pH-Responsive Super-Porous Hybrid Hydrogels for Gastroretentive Controlled-Release Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SPHHs
2.3. Drying of SPHHs
2.4. Drug (Amoxicillin Trihydrate) Loading of SPHHs-AT
2.5. pH-Responsive Swelling of SPHHs-AT
2.6. Density of SPHHs
2.7. Gelation Kinetics of SPHHs
2.8. Mechanical Stability of SPHHs
2.9. SEM
2.10. FTIRATR, X- RD and DSC
2.11. Drug Content and In Vitro Drug Release
3. Results and Discussion
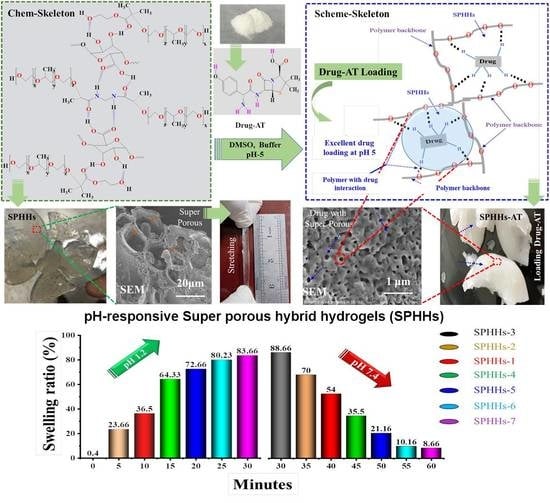
3.1. SPHHs and Swelling Studies
3.2. Density and Gelation Kinetics of SPHHs
3.3. Mechanical Performance of SPHHs
3.4. SPHHs’ Morphology
3.5. Comprehensive Study of SPHHs and SPHHs-AT
3.6. Drug Loading and Release Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karoyo, A.; Wilson, L. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Park, K. Superporous Hydrogels for Drug Delivery Systems. In Faculty Books and Book Chapters 15, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 688–704. [Google Scholar] [CrossRef]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, H.; Zhang, X.; Ma, Q.; Teng, L.; Qiu, L. Hydrosoluble collagen based biodegradable hybrid hydrogel for biomedical scaffold. J. Biomater. Sci. Polym. Ed. 2020, 31, 2199–2219. [Google Scholar] [CrossRef]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.; Thapa, P.; Maharjan, R.; Jeong, S.H. Current State and Future Perspectives on Gastroretentive Drug Delivery Systems. Pharmaceutics 2019, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Panteli, P.A.; Patrickios, C.S. Multiply Interpenetrating Polymer Networks: Preparation, Mechanical Properties, and Applications. Gels 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, H.; Alam, M.M.; Rahman, M.A.; Minami, H.; Gafur, M.A. Epoxide Functional Temperature-Sensitive Semi-IPN Hydrogel Microspheres for Isolating Inorganic Nanoparticles. Adv. Polym. Technol. 2018, 37, 94–103. [Google Scholar] [CrossRef]
- Chen, S.; Matsumoto, H.; Moro-Oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart Microneedle Fabricated with Silk Fibroin Combined Semi-interpenetrating Network Hydrogel for Glucose-Responsive Insulin Delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar] [CrossRef]
- Kameshwar, A.K.S.; Qin, W. Structural and functional properties of pectin and lignin–carbohydrate complexes de-esterases: A review. Bioresour. Bioprocess. 2018, 5, 43. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S.; et al. Dual pH-Responsive Hydrogel Actuator for Lipophilic Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef]
- Haleem, A.; Syaal, S.B.; Ajmal, M.; Ambreen, J.; Rauf, S.; Ali, N.; Muhammad, S.; Shah, A.; Zia, M.A.; Siddiq, M. Silver and palladium nanoparticle embedded poly(n-isopropylacrylamide-co-2-acrylamido-2-methylpropane sulfonic acid) hybrid microgel catalyst with pH and temperature dependent catalytic activity. Korean J. Chem. Eng. 2020, 37, 614–622. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Wu, W.; Zhou, S.; Siddiq, M. Engineering of Phenylboronic Acid Based Glucose-Sensitive Microgels with 4-Vinylpyridine for Working at Physiological pH and Temperature. Macromol. Chem. Phys. 2011, 212, 1510–1514. [Google Scholar] [CrossRef]
- Siddiq, M.; Bakhat, K.; Ajmal, M. Stimuli responsive microgel containing silver nanoparticles with tunable optical and catalytic properties. Pure Appl. Chem. 2020, 92, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Wen, G. Development history and synthesis of super-absorbent polymers: A review. J. Polym. Res. 2020, 27, 136. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Bingol, H.B.; Agopcan-Cinar, S.; Bal, T.; Oran, D.C.; Kizilel, S.; Kayaman-Apohan, N.; Avci, D. Stimuli-responsive poly(hydroxyethyl methacrylate) hydrogels from carboxylic acid-functionalized crosslinkers. J. Biomed. Mater. Res. Part A 2019, 107, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, D.; Zhang, T.; Wang, J.; Han, S.; Graham, D.Y.; Lu, H. PPI-amoxicillin dual therapy for Helicobacter pylori infection: An update based on a systematic review and meta-analysis. Helicobacter 2020, 25, e12692. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Li, L.; Zhang, C.; Tu, J.; Geng, X.; Wang, J.; Zhou, X.; Jing, J.; Pan, W. Systematic Review with Meta-analysis: Association of Helicobacter pylori Infection with Esophageal Cancer. Gastroenterol. Res. Pract. 2019, 2019, 1953497. [Google Scholar] [CrossRef] [Green Version]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Control Release 2005, 102, 3–12. [Google Scholar] [CrossRef]
- Desu, P.K.; Pasam, V.; Kotra, V. Implications of superporous hydrogel composites-based gastroretentive drug delivery systems with improved biopharmaceutical performance of fluvastatin. J. Drug Deliv. Sci. Technol. 2020, 57, 101668. [Google Scholar] [CrossRef]
- Mazi, H.; Surmelihindi, B. Temperature and Ph-sensıtıve Super absorbent Polymers based on Modıfıed Maleıc Anhydrıde. J. Chem. Sci. 2021, 133, 10. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K.; Kandalam, U.; Rocca, J.G. Swelling and Mechanical Properties of Modified HEMA-based Superporous Hydrogels. J. Bioact. Compat. Polym. 2010, 25, 483–497. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Elastic, Superporous Hydrogel Hybrids of Polyacrylamide and Sodium Alginate. Macromol. Biosci. 2006, 6, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Gulfam, M.; Jo, S.-H.; Park, S.-H.; Lim, K.T. Injectable and biocompatible alginate-derived porous hydrogels cross-linked by IEDDA click chemistry for reduction-responsive drug release application. Carbohydr. Polym. 2022, 278, 118964. [Google Scholar] [CrossRef]
- Hobiger, V.; Zahoranova, A.; Baudis, S.; Liska, R.; Krajnc, P. Thiol–Ene Cross-Linking of Poly(ethylene glycol) within High Internal Phase Emulsions: Degradable Hydrophilic PolyHIPEs for Controlled Drug Release. Macromolecules 2021, 54, 10370–10380. [Google Scholar] [CrossRef] [PubMed]
- Mastropietro, D.J.; Omidian, H.; Park, K. Drug delivery applications for superporous hydrogels. Expert Opin. Drug Deliv. 2012, 9, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Griveau, L.; Lafont, M.; le Goff, H.; Drouglazet, C.; Robbiani, B.; Berthier, A.; Sigaudo-Roussel, D.; Latif, N.; Le Visage, C.; Gache, V.; et al. Design and characterization of an in vivo injectable hydrogel with effervescently generated porosity for regenerative medicine applications. Acta Biomater. 2022, 140, 324–337. [Google Scholar] [CrossRef]
- El-Dib, F.; Eshaq, G.; ElMetwally, A.; Hefni, H.H. Enhancing the porous structure of swellable poly(acrylic acid-co-acrylamide) crosslinked by N-Maleyl chitosan via introducing foaming agents and non-ionic surfactant. Adv. Ind. Eng. Polym. Res. 2021, 4, 9–18. [Google Scholar] [CrossRef]
- Gümüşderelioğlu, M.; Erce, D.; Demirtaş, T.T. Superporous polyacrylate/chitosan IPN hydrogels for protein delivery. J. Mater. Sci. Mater. Med. 2011, 22, 2467–2475. [Google Scholar] [CrossRef]
- Maiti, S.; Khillar, P.S.; Mishra, D.; Nambiraj, N.A.; Jaiswal, A.K. Physical and self–crosslinking mechanism and characterization of chitosan-gelatin-oxidized guar gum hydrogel. Polym. Test. 2021, 97, 107155. [Google Scholar] [CrossRef]
- Radić, M.M.B.; Filipović, V.V.; Vukomanović, M.; Runić, J.N.; Tomić, S.L. Degradable 2-Hydroxyethyl Methacrylate/Gelatin/Alginate Hydrogels Infused by Nanocolloidal Graphene Oxide as Promising Drug Delivery and Scaffolding Biomaterials. Gels 2021, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Filipović, V.V.; Radić, M.M.B.; Vuković, J.S.; Vukomanović, M.; Rubert, M.; Hofmann, S.; Müller, R.; Tomić, S.L. Biodegradable Hydrogel Scaffolds Based on 2-Hydroxyethyl Methacrylate, Gelatin, Poly(β-amino esters), and Hydroxyapatite. Polymers 2021, 14, 18. [Google Scholar] [CrossRef]
- Carreño, G.; Pereira, A.; Ávila-Salas, F.; Marican, A.; Andrade, F.; Roca-Melendres, M.M.; Valdés, O.; Vijayakumar, S.; Schwartz, S.; Abasolo, I.; et al. Development of “on-demand” thermo-responsive hydrogels for anti-cancer drugs sustained release: Rational design, in silico prediction and in vitro validation in colon cancer models. Mater. Sci. Eng. C 2021, 131, 112483. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, M.; Singh, S.; Mishra, D. Synthesis characterization and in vitro drug release from acrylamide and sodium alginate based superporous hydrogel devices. Int. J. Pharm. Investig. 2013, 3, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.; Kim, D.; Hu, Y.; Kim, Y.; Hong, I.K.; Kim, M.S.; Jung, S. pH-Responsive Succinoglycan-Carboxymethyl Cellulose Hydrogels with Highly Improved Mechanical Strength for Controlled Drug Delivery Systems. Polymers 2021, 13, 3197. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Shadab; Abdullah, S.; Alhakamy, N.A.; Alharbi, W.S.; Ahmad, J.; Shaik, R.A.; Ansari, M.J.; Ibrahim, I.M.; Ali, J. Development, Optimization, and In Vitro Evaluation of Novel Oral Long-Acting Resveratrol Nanocomposite In-Situ Gelling Film in the Treatment of Colorectal Cancer. Gels 2021, 7, 276. [Google Scholar] [CrossRef]
- Danish, Z.; Ijaz, H.; Razzaque, G.; Aslam, M. Facile synthesis of three-dimensional porous hydrogel and its evaluation. Polym. Bull. 2022, 79, 7407–7428. [Google Scholar] [CrossRef]
- Koc, F.E.; Altıncekic, T.G. Investigation of gelatin/chitosan as potential biodegradable polymer films on swelling behavior and methylene blue release kinetics. Polym. Bull. 2021, 78, 3383–3398. [Google Scholar] [CrossRef]
- Hussain, M.A.; Kiran, L.; Haseeb, M.T.; Hussain, I.; Hussain, S.Z. Citric acid crosslinking of mucilage from Cydonia oblonga engenders a superabsorbent, pH-sensitive and biocompatible polysaccharide offering on-off swelling and zero-order drug release. J. Polym. Res. 2020, 27, 49. [Google Scholar] [CrossRef]
- Vo, N.T.N.; Huang, L.; Lemos, H.; Mellor, A.; Novakovic, K. Poly(ethylene glycol)-interpenetrated genipin-crosslinked chitosan hydrogels: Structure, pH responsiveness, gelation kinetics, and rheology. J. Appl. Polym. Sci. 2020, 137, 49259. [Google Scholar] [CrossRef]
- Seo, J.W.; Shin, S.R.; Lee, M.-Y.; Cha, J.M.; Min, K.H.; Lee, S.C.; Shin, S.Y.; Bae, H. Injectable hydrogel derived from chitosan with tunable mechanical properties via hybrid-crosslinking system. Carbohydr. Polym. 2021, 251, 117036. [Google Scholar] [CrossRef]
- Chen, J.; Park, K. Synthesis and characterization of superporous hydrogel composites. J. Control Release 2000, 65, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Liberman, G.N.; Ochbaum, G.; Bitton, R.; Arad, S. Antimicrobial hydrogels composed of chitosan and sulfated polysaccharides of red microalgae. Polymer 2021, 215, 123353. [Google Scholar] [CrossRef]
- Barajas-Ledesma, R.M.; Patti, A.F.; Wong, V.N.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose superabsorbent structure by controlling the drying rate. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 600, 124943. [Google Scholar] [CrossRef]
- Mittal, H.; Babu, R.; Alhassan, S.M. Utilization of gum xanthan based superporous hydrogels for the effective removal of methyl violet from aqueous solution. Int. J. Biol. Macromol. 2020, 143, 413–423. [Google Scholar] [CrossRef]
- Raza, M.A.; Lim, Y.-M.; Lee, S.-W.; Seralathan, K.-K.; Park, S.H. Synthesis and characterization of hydrogels based on carboxymethyl chitosan and poly(vinylpyrrolidone) blends prepared by electron beam irradiation having anticancer efficacy, and applications as drug carrier for controlled release of drug. Carbohydr. Polym. 2021, 258, 117718. [Google Scholar] [CrossRef]
- Bahsis, L.; Ablouh, E.-H.; Anane, H.; Taourirte, M.; Julve, M.; Stiriba, S.-E. Cu(ii)-alginate-based superporous hydrogel catalyst for click chemistry azide–alkyne cycloaddition type reactions in water. RSC Adv. 2020, 10, 32821–32832. [Google Scholar] [CrossRef]
- Kedir, C.N.; Salinas-Torres, D.; Quintero-Jaime, A.; Benyoucef, A.; Morallon, E. Hydrogels obtained from aniline and piperazine: Synthesis, characterization and their application in hybrid supercapacitors. J. Mol. Struct. 2022, 1248, 131445. [Google Scholar] [CrossRef]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, B. Radiation induced graft copolymerization of graphene oxide and carbopol onto sterculia gum polysaccharide to develop hydrogels for biomedical applications. Flatchem 2020, 19, 100151. [Google Scholar] [CrossRef]
- Mehra, S.; Nisar, S.; Chauhan, S.; Singh, G.; Singh, V.; Rattan, S. A dual stimuli responsive natural polymer based superabsorbent hydrogel engineered through a novel cross-linker. Polym. Chem. 2021, 12, 2404–2420. [Google Scholar] [CrossRef]
- Sarkar, N.; Sahoo, G.; Swain, S.K. Reduced graphene oxide decorated superporous polyacrylamide based interpenetrating network hydrogel as dye adsorbent. Mater. Chem. Phys. 2020, 250, 123022. [Google Scholar] [CrossRef]
- Sadeghian, A.; Kharaziha, M.; Khoroushi, M. Osteoconductive visible light-crosslinkable nanocomposite for hard tissue engineering. Colloids Surfaces A: Physicochem. Eng. Asp. 2022, 632, 127761. [Google Scholar] [CrossRef]
- Benhabbour, S.R.; Kovarova, M.; Jones, C.; Copeland, D.J.; Shrivastava, R.; Swanson, M.D.; Sykes, C.; Ho, P.T.; Cottrell, M.L.; Sridharan, A.; et al. Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nat. Commun. 2019, 10, 4324. [Google Scholar] [CrossRef] [Green Version]
- Kamaci, M. Polyurethane-based hydrogels for controlled drug delivery applications. Eur. Polym. J. 2020, 123, 109444. [Google Scholar] [CrossRef]
- Balamuralidhara, V.; Pramod Kumar, T.; Vishal Gupta, N.; Getyala, A.; Gangadharappa, H. Development of a novel biodegradable superporous hydrogel for gastroretentive application. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 524–532. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Hibbins, A.R.; Kumar, P.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Du Toit, L.C.; Pillay, V. Design of a versatile pH-responsive hydrogel for potential oral delivery of gastric-sensitive bioactives. Polymers 2017, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Park, K. Superporous IPN hydrogels having enhanced mechanical properties. AAPS PharmSciTech 2003, 4, 406–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Hodges, C.S.; Mishra, P.K.; Yoon, J.Y.; Hunter, T.N.; Lee, J.W.; Harbottle, D. Bio-Inspired Preparation of Clay–Hexacyanoferrate Composite Hydrogels as Super Adsorbents for Cs+. ACS Appl. Mater. Interfaces 2020, 12, 33173–33185. [Google Scholar] [CrossRef] [PubMed]
- Farid-ul-Haq, M.; Haseeb, M.T.; Hussain, M.A.; Ashraf, M.U.; Naeem-ul-Hassan, M.; Hussain, S.Z.; Hussain, I. A smart drug delivery system based on Artemisia vulgaris hydrogel: Design, on-off switching, and real-time swelling, transit detection, and mechanistic studies. J. Drug Deliv. Sci. Technol. 2020, 58, 101795. [Google Scholar] [CrossRef]
- Hussain, M.A.; Rana, A.I.; Haseeb, M.T.; Muhammad, G.; Kiran, L. Citric acid cross-linked glucuronoxylans: A pH-sensitive polysaccharide material for responsive swelling-deswelling vs various biomimetic stimuli and zero-order drug release. J. Drug Deliv. Sci. Technol. 2020, 55, 101470. [Google Scholar] [CrossRef]
- Hao, N.; Jayawardana, K.W.; Chen, X.; Yan, M. One-step synthesis of amine-functionalized hollow mesoporous silica nanoparticles as efficient antibacterial and anticancer materials. ACS Appl. Mater. Interfaces 2015, 7, 1040–1045. [Google Scholar] [CrossRef] [Green Version]
- Bardonnet, P.; Faivre, V.; Pugh, W.; Piffaretti, J.; Falson, F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J. Control. Release 2006, 111, 1–18. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juthi, A.Z.; Li, F.; Wang, B.; Alam, M.M.; Talukder, M.E.; Qiu, B. pH-Responsive Super-Porous Hybrid Hydrogels for Gastroretentive Controlled-Release Drug Delivery. Pharmaceutics 2023, 15, 816. https://doi.org/10.3390/pharmaceutics15030816
Juthi AZ, Li F, Wang B, Alam MM, Talukder ME, Qiu B. pH-Responsive Super-Porous Hybrid Hydrogels for Gastroretentive Controlled-Release Drug Delivery. Pharmaceutics. 2023; 15(3):816. https://doi.org/10.3390/pharmaceutics15030816
Chicago/Turabian StyleJuthi, Ajkia Zaman, Fenfen Li, Bo Wang, Md Mofasserul Alam, Md Eman Talukder, and Bensheng Qiu. 2023. "pH-Responsive Super-Porous Hybrid Hydrogels for Gastroretentive Controlled-Release Drug Delivery" Pharmaceutics 15, no. 3: 816. https://doi.org/10.3390/pharmaceutics15030816