P.; Psycharis, V.; Tsilibary, E.C.; Silvestros, S.; Sagnou, M. Synthesis of hydroxyapatite, β-tricalcium phosphate and biphasic calcium phosphate particles to act as local delivery carriers of curcumin: Loading, release and in vitro studies. Materials 2018, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 78, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.M.; Roncero-Martin, R.; Rodriguez-Velasco, F.J.; Calderon-Garcia, J.F.; Rey-Sanchez, P.; Vera, V.; Canal-Macias, M.L.; Pedrera-Zamorano, J.D. Effects of curcumin on the proliferation and mineralization of human osteoblast-like cells: Implications of nitric oxide. Int. J. Mol. Sci. 2012, 13, 16104–16118. [Google Scholar] [CrossRef]
- Li, G.; Bu, J.; Zhu, Y.; Xiao, X.; Liang, Z.; Zhang, R. Curcumin improves bone microarchitecture in glucocorticoid-induced secondary osteoporosis mice through the activation of microRNA-365 via regulating MMP-9. Int. J. Clin. Exp. Pathol. 2015, 8, 15684–15695. [Google Scholar] [PubMed]
- Chen, Z.; Xue, J.; Shen, T.; Mu, S.; Fu, Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int. J. Mol. Med. 2016, 37, 329–338. [Google Scholar] [CrossRef]
- Moon, H.J.; Ko, W.K.; Han, S.W.; Kim, D.S.; Hwang, Y.S.; Park, H.K.; Kwon, I.K. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem. Biophys. Res. Commun. 2012, 418, 247–253. [Google Scholar] [CrossRef]
- Bharti, A.C.; Takada, Y.; Aggarwal, B.B. Curcumin (Diferuloylmethane) inhibits receptor activator of nf-κb ligand induced nf-κb activation in osteoclast precursors and suppresses osteoclastogenesis. J. Immunol. 2004, 172, 5940–5947. [Google Scholar] [CrossRef]
- Yeh, C.C.; Su, Y.H.; Lin, Y.J.; Chen, P.J.; Shi, C.S.; Chen, C.N.; Chang, H.I. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug. Des. Devel Ther. 2015, 9, 2285–2300. [Google Scholar] [CrossRef]
- Xiao, C.J.; Yu, X.J.; Xie, J.L.; Liu, S.; Li, S. Protective effect and related mechanisms of curcumin in rat experimental periodontitis. Head. Face Med. 2018, 14, 12. [Google Scholar] [CrossRef]
- Querido, W.; Rossi, A.L.; Farina, M. The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Review. Micron. 2016, 80, 122–134. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, K.; Yuan, X.; Zhang, X.; Qian, Y.; Cheng, T. Curcumin has immunomodulatory effects on RANKL-stimulated osteoclastogenesis in vitro and titanium nanoparticle-induced bone loss in vivo. J. Cell. Mol. Med. 2020, 24, 1553–1567. [Google Scholar] [CrossRef] [PubMed]











| Description of the Sample | Labels Used in Preliminary Tests | Labels Used in Complete Tests |
|---|---|---|
| Strontium-substituted DCPA (before loading-sample without curcumin) | SrDCPA | SA0 |
| Strontium-substituted DCPD (before loading-sample without curcumin) | SrDCPD | SD0 |
| SrDCPA in 3 mM curcumin solution for 6 h | SrDCPA3_6 h * | SA3 |
| SrDCPD in 3 mM curcumin solution for 6 h | SrDCPD3_6 h * | SD3 |
| SrDCPA in 5 mM curcumin solution for 72 h | SrDCPA5_72 h * | SA5 |
| SrDCPD in 5 mM curcumin solution for 72 h | SrDCPD5_72 h * | SD5 |
| Sample | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Sr Content (at%) |
|---|---|---|---|---|---|---|---|
| SrDCPA | 6.936 (5) | 6.644 (4) | 7.015 (5) | 96.1 | 104.1 | 88.5 | 11.2 |
| SrDCPD | 6.394 (3) | 15.239 (3) | 5.830 (4) | 90 | 118.4 | 90 | 9.4 |
| SA0 | SA3 | SA5 | SD0 | SD3 | SD5 | |
|---|---|---|---|---|---|---|
| Rs (nm) | 172 ± 58 | 120 ± 30 | 149 ± 22 | 86 ± 35 | 87 ± 13 | 106 ± 11 |
| hmax (nm) | 25.5 ± 6.6 | 27.3 ± 7.9 | 31.7 ± 9.2 | 26.6 ± 6.6 | 28.0 ± 10.2 | 30.5 ± 10.5 |
| ηel | 0.654 ± 0.127 | 0.572 ± 0.188 | 0.579 ± 0.173 | 0.730 ± 0.106 | 0.778 ± 0.094 | 0.616 ± 0.158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silingardi, F.; Pagani, S.; Gambardella, A.; Giavaresi, G.; Bigi, A.; Boanini, E. Anti-Oxidant Multi-Functionalized Materials: Strontium-Substituted Monetite and Brushite as Delivery Systems for Curcumin. Pharmaceutics 2023, 15, 1344. https://doi.org/10.3390/pharmaceutics15051344
Silingardi F, Pagani S, Gambardella A, Giavaresi G, Bigi A, Boanini E. Anti-Oxidant Multi-Functionalized Materials: Strontium-Substituted Monetite and Brushite as Delivery Systems for Curcumin. Pharmaceutics. 2023; 15(5):1344. https://doi.org/10.3390/pharmaceutics15051344
Chicago/Turabian StyleSilingardi, Francesca, Stefania Pagani, Alessandro Gambardella, Gianluca Giavaresi, Adriana Bigi, and Elisa Boanini. 2023. "Anti-Oxidant Multi-Functionalized Materials: Strontium-Substituted Monetite and Brushite as Delivery Systems for Curcumin" Pharmaceutics 15, no. 5: 1344. https://doi.org/10.3390/pharmaceutics15051344