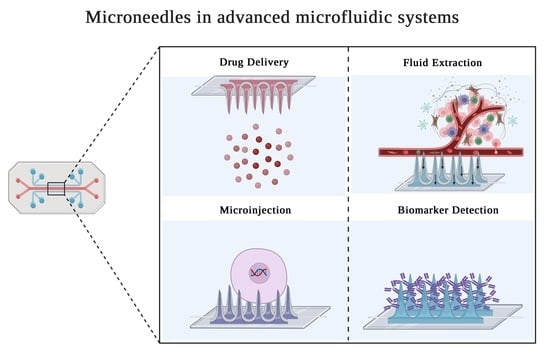
Microneedles in Advanced Microfluidic Systems: A Systematic Review throughout Lab and Organ-on-a-Chip Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Validity Assessment
2.3. Inclusion and Exclusion Criteira
- Published since 2000;
- Use of microfluidic platforms or organs-on-a-chip in combination with MNs;
- Use of microfluidic platforms or lab-on-a-chip in combination with MNs;
- MNs for media/ISF collection;
- MNs for cell injection;
- MNs for biomarkers detection.
- MNs for biofluid extraction, microneedle sensors, and analyte-capturing MNs, or combinations thereof.
3. Results
3.1. Data Collection Results
3.2. MNs Applied in Advanced Microfluidic Devices
| MNType | In/Out-of-Plane | MN–Chip Connection | Fabrication Strategy | Material Chip/MN | Forces | Function | Application | Microfluidic System | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Porous MNs | Out-of-plane | MN integrated in the inlets of the microdevice | Microfabrication + Leach method | Polylactic acid (PLA)/PDMS | Pump | Biomarker detection | ISF collection and glucose detection | Lab-on-a-chip | [61,62] |
| Porous MNs | Out-of-plane | Integrated as MN patch | Mold + Leach method | PDMS/Ethoxylated trimethylolpropane triacrylate (ETPTA) | Capillary Action | Biomarker detection | Extraction and detection of skin interstitial fluid biomarkers | Lab-on-a-chip | [64] |
| Solid MNs | Out-of-plane | MN integrated in the inlets of the microdevice | Microfabrication (SU-8) | PDMS/SU-8 | Pressure | Drug delivery | Delivery functions for inflammation treatment | Lab-on-a-chip | [73] |
| Solid MNs | In-of-plane | MN integrated perpendicular to the microfluidic channel | Microfabrication | Oxide layer + metallic layer/Silicon | - | Biomarker Detection | Microneedle biosensor for direct label-free real-time protein detection | Lab-on-a-chip | [65] |
| Solid MNs | Out-of-plane | MN integrated perpendicular to the microfluidic channel | - | PDMS/Tungsten + parylene | Syringe pump | Cell Detection | Detection of cells in suspension | Lab-on-a-chip | [85] |
| Coated MNs | Out-of-plane | MN integrated above microfluidic channel | Microfabrication | PDMS/Silicon + Cr/AU | Capillary Forces | Delivery | Chemical delivery capability | Lab-on-a-chip | [71] |
| Coated MNs | Out-of-plane | MN integrated above chamber | Microfabrication (two-photon lithography) | PDMS/Gold + enzyme layer | Syringe pump | Biomarker detection/Biosensor | 3D microspike array-based glucose and lactate biosensor | Lab-on-a-chip | [67] |
| Coated MNs | Out-of-plane | MN integrated above microfluidic channel | SU-8 | PDMS/SU-8 resin | Syringe pump | Biomarker detection/Biosensor | Drug delivery and body fluid sampling applications | Lab-on-a-chip | [68] |
| Hollow MNs | Out-of-plane | MN integrated above microfluidic channel | Microfabrication | - | Micropump | Biomarker detection | Nonenzymatic microfluidic glucose sensor | Lab-on-a-chip | [60] |
| Hollow MNs | Out-of-plane | MN integrated in organoid chamber | Microfabrication (Photolithography) | PMMA/Silicon | Pneumatic interface | Biomarker detection | Microfluidic sampling system for tissue analytics | Organ-on-a-chip | [87] |
| Hollow MNs | Out-of-plane | MN integrated above microfluidic channel | Microfabrication | Pyrex/Silicon | Capillary action and evaporation | Biomarkerdetection | Microneedle-based glucose monitor | Lab-on-a-chip | [58] |
| Hollow MNs | Out-of-plane | MN integrated above microfluidic channel | Microfabrication/DRIE | Aluminum + Silicon/Silicon | Capillary forces | Extraction | ISF extraction | Lab-on-a-chip | [81] |
| Hollow MNs | Out-of-plane | MN integrated above microfluidic channel | Microfabrication (two-photon lithography) | PDMS/Eshell 300 | Pump | Analysis | Sensor for on-chip potentiometric determination of K+ | Lab-on-a-chip | [63] |
| Hollow MNs | Out-of-plane | MN integrated perpendicular to the microfluidic channel | Soft lithography | PDMS + SU-8/Glass | Valve actuation | Micro-injection | Single cells microinjection system | Organ-on-a-chip | [74] |
| Hollow MNs | Out-of-plane | MN integrated above microfluidic channel | Direct laser writing | PMMA/Photosensitive material | Syringe | Extraction/ delivery | A system for fluid injection and extraction | Lab-on-a-chip | [86] |
| Hollow and sharp MNs | Out-of-plane | MN integrated above microfluidic channel | Laser Ablation | Glass/SU-8 | Syringe pump | Perfusion | 3D micro perfusion system | Organ-on-a-chip | [89] |
| Hollow MNs | Out-of-plane | Integrated as MN patch | Soft lithography | PDMS/metal | Pressure | Extraction | Extraction and transport of blood | Lab-on-a-chip | [90] |
| Hollow MNs | Out-of-plane | Integrated as MN patch | Soft lithography | PDMS + paper sensor | Pressure | Biomarker detection | POCT biosensors for quantification of glucose and cholesterol in blood | Lab-on-a-chip | [66] |
| Hollow MNs | Out-of-plane | MN integrated perpendicular to the microfluidic channel | 3D printing + DRIE | PDMS/Glass | Vacuum pump | Microinjection | Microfluidic device for localized microinjection | Lab-on-a-chip | [75] |
| MN with open capillary | In-plane | Connected with microfluidic device | DRIE + photolithography | Silicon | Pressure | Insertion into skin | Extraction/delivery | Lab-on-a-chip | [76,77] |
| MN with open capillary | In-plane | Connected with microfluidic device | DRIE + photolithography | Titanium | Pressure | Insertion into skin | Extraction/delivery | Lab-on-a-chip | [78] |
| MN with open capillary | In-plane | Connected with microfluidic device | MEMS + glass cover on silicon technology | Silicon | Syring pump | Drug Infusion | System for brain drug infusion | Lab-on-a-chip/organ-on-a-chip | [72] |
| MN with open capillary | In-plane | Connected with microfluidic device | MEMS + DRIE | Silicon | Planar Micropump | Drug Delivery | Continuous on-chip micropumping for microneedle enhanced drug delivery | Lab-on-a-chip | [70] |
3.2.1. Devices for Extraction and Biomarker Detection
3.2.2. Devices for Drug Delivery and Microinjection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patabadige, D.E.W.; Jia, S.; Sibbitts, J.; Sadeghi, J.; Sellens, K.; Culbertson, C.T. Micro Total Analysis Systems: Fundamental Advances and Applications. Anal. Chem. 2016, 88, 320–338. [Google Scholar] [CrossRef]
- Lafleur, J.P.; Jönsson, A.; Senkbeil, S.; Kutter, J.P. Recent advances in lab-on-a-chip for biosensing applications. Biosens. Bioelectron. 2016, 76, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.I.; Haswell, S.; Gibson, I. Lab-on-a-chip or Chip-in-a-lab: Challenges of Commercialization Lost in Translation. Procedia Technol. 2015, 20, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.; Han, J.; Choi, J.-W.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Samper, I.C.; Gowers, S.A.N.; Booth, M.A.; Wang, C.; Watts, T.; Phairatana, T.; Vallant, N.; Sandhu, B.; Papalois, V.; Boutelle, M.G. Portable Microfluidic Biosensing System for Real-Time Analysis of Microdialysate in Transplant Kidneys. Anal. Chem. 2019, 91, 14631–14638. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Z.; Bachman, H.; Zhang, P.; Huang, T.J. A Cell-Phone-Based Acoustofluidic Platform for Quantitative Point-of-Care Testing. ACS Nano 2020, 14, 3159–3169. [Google Scholar] [CrossRef]
- Yan, J.; Li, Z.; Guo, J.; Liu, S.; Guo, J. Organ-on-a-chip: A new tool for in vitro research. Biosens. Bioelectron. 2022, 216, 114626. [Google Scholar] [CrossRef]
- Ribas, J.; Pawlikowska, J.; Rouwkema, J. Microphysiological systems: Analysis of the current status, challenges and commercial future. Microphysiol. Syst. 2018, 2, 10. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Syama, S.; Mohanan, P.V. Microfluidic based human-on-a-chip: A revolutionary technology in scientific research. Trends Food Sci. Technol. 2021, 110, 711–728. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, L.; Wu, J.; Wang, Y.; Meng, X.; Hu, P.; Liang, Q.; Xie, Y.; Luo, G. Design and fabrication of an integrated 3D dynamic multicellular liver-on-a-chip and its application in hepatotoxicity screening. Talanta 2022, 241, 123262. [Google Scholar]
- Zhang, F.; Qu, K.-Y.; Zhou, B.; Luo, Y.; Zhu, Z.; Pan, D.-J.; Cui, C.; Zhu, Y.; Chen, M.-L.; Huang, N.-P. Design and fabrication of an integrated heart-on-a-chip platform for construction of cardiac tissue from human iPSC-derived cardiomyocytes and in situ evaluation of physiological function. Biosens. Bioelectron. 2021, 179, 113080. [Google Scholar]
- Haring, A.P.; Sontheimer, H.; Johnson, B.N. Microphysiological Human Brain and Neural Systems-on-a-Chip: Potential Alternatives to Small Animal Models and Emerging Platforms for Drug Discovery and Personalized Medicine. Stem Cell Rev. Rep. 2017, 13, 381–406. [Google Scholar] [PubMed]
- Nieskens, T.T.; Magnusson, O.; Persson, M.; Andersson, P.; Söderberg, M.; Sjögren, A. Development of a kidney-on-a-chip model that replicates an antisense oligonucleotide-induced kidney injury biomarker response. Toxicol. Lett. 2021, 350, S58. [Google Scholar] [CrossRef]
- Joseph, X.; Akhil, V.; Arathi, A.; Mohanan, P.V. Comprehensive Development in Organ-On-A-Chip Technology. J. Pharm. Sci. 2022, 111, 18–31. [Google Scholar] [PubMed]
- Rodrigues, R.O.; Sousa, P.C.; Gaspar, J.; Bañobre-López, M.; Lima, R.; Minas, G. Organ-on-a-Chip: A Preclinical Microfluidic Platform for the Progress of Nanomedicine. Small 2020, 16, 2003517. [Google Scholar]
- Meng, X.; Zhang, Z.; Li, L. Micro/nano needles for advanced drug delivery. Prog. Nat. Sci. Mater. Int. 2020, 30, 589–596. [Google Scholar]
- Angkawinitwong, U.; Courtenay, A.J.; Rodgers, A.M.; Larrañeta, E.; McCarthy, H.O.; Brocchini, S.; Donnelly, R.F.; Williams, G.R. A Novel Transdermal Protein Delivery Strategy via Electrohydrodynamic Coating of PLGA Microparticles onto Microneedles. ACS Appl. Mater. Interfaces 2020, 12, 12478–12488. [Google Scholar] [CrossRef]
- Chiappini, C.; De Rosa, E.; Martinez, J.O.; Liu, X.; Steele, J.; Stevens, M.M.; Tasciotti, E. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat. Mater 2015, 14, 532–539. [Google Scholar] [PubMed]
- Zhuang, J.; Rao, F.; Wu, D.; Huang, Y.; Xu, H.; Gao, W.; Zhang, J.; Sun, J. Study on the fabrication and characterization of tip-loaded dissolving microneedles for transdermal drug delivery. Eur. J. Pharm. Biopharm. 2020, 157, 66–73. [Google Scholar] [PubMed]
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.R.; Khademhosseini, A. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 2014, 190, 82–93. [Google Scholar] [PubMed] [Green Version]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [PubMed]
- Chang, K.-T.; Shen, Y.-K.; Fan, F.-Y.; Lin, Y.; Kang, S.-C. Optimal design and fabrication of a microneedle arrays patch. J. Manuf. Process. 2020, 54, 274–285. [Google Scholar]
- Sonetha, V.; Majumdar, S.; Shah, S. Step-wise micro-fabrication techniques of microneedle arrays with applications in transdermal drug delivery—A review. J. Drug Deliv. Sci. Technol. 2022, 68, 103119. [Google Scholar]
- Matsumoto, D.; Rao Sathuluri, R.; Kato, Y.; Silberberg, Y.R.; Kawamura, R.; Iwata, F.; Kobayashi, T.; Nakamura, C. Oscillating high-aspect-ratio monolithic silicon nanoneedle array enables efficient delivery of functional bio-macromolecules into living cells. Sci. Rep. 2015, 5, 15325. [Google Scholar] [PubMed] [Green Version]
- Kaur, R.; Arora, S.; Goswami, M. Advancement in microneedles as minimally invasive delivery system for pharmaceutical and biomedical application: A review. Mater. TodayProc. 2022. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Chen, H.; Mei, L.; Zeng, X. Polymeric microneedle-mediated sustained release systems: Design strategies and promising applications for drug delivery. Asian J. Pharm. Sci. 2022, 17, 70–86. [Google Scholar]
- McAlister, E.; Kirkby, M.; Donnelly, R.F. 6—Microneedles for drug delivery and monitoring. In Microfluidic Devices for Biomedical Applications, 2nd ed.; Li, X. (James), Zhou, Y., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2021; pp. 225–260. ISBN 978-0-12-819971-8. [Google Scholar]
- Nguyen, N.-T.; Shaegh, S.A.M.; Kashaninejad, N.; Phan, D.-T. Design, fabrication and characterization of drug delivery systems based on lab-on-a-chip technology. Adv. Drug Deliv. Rev. 2013, 65, 1403–1419. [Google Scholar]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 2018, 128, 3–28. [Google Scholar]
- Sawon, M.A.; Samad, M.F. Design and optimization of a microneedle with skin insertion analysis for transdermal drug delivery applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102477. [Google Scholar]
- Chow, Y.T.; Chen, S.; Liu, C.; Liu, C.; Li, L.; Kong, C.W.M.; Cheng, S.H.; Li, R.A.; Sun, D. A High-Throughput Automated Microinjection System for Human Cells With Small Size. IEEE/ASME Trans. Mechatron. 2016, 21, 838–850. [Google Scholar]
- Naveen, N.R.; Goudanavar, P.S.; Ramesh, B.; Kumar, G.K. Prospection of fabrication techniques and material selection of microneedles for transdermal drug delivery: An update on clinical trials. Mater. Today Proc. 2022, 69, 187–192. [Google Scholar]
- Bhadale, R.S.; Londhe, V.Y. A systematic review of carbohydrate-based microneedles: Current status and future prospects. J. Mater. Sci. Mater. Med. 2021, 32, 89. [Google Scholar] [PubMed]
- Meng, F.; Hasan, A.; Mahdi Nejadi Babadaei, M.; Hashemi Kani, P.; Jouya Talaei, A.; Sharifi, M.; Cai, T.; Falahati, M.; Cai, Y. Polymeric-based microneedle arrays as potential platforms in the development of drugs delivery systems. J. Adv. Res. 2020, 26, 137–147. [Google Scholar] [PubMed]
- Singh, J.; Rathi, A.; Rawat, M.; Gupta, M. Graphene: From synthesis to engineering to biosensor applications. Front. Mater. Sci. 2018, 12, 1–20. [Google Scholar]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A review of recent advances in microneedle technology for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar]
- Tarbox, T.N.; Watts, A.B.; Cui, Z.; Williams, R.O. An update on coating/manufacturing techniques of microneedles. Drug Deliv. Transl. Res. 2018, 8, 1828–1843. [Google Scholar]
- Takahashi, H.; Jung Heo, Y.; Arakawa, N.; Kan, T.; Matsumoto, K.; Kawano, R.; Shimoyama, I. Scalable fabrication of microneedle arrays via spatially controlled UV exposure. Microsyst. Nanoeng. 2016, 2, 16049. [Google Scholar]
- Jung, P.G.; Lee, T.W.; Oh, D.J.; Hwang, S.J.; Jung, I.; Lee, S.; Ko, J. Nickel Microneedles Fabricated by Sequential Copper and Nickel Electroless Plating and Copper Chemical Wet Etching. Sens. Mater. 2008, 20, 45–53. [Google Scholar]
- Wang, Q.L.; Zhu, D.D.; Liu, X.B.; Chen, B.Z.; Guo, X.D. Microneedles with Controlled Bubble Sizes and Drug Distributions for Efficient Transdermal Drug Delivery. Sci. Rep. 2016, 6, 38755. [Google Scholar] [PubMed] [Green Version]
- Aoyagi, S.; Izumi, H.; Isono, Y.; Fukuda, M.; Ogawa, H. Laser fabrication of high aspect ratio thin holes on biodegradable polymer and its application to a microneedle. Sens. Actuators A Phys. 2007, 139, 293–302. [Google Scholar] [CrossRef]
- Kim, J.D.; Kim, M.; Yang, H.; Lee, K.; Jung, H. Droplet-born air blowing: Novel dissolving microneedle fabrication. J. Control. Release 2013, 170, 430–436. [Google Scholar] [PubMed]
- Faraji Rad, Z.; Prewett, P.D.; Davies, G.J. Rapid prototyping and customizable microneedle design: Ultra-sharp microneedle fabrication using two-photon polymerization and low-cost micromolding techniques. Manuf. Lett. 2021, 30, 39–43. [Google Scholar]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [PubMed] [Green Version]
- Häfeli, U.O.; Mokhtari, A.; Liepmann, D.; Stoeber, B. In vivo evaluation of a microneedle-based miniature syringe for intradermal drug delivery. Biomed. Microdevices 2009, 11, 943–950. [Google Scholar]
- Valdés-Ramírez, G.; Windmiller, J.R.; Claussen, J.C.; Martinez, A.G.; Kuralay, F.; Zhou, M.; Zhou, N.; Polsky, R.; Miller, P.R.; Narayan, R.; et al. Multiplexed and switchable release of distinct fluids from microneedle platforms via conducting polymer nanoactuators for potential drug delivery. Sens. Actuators B Chem. 2012, 161, 1018–1024. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Ventrelli, L.; Marsilio Strambini, L.; Barillaro, G. Microneedles for Transdermal Biosensing: Current Picture and Future Direction. Adv. Healthc. Mater. 2015, 4, 2606–2640. [Google Scholar]
- Li, C.G.; Lee, C.Y.; Lee, K.; Jung, H. An optimized hollow microneedle for minimally invasive blood extraction. Biomed. Microdevices 2013, 15, 17–25. [Google Scholar] [PubMed]
- Wang, Z.; Luan, J.; Seth, A.; Liu, L.; You, M.; Gupta, P.; Rathi, P.; Wang, Y.; Cao, S.; Jiang, Q.; et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 2021, 5, 64–76. [Google Scholar] [PubMed]
- Takeuchi, K.; Kim, B. Functionalized microneedles for continuous glucose monitoring. Nano Converg. 2018, 5, 28. [Google Scholar]
- Dardano, P.; Rea, I.; De Stefano, L. Microneedles-based electrochemical sensors: New tools for advanced biosensing. Curr. Opin. Electrochem. 2019, 17, 121–127. [Google Scholar]
- Kusama, S.; Sato, K.; Matsui, Y.; Kimura, N.; Abe, H.; Yoshida, S.; Nishizawa, M. Transdermal electroosmotic flow generated by a porous microneedle array patch. Nat. Commun. 2021, 12, 658. [Google Scholar]
- Azizgolshani, H.; Coppeta, J.R.; Vedula, E.M.; Marr, E.E.; Cain, B.P.; Luu, R.J.; Lech, M.P.; Kann, S.H.; Mulhern, T.J.; Tandon, V.; et al. High-throughput organ-on-chip platform with integrated programmable fluid flow and real-time sensing for complex tissue models in drug development workflows. Lab Chip 2021, 21, 1454–1474. [Google Scholar] [PubMed]
- Zimmermann, S.; Fienbork, D.; Stoeber, B.; Flounders, A.W.; Liepmann, D. A microneedle-based glucose monitor: Fabricated on a wafer-level using in-device enzyme immobilization. In Proceedings of the TRANSDUCERS ’03, 12th International Conference on Solid-State Sensors, Actuators and Microsystems, Digest of Technical Papers (Cat. No.03TH8664), Boston, MA, USA, 8–12 June 2003; Volume 1, pp. 99–102. [Google Scholar]
- Najmi, A.; Saidi, M.S.; Shahrokhian, S.; Hosseini, H.; Kazemzadeh Hannani, S. Fabrication of a microdialysis-based nonenzymatic microfluidic sensor for regular glucose measurement. Sens. Actuators B Chem. 2021, 333, 129569. [Google Scholar]
- Najmi, A.; Saidi, M.S.; Kazemzadeh Hannani, S. Design of the micropump and mass-transfer compartment of a microfluidic system for regular nonenzymatic glucose measurement. Biotechnol. Rep. 2022, 34, e00723. [Google Scholar]
- Takeuchi, K.; Takama, N.; Kim, B.; Sharma, K.; Paul, O.; Ruther, P. Microfluidic chip to interface porous microneedles for ISF collection. Biomed. Microdevices 2019, 21, 28. [Google Scholar]
- Takeuchi, K.; Takama, N.; Sharma, K.; Paul, O.; Ruther, P.; Suga, T.; Kim, B. Microfluidic chip connected to porous microneedle array for continuous ISF sampling. Drug Deliv. Transl. Res. 2022, 12, 435–443. [Google Scholar] [CrossRef]
- Miller, P.R.; Xiao, X.; Brener, I.; Burckel, D.B.; Narayan, R.; Polsky, R. Microneedle-Based Transdermal Sensor for On-Chip Potentiometric Determination of K +. Adv. Healthc. Mater. 2014, 3, 876–881. [Google Scholar] [PubMed]
- Yi, K.; Wang, Y.; Shi, K.; Chi, J.; Lyu, J.; Zhao, Y. Aptamer-decorated porous microneedles arrays for extraction and detection of skin interstitial fluid biomarkers. Biosens. Bioelectron. 2021, 190, 113404. [Google Scholar] [PubMed]
- Esfandyarpour, R.; Esfandyarpour, H.; Javanmard, M.; Harris, J.S.; Davis, R.W. Microneedle biosensor: A method for direct label-free real time protein detection. Sens. Actuators B Chem. 2013, 177, 848–855. [Google Scholar] [PubMed] [Green Version]
- Li, C.G.; Joung, H.-A.; Noh, H.; Song, M.-B.; Kim, M.-G.; Jung, H. One-touch-activated blood multidiagnostic system using a minimally invasive hollow microneedle integrated with a paper-based sensor. Lab Chip 2015, 15, 3286–3292. [Google Scholar] [PubMed]
- Trzebinski, J.; Sharma, S.; Radomska-Botelho Moniz, A.; Michelakis, K.; Zhang, Y.; Cass, A.E.G. Microfluidic device to investigate factors affecting performance in biosensors designed for transdermal applications. Lab Chip 2012, 12, 348–352. [Google Scholar]
- Kim, K.; Lee, J.-B. High aspect ratio tapered hollow metallic microneedle arrays with microfluidic interconnector. Microsyst. Technol. 2006, 13, 231–235. [Google Scholar]
- Singh, R.R.T.; Tekko, I.; McAvoy, K.; McMillan, H.; Jones, D.; Donnelly, R.F. Minimally invasive microneedles for ocular drug delivery. Expert Opin. Drug Deliv. 2017, 14, 525–537. [Google Scholar]
- Zahn, J.D.; Deshmukh, A.; Pisano, A.P.; Liepmann, D. Continuous On-Chip Micropumping for Microneedle Enhanced Drug Delivery. Biomed. Microdevices 2004, 6, 183–190. [Google Scholar] [CrossRef]
- Kang, Y.N.; Chou, N.; Jang, J.-W.; Choe, H.K.; Kim, S. A 3D flexible neural interface based on a microfluidic interconnection cable capable of chemical delivery. Microsyst. Nanoeng. 2021, 7, 66. [Google Scholar]
- Lee, H.J.; Son, Y.; Kim, D.; Kim, Y.K.; Choi, N.; Yoon, E.-S.; Cho, I.-J. A new thin silicon microneedle with an embedded microchannel for deep brain drug infusion. Sens. Actuators B Chem. 2015, 209, 413–422. [Google Scholar]
- Xiang, Z.; Wang, H.; Pastorin, G.; Lee, C. Development of a Flexible and Disposable Microneedle-Fluidic-System With Finger-Driven Drug Loading and Delivery Functions for Inflammation Treatment. J. Microelectromech. Syst. 2015, 24, 565–574. [Google Scholar] [CrossRef]
- Adamo, A.; Jensen, K.F. Microfluidic based single cell microinjection. Lab Chip 2008, 8, 1258. [Google Scholar] [CrossRef] [PubMed]
- Zabihihesari, A.; Hilliker, A.J.; Rezai, P. Localized microinjection of intact Drosophila melanogaster larva to investigate the effect of serotonin on heart rate. Lab Chip 2020, 20, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Yang, R.; Tazrin, F.; Zhu, C.; Kaddoura, M.; Blondeel, E.J.M.; Cui, B. In-plane silicon microneedles with open capillary microfluidic networks by deep reactive ion etching and sacrificial layer based sharpening. Sens. Actuators A Phys. 2019, 292, 149–157. [Google Scholar] [CrossRef]
- Jung, M.; Jeong, D.; Yun, S.-S.; Lee, J.-H. Fabrication of a 2-D in-plane micro needle array integrated with microfluidic components using crystalline wet etching of (110) silicon. Microsyst. Technol. 2016, 22, 2287–2294. [Google Scholar] [CrossRef]
- Parker, E.R.; Rao, M.P.; Turner, K.L.; Meinhart, C.D.; MacDonald, N.C. Bulk Micromachined Titanium Microneedles. J. Microelectromech. Syst. 2007, 16, 289–295. [Google Scholar] [CrossRef]
- Griss, P.; Stemme, G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J. Microelectromech. Syst. 2003, 12, 296–301. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, Y.; Wang, H.; Li, Y.; Chen, X.; Chen, D. Fabricating process of hollow out-of-plane Ni microneedle arrays and properties of the integrated microfluidic device. J. Micro/Nanolith. MEMS MOEMS 2013, 12, 033019. [Google Scholar] [CrossRef]
- Mukerjee, E.V.; Collins, S.D.; Isseroff, R.R.; Smith, R.L. Microneedle array for transdermal biological fluid extraction and in situ analysis. Sens. Actuators A Phys. 2004, 114, 267–275. [Google Scholar] [CrossRef]
- Dixon, R.V.; Skaria, E.; Lau, W.M.; Manning, P.; Birch-Machin, M.A.; Moghimi, S.M.; Ng, K.W. Microneedle-based devices for point-of-care infectious disease diagnostics. Acta Pharm. Sin. B 2021, 11, 2344–2361. [Google Scholar] [CrossRef]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N. Micro Electromechanical Systems (MEMS) Based Microfluidic Devices for Biomedical Applications. IJMS 2011, 12, 3648–3704. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Guo, M.; Lyu, K.; Chu, T.; He, B. Intelligent Silk Fibroin Based Microneedle Dressing (i-SMD). Adv. Funct. Mater. 2021, 31, 2006839. [Google Scholar] [CrossRef]
- Mansor, M.; Takeuchi, M.; Nakajima, M.; Hasegawa, Y.; Ahmad, M. Electrical Impedance Spectroscopy for Detection of Cells in Suspensions Using Microfluidic Device with Integrated Microneedles. Appl. Sci. 2017, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Trautmann, A.; Roth, G.-L.; Nujiqi, B.; Walther, T.; Hellmann, R. Towards a versatile point-of-care system combining femtosecond laser generated microfluidic channels and direct laser written microneedle arrays. Microsyst. Nanoeng. 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Hokkanen, A.; Stuns, I.; Schmid, P.; Kokkonen, A.; Gao, F.; Steinecker, A.; Budczies, J.; Heimala, P.; Hakalahti, L. Microfluidic sampling system for tissue analytics. Biomicrofluidics 2015, 9, 054109. [Google Scholar] [CrossRef] [Green Version]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-printed microneedles in biomedical applications. iScience 2021, 24, 102012. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; McClain, M.A.; LaPlaca, M.C.; Frazier, A.B.; Allen, M.G. Three dimensional MEMS microfluidic perfusion system for thick brain slice cultures. Biomed. Microdevices 2007, 9, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Lee, K.; Lee, C.Y.; Dangol, M.; Jung, H. A Minimally Invasive Blood-Extraction System: Elastic Self-Recovery Actuator Integrated with an Ultrahigh- Aspect-Ratio Microneedle. Adv. Mater. 2012, 24, 4583–4586. [Google Scholar] [CrossRef]
- Ju, J.; Li, L.; Regmi, S.; Zhang, X.; Tang, S. Microneedle-Based Glucose Sensor Platform: From Vitro to Wearable Point-of-Care Testing Systems. Biosensors 2022, 12, 606. [Google Scholar] [CrossRef]
- Sarabi, M.R.; Ahmadpour, A.; Yetisen, A.K.; Tasoglu, S. Finger-Actuated Microneedle Array for Sampling Body Fluids. Appl. Sci. 2021, 11, 5329. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, D.; Li, J.; Wu, Y.; Du, L.; Xia, X.-H.; Li, X.; Deng, Y.; Li, Z.; Huang, Y. Continuous Vector-free Gene Transfer with a Novel Microfluidic Chip and Nanoneedle Array. CDD 2018, 16, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.; Douville, N.J.; Takayama, S.; ElSayed, M. Quantitative Analysis of Molecular Absorption into PDMS Microfluidic Channels. Ann. Biomed. Eng. 2012, 40, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Minas, H.; Maria, G.; Rodrigues, R.O.; de Sousa, T.; Jorge, P.; De Lima, M.M.; Alberto, R.; De Sousa, S.; Catarina, P.; Cabanas, C.; et al. WO2022180595—Multiorgan-on-Chip Device with Integrated Microbiosensors, Methods and Uses Thereof. WO/2022/180595. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022180595&_cid=P20-L7J87M-45311-1 (accessed on 10 January 2023).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, R.; Carvalho, V.; Lima, R.; Minas, G.; Rodrigues, R.O. Microneedles in Advanced Microfluidic Systems: A Systematic Review throughout Lab and Organ-on-a-Chip Applications. Pharmaceutics 2023, 15, 792. https://doi.org/10.3390/pharmaceutics15030792
Maia R, Carvalho V, Lima R, Minas G, Rodrigues RO. Microneedles in Advanced Microfluidic Systems: A Systematic Review throughout Lab and Organ-on-a-Chip Applications. Pharmaceutics. 2023; 15(3):792. https://doi.org/10.3390/pharmaceutics15030792
Chicago/Turabian StyleMaia, Renata, Violeta Carvalho, Rui Lima, Graça Minas, and Raquel O. Rodrigues. 2023. "Microneedles in Advanced Microfluidic Systems: A Systematic Review throughout Lab and Organ-on-a-Chip Applications" Pharmaceutics 15, no. 3: 792. https://doi.org/10.3390/pharmaceutics15030792