Ciprofloxacin-Loaded Mixed Polymeric Micelles as Antibiofilm Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cationic Triblock Copolymer
2.1.2. Preparation of Polymeric Micelles
2.1.3. Loading of Polymeric Micelles with Ciprofloxacin
2.1.4. In Vitro Release of CF
2.2. Methods
2.2.1. Dynamic and Electrophoretic Light Scattering
2.2.2. Determination of Critical Micellar Concentration
2.2.3. High-Performance Liquid Chromatography
2.2.4. UV–VIS Spectrophotometry
2.2.5. Biofilm Experiments
2.2.6. Biofilm Metabolic Activity
2.2.7. Cytotoxicity of the Micelles
3. Results and Discussion
3.1. Preparation of Mixed Polymeric Micelles
3.2. Loading of Micelles with CF
3.3. CF In Vitro Release
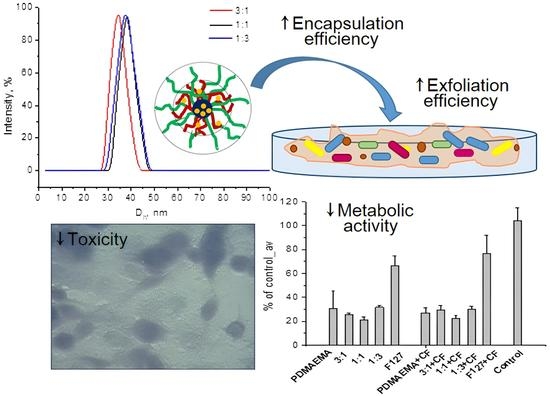
3.4. Effects of the Micelles on the Biofilm Biomass and Metabolic Activity
3.5. Cytotoxicity and Cellular Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schönborn, S.; Krömker, V. Detection of the biofilm component polysaccharide intercellular adhesin in Staphylococcus aureus infected cow udders. Vet. Microbiol. 2016, 196, 126–128. [Google Scholar] [CrossRef]
- Wood, T.K. Biofilm dispersal: Deciding when it is better to travel. Mol. Microbiol. 2014, 94, 747–750. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Cornel, E.J.; Du, J. Advances and Prospects of Polymeric Particles for the Treatment of Bacterial Biofilms. ACS Appl. Polym. Mater. 2021, 3, 2218–2232. [Google Scholar] [CrossRef]
- Bi, Y.; Xia, G.; Shi, C.; Wan, J.; Liu, L.; Chen, Y.; Wu, Y.; Zhang, W.; Zhou, M.; He, H.; et al. Therapeutic strategies against bacterial biofilms. Fundam. Res. 2021, 1, 193–212. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Assefa, M.; Amare, A. Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review. Infect. Drug Resist. 2022, 15, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C. Biofouling and me: My Stockholm syndrome with biofilms. Water. Res. 2020, 173, 115576. [Google Scholar] [CrossRef] [PubMed]
- Stoitsova, S.; Paunova-Krasteva, T.; Dimitrova, P.D.; Damyanova, T. The concept for the antivirulence therapeutics approach as alternative to antibiotics: Hope or still a fiction? Biotechnol. Biotechnol. 2022, 36, 697–705. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Z.; Sun, G.; Su, H.; Liu, J.; Hu, B.; Zhou, X.; Lou, L. Formation of biofilms from new pipelines at both ends of the drinking water distribution system and comparison of disinfection by-products formation potential. Environ. Res. 2020, 182, 109150. [Google Scholar] [CrossRef]
- Afonso, T.B.; Simões, L.C.; Lima, N. Occurrence of filamentous fungi in drinking water: Their role on fungal-bacterial biofilm formation. Res. Microbiol. 2021, 172, 103791. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, C.; Shi, B. The control of red water occurrence and opportunistic pathogens risks in drinking water distribution systems: A review. J. Environ. Sci. 2021, 110, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sharan, M.; Vijay, D.; Dhaka, P.; Bedi, J.S.; Gill, J.P.S. Biofilms as a microbial hazard in the food industry: A scoping review. J. Appl. Microbiol. 2022, 133, 2210–2234. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Liu, M.; Xie, Z.; Chen, X.; Li, G.; Wang, X. Targeted Anti-Biofilm Therapy: Dissecting Targets in the Biofilm Life Cycle. Pharmaceuticals 2022, 15, 1253. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.-T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.U. Nanobiotics against antimicrobial resistance: Harnessing the power of nanoscale materials and technologies. J. Nanobiotechnol. 2022, 20, 375. [Google Scholar] [CrossRef]
- Lv, X.; Wang, L.; Mei, A.; Xu, Y.; Ruan, X.; Wang, W.; Shao, J.; Yang, D.; Dong, X. Recent Nanotechnologies to Overcome the Bacterial Biofilm Matrix Barriers. Small 2023, 19, 2206220. [Google Scholar] [CrossRef]
- Hu, Y.; Ruan, X.; Lv, X.; Xu, Y.; Wang, W.; Cai, Y.; Ding, M.; Dong, H.; Shao, J.; Yang, D.; et al. Biofilm microenvironment-responsive nanoparticles for the treatment of bacterial infection. Nano Today 2022, 46, 101602. [Google Scholar] [CrossRef]
- Xiu, W.; Shan, J.; Yang, K.; Xiao, H.; Yuwen, L.; Wang, L. Recent development of nanomedicine for the treatment of bacterial biofilm infections. VIEW 2021, 2, 20200065. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Shi, L. Controlled drug delivery systems in eradicating bacterial biofilm-associated infections. J. Control. Release 2021, 329, 1102–1116. [Google Scholar] [CrossRef]
- Caço, A.I.; Varanda, F.; Pratas de Melo, M.J.; Dias, A.M.A.; Dohrn, R.; Marrucho, I.M. Solubility of Antibiotics in Different Solvents. Part II. Non-Hydrochloride Forms of Tetracycline and Ciprofloxacin. Ind. Eng. Chem. Res. 2008, 47, 8083–8089. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Jain, S.; Pahwa, R.; Yar, M.S. Ciprofloxacin: Review on developments in synthetic, analytical, and medicinal aspects. J. Enzyme Inhib. Med. Chem. 2010, 25, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric micelles for nano-scale drug delivery. React. Funct. Polym. 2011, 71, 227–234. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Zhu, Y.H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef]
- Toscanini, M.A.; Limeres, M.J.; Garrido, A.V.; Cagel, M.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A.; Cuestas, M.L. Polymeric micelles and nanomedicines: Shaping the future of next generation therapeutic strategies for infectious diseases. J. Drug Deliv. Sci. Technol. 2021, 66, 102927. [Google Scholar] [CrossRef]
- Chen, M.; Wei, J.; Xie, S.; Tao, X.; Zhang, Z.; Ran, P.; Li, X. Bacterial biofilm destruction by size/surface charge-adaptive micelles. Nanoscale 2019, 11, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Liu, Q.; Du, J. Antibacterial polymeric nanostructures for biomedical applications. Chem. Commun. 2014, 50, 14482–14493. [Google Scholar] [CrossRef]
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial polymeric nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Kuroda, K.; Caputo, G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 49–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milović, N.M.; Wang, J.; Lewis, K.; Klibanov, A.M. Immobilized N-alkylated polyethylenimine avidly kills bacteria by rupturing cell membranes with no resistance developed. Biotechnol. Bioeng. 2005, 90, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, D.; Luo, W.; Liu, Y.; Zhu, M.; Li, X.; Liu, M. Functionalization of chitosan viasingle electron transfer living radical polymerization in an ionic liquid andits antimicrobial activity. J. Appl. Polym. Sci. 2015, 132, 42754. [Google Scholar] [CrossRef]
- Yuan, W.; Wei, J.; Lu, H.; Fan, L.; Du, J. Water-dispersible and biodegradable polymer micelles with good antibacterial efficacy. Chem. Commun. 2012, 48, 6857–6859. [Google Scholar] [CrossRef]
- Gao, Y.F.; Wang, J.; Chai, M.Y.; Li, X.; Deng, Y.Y.; Jin, Q.; Ji, J. Size and Charge Adaptive Clustered Nanoparticles Targeting the Biofilm Microenvironment for Chronic Lung Infection Management. ACS Nano 2020, 14, 5686–5699. [Google Scholar] [CrossRef]
- Wen, Y.; Tan, Z.; Sun, F.; Sheng, L.; Zhang, X.; Yao, F. Synthesis and characterization of quaternized carboxymethyl chitosan/poly(amidoamine) dendrimer core-shell nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Leng, M.; Hu, S.; Lu, A.; Cai, M.; Luo, X. The anti-bacterialpoly(caprolactone)-poly(quaternary ammonium salt) as drug delivery carriers. Appl. Microbiol. Biotechnol. 2016, 100, 3049–3059. [Google Scholar] [CrossRef]
- Lenoir, S.; Pagnoulle, C.; Detrembleur, C.; Galleni, M.; Jerome, R. New antibacterialcationic surfactants prepared by atom transfer radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1214–1224. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Y.; Li, Y.; Su, L.; Zhang, Y.; Huang, F.; Liu, J.; Liu, J.; van Kooten, T.G.; An, Y.; et al. Nanocarriers with conjugated antimicrobials to eradicate pathogenic biofilms evaluated in murine in vivo and human ex vivo infection models. Acta Biomater. 2018, 79, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Northeved, H.; Kumar, P.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, A.B.E.; Ong, Z.Y.; Hedrick, J.L.; Lee, P.P.; Ee, P.R.L.; Hammond, P.T.; Yang, Y.-Y. Mixed micelles self-assembled from block copolymers for drug delivery. Curr. Opin. Colloid Interface Sci. 2011, 16, 182–194. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Europ. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Kamenova, K.; Haladjova, E.; Grancharov, G.; Kyulavska, M.; Tzankova, V.; Aluani, D.; Yoncheva, K.; Pispas, S.; Petrov, P. Co-assembly of block copolymers as a tool for developing novel micellar carriers of insulin for controlled drug delivery. Eur. Polym. J. 2018, 104, 1–9. [Google Scholar] [CrossRef]
- Borisova, D.; Haladjova, E.; Kyulavska, M.; Petrov, P.; Pispas, S.; Stoitsova, S.; Paunova-Krasteva, T. Application of cationic polymer micelles for the dispersal of bacterial biofilms. Eng. Life Sci. 2018, 18, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Paunova-Krasteva, T.; Haladjova, E.; Petrov, P.; Forys, A.; Trzebicka, B.; Topouzova-Hristova, T.; Stoitsova, S. Destruction of Pseudomonas aeruginosa pre-formed biofilms by cationic polymer micelles bearing silver nanoparticles. Biofouling 2020, 36, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef] [Green Version]
- Haladjova, E.; Kyulavska, M.; Doumanov, J.; Topouzova-Hristova, T.; Petrov, P. Polymeric vehicles for transport and delivery of DNA via cationic micelle template method. Coll. Polymer Sci. 2017, 259, 2197–2205. [Google Scholar] [CrossRef]
- Kamenova, K.; Trzebicka, B.; Momekova, D.; Petrov, P. Double stimuli responsive mixed aggregates from poly(acrylic acid)-block-poly(ε-caprolactone)-block-poly(acrylic acid) and poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) triblock copolymers. Polym. Bull. 2017, 74, 707–720. [Google Scholar] [CrossRef]
- Xiong, X.-B.; Falamarzian, A.; Garg, S.M.; Lavasanifar, A. Engineering of amphiphilic block copolymers for polymeric micellar drug and gene delivery. J. Control. Release 2011, 155, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Tam, K.C.; Gan, L.H. Synthesis and thermally responsive properties of novel Pluronic F87/polycaprolactone (PCL) block copolymers with short PCL blocks. J. Appl. Polym. Sci. 2006, 100, 4163–4172. [Google Scholar] [CrossRef]
- Grancharov, G.; Atanasova, M.D.; Aluani, D.; Yoncheva, K.; Tzankova, V.; Trusheva, B.; Forys, A.; Trzebicka, B.; Petrov, P. Functional block copolymers bearing pendant cinnamyl groups for enhanced solubilization of caffeic acid phenethyl ester. Polym. J. 2020, 52, 435–447. [Google Scholar] [CrossRef]
- Wu, Y.; Lv, S.; Li, Y.; He, H.; Ji, H.; Zheng, M.; Liu, Y.; Yin, L. Co-delivery of dual chemo-drugs with precisely controlled, high drug loading polymeric micelles for synergistic anti-cancer therapy. Biomater. Sci. 2020, 8, 949–959. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, P. Reduction-responsive core–shell–corona micelles based on triblock copolymers: Novel synthetic strategy, characterization, and application as a tumor microenvironment-responsive drug delivery system. ACS Appl. Mater. Interfaces 2015, 7, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wu, Y.; Cai, K.; He, H.; Li, Y.; Lan, M.; Chen, X.; Cheng, J.; Yin, L. High Drug Loading and Sub-Quantitative Loading Efficiency of Polymeric Micelles Driven by Donor–Receptor Coordination Interactions. J. Am. Chem. Soc. 2018, 140, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, A.J.; Bruland, G.L.; MacKay, A.A.; Vasudevan, D. Sorption of ciprofloxacin and oxytetracycline zwitterions to soils and soil minerals: Influence of compound structure. Environ. Sci. Technol. 2008, 42, 7634–7642. [Google Scholar] [CrossRef] [PubMed]
- Bonkovoski, L.C.; Martins, A.F.; Bellettini, I.C.; Garcia, F.P.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Polyelectrolyte complexes of poly[(2-dimethylamino) ethyl methacrylate]/chondroitin sulfate obtained at different pHs: I. Preparation, characterization, cytotoxicity and controlled release of chondroitin sulfate. Intern. J. Pharm. 2014, 477, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haladjova, E.; Stancheva, R.; Pispas, S.; Rangelov, S. Effect of concentration on the physicochemical properties and drug release profile of cationic block copolymer aggregates. C. R. Acad. Bulg. Sci. 2021, 74, 1749–1756. [Google Scholar]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. Curr. Top. Microbiol. Immunol. 2017, 404, 1–44. [Google Scholar]
- Sajid, M.; Akash, H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar]
- Batrakova, E.; Li, S.; Brynskikh, A.; Sharma, A.; Li, Y.; Boska, M.; Gong, N.; Lee Mosley, R.; Alakhov, V.Y.; Gendelman, H.; et al. Effects of pluronic and doxorubicin on drug uptake, cellular metabolism, apoptosis and tumor inhibition in animal models of MDR cancers. J. Control. Release 2010, 143, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Griffin, W.C. Calculation of HLB Values of Non-Ionic Surfactants. J. Soc. Cosmet. Chem. 1954, 5, 259. [Google Scholar]











| Micellar Composition | CMC, mg·mL−1 | ζ Potential, mV | Dh, nm |
|---|---|---|---|
| PDMAEMA | 0.056 | 29.1 ± 5.2 | 33.2 ± 3.1 |
| 3:1 | 0.091 | 25.9 ± 6.3 | 34.6 ± 2.3 |
| 1:1 | 0.124 | 17.6 ± 5.6 | 37.1 ± 2.8 |
| 1:3 | 0.131 | 15.2 ± 6.1 | 36.4 ± 2.6 |
| F127 | 0.161 | −4.9 ± 5.9 | 14.9 ± 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancheva, R.; Paunova-Krasteva, T.; Topouzova-Hristova, T.; Stoitsova, S.; Petrov, P.; Haladjova, E. Ciprofloxacin-Loaded Mixed Polymeric Micelles as Antibiofilm Agents. Pharmaceutics 2023, 15, 1147. https://doi.org/10.3390/pharmaceutics15041147
Stancheva R, Paunova-Krasteva T, Topouzova-Hristova T, Stoitsova S, Petrov P, Haladjova E. Ciprofloxacin-Loaded Mixed Polymeric Micelles as Antibiofilm Agents. Pharmaceutics. 2023; 15(4):1147. https://doi.org/10.3390/pharmaceutics15041147
Chicago/Turabian StyleStancheva, Rumena, Tsvetelina Paunova-Krasteva, Tanya Topouzova-Hristova, Stoyanka Stoitsova, Petar Petrov, and Emi Haladjova. 2023. "Ciprofloxacin-Loaded Mixed Polymeric Micelles as Antibiofilm Agents" Pharmaceutics 15, no. 4: 1147. https://doi.org/10.3390/pharmaceutics15041147