Influence of Intramuscular Injection Sites on Pharmacokinetics of Amoxicillin in Olive Flounder (Paralichthys olivaceus) and Its Implication for Antibacterial Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drug and Chemicals
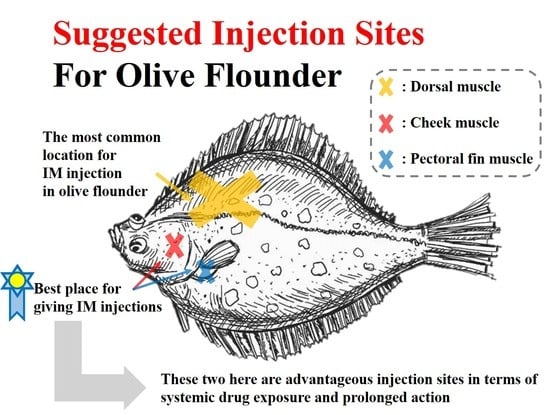
2.2. Experimental Animals
2.3. Experimental Design
2.4. HPLC-MS/MS and Sample Preparation
2.5. Pharmacokinetic Analysis
2.6. Pharmacokinetic/Pharmacodynamic Relationships
2.7. Statistical Analysis
3. Results
3.1. Serum Pharmacokinetics
3.2. Pharmacokinetic/Pharmacodynamic Relationships
3.3. Muscle Residue Depletion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.H.; Seo, J.S.; Kim, G.W.; Kwon, M.G.; Kim, D.H.; Park, C.I.; Kim, K.T.; Park, J. Effect of lincomycin, an injectable lincosamide antibiotic, against streptococcosis in cultured olive flounder Paralichthys olivaceus and its pharmacokinetic-pharmacodynamic profile. Aquaculture 2022, 548, 73766. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, G.W.; Kwon, M.G.; Seo, J.S. Pharmacokinetic-Pharmacodynamic Profile, Bioavailability, and Withdrawal Time of Tylosin Tartrate Following a Single Intramuscular Administration in Olive Flounder (Paralichthys olivaceus). Animals 2021, 11, 2468. [Google Scholar] [CrossRef] [PubMed]
- NFQS. Available online: https://www.nfqs.go.kr/apms/ebook/mice_ebook/index.html#page=1 (accessed on 25 February 2023).
- Lim, J.W.; Jung, M.H.; Jung, S.J.; Kim, D.H.; Park, K.H.; Kang, S.Y. The efficacy of amoxicillin sodium against streptococcosis in cultured olive flounder Paralichthys olivaceus and its pharmacokinetics. J. Vet. Pharmacol. Ther. 2017, 40, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Awji, E.G.; Suh, J.W.; Park, S.C. Pharmacokinetics, pharmacokinetic–pharmacodynamic relationship, and withdrawal period of amoxicillin sodium in olive flounder (Paralichthys olivaceus). Xenobiotica 2016, 46, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Jeon, E.J.; Jung, S.H.; Park, M.A.; Kim, N.Y. Pharmacokinetics of amoxicillin trihydrate in cultured olive flounder (Paralichthys olivaceus). J. Vet. Pharmacol. Ther. 2015, 38, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Baggot, J.D. The bioavailability and disposition of antimicrobial agents in neonatal animals. In The Physiological Basis of Veterinary Clinical Pharmacology, 1st ed.; Blackwell Publishing Sciences Ltd.: Oxford, UK, 2008; pp. 252–266. [Google Scholar]
- Horsberg, T.E. Experimental methods for pharmacokinetic studies in salmonids. Annu. Rev. Fish Dis. 1994, 4, 345–358. [Google Scholar] [CrossRef]
- Kleinow, K.M.; James, M.O.; Lech, J.J. Drug pharmacokinetics and metabolism in food-producing fish and crustaceans: Methods and examples. Xenobiot. Food-Prod. Anim. 1992, 8, 98–130. [Google Scholar] [CrossRef]
- MFDS. Available online: https://residue.foodsafetykorea.go.kr/vd/analysis (accessed on 26 February 2023).
- FDA. Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/media/70858/download (accessed on 26 February 2023).
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lees, P.; Pelligand, L.; Illambas, J.; Potter, T.; Lacroix, M.; Rycroft, A.; Toutain, P.L. Pharmacokinetic/pharmacodynamic integration and modelling of amoxicillin for the calf pathogens Mannheimia haemolytica and Pasteurella multocida. J. Vet. Pharmacol. Ther. 2015, 38, 457–470. [Google Scholar] [CrossRef] [PubMed]
- MFDS. Available online: https://residue.foodsafetykorea.go.kr/vd/mrl (accessed on 26 February 2023).
- Song, I.B.; Kim, T.W.; Lee, H.G.; Kim, M.S.; Hwang, Y.H.; Park, B.K.; Lim, J.H.; Yun, H.I. Influence of the injection site on the pharmacokinetics of cefquinome following intramuscular injection in piglets. J. Vet. Med. Sci. 2013, 75, 89–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delis, G.; Batzias, G.; Theodosiadou, E.; Kounenis, G.; Koutsoviti-Papadopoulou, M. Influence of the injection site on the pharmacokinetics of amoxicillin after intramuscular administration of a conventional and a long-acting formulation in sheep. J. Vet. Pharmacol. Ther. 2009, 32, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Firth, E.C.; Nouws, J.F.; Driessens, F.; Schmaetz, P.; Peperkamp, K.; Klein, W.R. Effect of the injection site on the pharmacokinetics of procaine penicillin G in horses. Am. J. Vet. Res. 1986, 47, 2380–2384. [Google Scholar] [PubMed]
- Abo-El-Sooud, K.; Swielim, G.A.; El-Gammal, S.M.; Ramsis, M.N. Comparative Pharmacokinetics and bioavailability of marbofloxacin in geese (Anser Anser domesticus) after two sites of intramuscular administrations. J. Vet. Pharmacol. Ther. 2020, 43, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, J.Y.; Kim, J.W.; Kim, H.C.; Noh, J.K.; Kim, Y.O.; Hwang, H.K.; Kim, W.J.; Yeo, S.Y.; An, C.M.; et al. CRISPR/Cas9-mediated myostatin disruption enhances muscle mass in the olive flounder Paralichthys olivaceus. Aquaculture 2019, 512, 734336. [Google Scholar] [CrossRef]
- Cassens, R.G.; Cooper, C.C. Red and white muscle. Adv. Food Res. 1971, 19, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.D. The effect of exercise on the distribution of blood to various organs in rainbow trout. Comp. Biochem. Physiol. 1968, 25, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Harder, W.; Sokoloff, S. Anatomy of Fishes; Schweizerbart: Stuttgart, Germany, 1976; ISBN 978-3-510-65067-5. [Google Scholar]
- Papich, M.G. Pharmacokinetic–pharmacodynamic (PK–PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Vet. Microbiol. 2014, 171, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.R. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 2001, 7, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| PK Parameters | Unit | Dorsal IM | Cheek IM | Pectoral Fin IM |
|---|---|---|---|---|
| λz | h−1 | 0.08 | 0.07 | 0.07 |
| t1/2λz | h | 8.89 | 10.12 | 10.33 |
| Tmax | h | 3.00 | 3.00 | 3.00 |
| Cmax * | µg/mL | 202.79 ± 24.88 | 203.96 ± 58.26 | 229.59 ± 26.70 |
| AUC0–24 | µg/mL·h | 1227.19 | 1618.71 | 1540.42 |
| AUC0-t | µg/mL·h | 1697.23 | 2006.71 | 1846.61 |
| AUC0-inf | µg/mL·h | 1706.42 | 2029.39 | 1854.69 |
| AUMC0-inf | µg/mL·h2 | 24,469.78 | 24,582.22 | 19,622.66 |
| MRT0-inf | h | 14.34 | 12.11 | 10.58 |
| PK/PD Parameters | Dorsal IM | Cheek IM | Pectoral Fin IM |
|---|---|---|---|
| Streptococcus iniae | |||
| Cmax/MIC90 | 6499.68 | 6537.18 | 7358.65 |
| AUC0–24/MIC90 (h) | 39,333.01 | 51,881.73 | 49,372.44 |
| AUC0-t/MIC90 (h) | 54,398.40 | 64,317.63 | 59,186.22 |
| AUC0-inf/MIC90 (h) | 54,692.95 | 65,044.55 | 59,445.19 |
| T > MIC90 (h) | 111.61 | 132.37 | 118.28 |
| Streptococcus parauberis | |||
| Cmax/MIC90 | 405.58 | 407.92 | 459.18 |
| AUC0–24/MIC90 (h) | 2454.38 | 3237.42 | 3080.84 |
| AUC0-t/MIC90 (h) | 3394.46 | 4013.42 | 3693.22 |
| AUC0-inf/MIC90 (h) | 3412.84 | 4058.78 | 3709.38 |
| T > MIC90 (h) | 76.05 | 85.35 | 76.87 |
| Time (day) | Amoxicillin Concentration (mg/kg) | ||
|---|---|---|---|
| Dorsal IM | Cheek IM | Pectoral Fin IM | |
| 1 | 1.28 ± 0.14 (7/7) | 2.20 ± 1.17 (7/7) | 1.04 ± 0.45 (7/7) |
| 2 | 0.27 ± 0.12 (7/7) | 0.24 ± 0.09 (7/7) | 0.18 ± 0.06 (7/7) |
| 3 | 0.06 ± 0.03 (5/7) | 0.11 ± 0.04 (6/7) | 0.08 ± 0.03 (6/7) |
| 7 | Not detected | Not detected | 0.01 ± 0.01 (0/7) |
| 20 | Not detected | 0.01 ± 0.02 (0/7) | Not detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kim, G.-W.; Kang, H.-W.; Hong, J.-W.; Lee, H.-E.; Kwon, M.-G.; Seo, J.-S. Influence of Intramuscular Injection Sites on Pharmacokinetics of Amoxicillin in Olive Flounder (Paralichthys olivaceus) and Its Implication for Antibacterial Efficacy. Pharmaceutics 2023, 15, 1153. https://doi.org/10.3390/pharmaceutics15041153
Lee J-H, Kim G-W, Kang H-W, Hong J-W, Lee H-E, Kwon M-G, Seo J-S. Influence of Intramuscular Injection Sites on Pharmacokinetics of Amoxicillin in Olive Flounder (Paralichthys olivaceus) and Its Implication for Antibacterial Efficacy. Pharmaceutics. 2023; 15(4):1153. https://doi.org/10.3390/pharmaceutics15041153
Chicago/Turabian StyleLee, Ji-Hoon, Ga-Won Kim, Hyun-Woo Kang, Joo-Won Hong, Hyo-Eun Lee, Mun-Gyeong Kwon, and Jung-Soo Seo. 2023. "Influence of Intramuscular Injection Sites on Pharmacokinetics of Amoxicillin in Olive Flounder (Paralichthys olivaceus) and Its Implication for Antibacterial Efficacy" Pharmaceutics 15, no. 4: 1153. https://doi.org/10.3390/pharmaceutics15041153