Polymeric Nanoparticles as Tunable Nanocarriers for Targeted Delivery of Drugs to Skin Tissues for Treatment of Topical Skin Diseases
Abstract
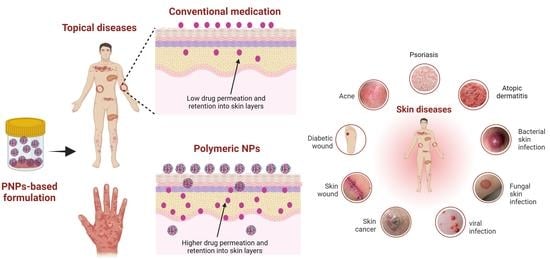
:1. Introduction
1.1. Skin Structure
1.2. Topical Drug Delivery through the Skin
2. Physicochemical Properties and Types of PNPs
3. Topical Application of PNPs for Treatment of Skin Diseases
3.1. Natural Polymers Used to Prepare PNPs for Treatment of Skin Diseases
3.2. Psoriasis
3.2.1. Pathogenesis of Psoriasis
3.2.2. Conventional Treatments for Psoriasis and Their Limitations
3.2.3. PNPs-Based Topical Therapies for Treatment of Psoriasis
3.3. Atopic Dermatitis
3.3.1. Pathogenesis of Atopic Dermatitis
3.3.2. Conventional Treatments for Atopic Dermatitis and Their Limitations
3.3.3. PNPs-Based Topical Therapies for Treatment of Atopic Dermatitis
3.4. Skin Cancer
3.4.1. Pathogenesis of Skin Cancer
3.4.2. Conventional Treatments for Skin Cancer and Their Limitations
3.4.3. PNPs-Based Topical Therapies for Treatment of Skin Cancer
3.5. Skin Infections
3.5.1. Pathogenesis of Skin Infection
3.5.2. Conventional Treatments for Skin Infections and Limitations
3.5.3. PNPs-Based Topical Therapies for Treatment of Skin Infections
3.6. Skin Wounds
3.6.1. Pathogenesis of Skin Wounds
3.6.2. Conventional Therapies for Acute-to-Chronic Wounds and Limitations
3.6.3. PNPs-Based Topical Therapies for Wound Healing
3.7. Acne
3.7.1. Pathogenesis of Acne
3.7.2. Conventional Treatments for Acne and Limitations
3.7.3. PNPs-Based Topical Therapies for Treatment of Acne
4. Conclusions and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferreira, I.G.; Weber, M.B.; Bonamigo, R.R. History of Dermatology: The Study of Skin Diseases over the Centuries. An. Bras. Dermatol. 2021, 96, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Rabindranathnambi, A.; Abid, M. Topical Treatments in Dermatology. Br. J. Hosp. Med. 2021, 82, 1–9. [Google Scholar] [CrossRef]
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. On the Role of Skin in the Regulation of Local and Systemic Steroidogenic Activities. Steroids 2015, 103, 72–88. [Google Scholar] [CrossRef] [Green Version]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin Barrier Immunity and Ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bragazzi, N.L.; Sellami, M.; Salem, I.; Conic, R.; Kimak, M.; Pigatto, P.D.M.; Damiani, G. Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature. Nutrients 2019, 11, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M. Stratum Corneum Defensive Functions: An Integrated View. J. Investig. Dermatol. 2005, 125, 183–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.Q.; Yang, X.; Wu, X.F.; Fan, Y. Bin Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Tsai, P.-C.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric Nanoparticles-Based Topical Delivery Systems for the Treatment of Dermatological Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for Topical, Dermal, and Transdermal Drug Delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and Nanofibers for Topical Drug Delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Zoabi, A.; Touitou, E.; Margulis, K. Recent Advances in Nanomaterials for Dermal and Transdermal Applications. Colloids Interfaces 2021, 5, 18. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Ayub, A.; Wettig, S. An Overview of Nanotechnologies for Drug Delivery to the Brain. Pharmaceutics 2022, 14, 224. [Google Scholar] [CrossRef]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From Properties to Perspective of Mucosal Drug Delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Wong, T.W. Microwave as Skin Permeation Enhancer for Transdermal Drug Delivery of Chitosan-5-Fluorouracil Nanoparticles. Carbohydr. Polym. 2017, 157, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Tacrolimus-Loaded Chitosan Nanoparticles for Enhanced Skin Deposition and Management of Plaque Psoriasis. Carbohydr. Polym. 2021, 268, 118238. [Google Scholar] [CrossRef]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-Known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Sosnik, A. Alginate Particles as Platform for Drug Delivery by the Oral Route: State-of-the-Art. ISRN Pharm. 2014, 2014, 926157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakil, S.N.A.; Kamal, H.; Abdullah, H.Z.; Idris, M.I. Sodium Alginate-Zinc Oxide Nanocomposite Film for Antibacterial Wound Healing Applications. Biointerface Res. Appl. Chem. 2020, 10, 6245–6252. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles-An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.; Muangnoi, C.; Sorasitthiyanukarn, F.N.; Wongpiyabovorn, J.; Rojsitthisak, P.; Rojsitthisak, P. Synergistic Effects of Photo-Irradiation and Curcumin-Chitosan/Alginate Nanoparticles on Tumor Necrosis Factor-Alpha-Induced Psoriasis-like Proliferation of Keratinocytes. Molecules 2019, 24, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhaščik, M.; Kováčik, A.; Huerta-Ángeles, G. Recent Advances of Hyaluronan for Skin Delivery: From Structure to Fabrication Strategies and Applications. Polymers 2022, 14, 4833. [Google Scholar] [CrossRef]
- Tolentino, S.; Pereira, M.N.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Targeted Clindamycin Delivery to Pilosebaceous Units by Chitosan or Hyaluronic Acid Nanoparticles for Improved Topical Treatment of Acne Vulgaris. Carbohydr. Polym. 2021, 253, 117295. [Google Scholar] [CrossRef]
- Hussain, Z.; Pandey, M.; Choudhury, H.; Ying, P.C.; Xian, T.M.; Kaur, T.; Jia, G.W.; Gorain, B. Hyaluronic Acid Functionalized Nanoparticles for Simultaneous Delivery of Curcumin and Resveratrol for Management of Chronic Diabetic Wounds: Fabrication, Characterization, Stability and in Vitro Release Kinetics. J. Drug Deliv. Sci. Technol. 2020, 57, 101747. [Google Scholar] [CrossRef]
- Ghosh, N.; Mitra, S.; Banerjee, E.R. Therapeutic Effects of Topically-Administered Guar Gum Nanoparticles in Oxazolone-Induced Atopic Dermatitis in Mice. Biomed. Res. Ther. 2018, 5, 2305–2325. [Google Scholar] [CrossRef]
- Pervaiz, F.; Mushtaq, R.; Noreen, S. Formulation and Optimization of Terbinafine HCl Loaded Chitosan/Xanthan Gum Nanoparticles Containing Gel: Ex-Vivo Permeation and in-Vivo Antifungal Studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102935. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Abdullah, F.; Mukherjee, A. Fabrication and Fluorescent Labeling of Guar Gum Nanoparticles in a Surfactant Free Aqueous Environment. Mater. Sci. Eng. C 2015, 46, 521–529. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Michopoulou, A.; Palamidi, A.; Koliakou, E.; Bikiaris, D. Preparation and Evaluation of Collagen-Based Patches as Curcumin Carriers. Polymers 2020, 12, 2393. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J.; et al. Chitosan-Based Nanoformulated (-)-Epigallocatechin-3-Gallate (EGCG) Modulates Human Keratinocyte-Induced Responses and Alleviates Imiquimod-Induced Murine Psoriasiform Dermatitis. Int. J. Nanomed. 2018, 13, 4189–4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shandil, A.; Yadav, M.; Sharma, N.; Nagpal, K.; Jindal, D.K.; Deep, A.; Kumar, S. Targeting Keratinocyte Hyperproliferation, Inflammation, Oxidative Species and Microbial Infection by Biological Macromolecule-Based Chitosan Nanoparticle-Mediated Gallic Acid–Rutin Combination for the Treatment of Psoriasis. Polym. Bull. 2020, 77, 4713–4738. [Google Scholar] [CrossRef]
- Malgarim Cordenonsi, L.; Faccendini, A.; Catanzaro, M.; Bonferoni, M.C.; Rossi, S.; Malavasi, L.; Platcheck Raffin, R.; Scherman Schapoval, E.E.; Lanni, C.; Sandri, G.; et al. The Role of Chitosan as Coating Material for Nanostructured Lipid Carriers for Skin Delivery of Fucoxanthin. Int. J. Pharm. 2019, 567, 118487. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Singh, J.K.A.K.; Waqas, M.; Pandey, M.; Choudhury, H.; Habib, H.; Hussain, F.; Hussain, Z. Nanoencapsulation of Betamethasone Valerate Using High Pressure Homogenization–Solvent Evaporation Technique: Optimization of Formulation and Process Parameters for Efficient Dermal Targeting. Drug Dev. Ind. Pharm. 2019, 45, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Gunasegaran, T.A.P.; Nathan, S.S.; Md, S.; Gorain, B.; Tripathy, M.; Hussain, Z. Hyaluronic Acid-Modified Betamethasone Encapsulated Polymeric Nanoparticles: Fabrication, Characterisation, in Vitro Release Kinetics, and Dermal Targeting. Drug Deliv. Transl. Res. 2019, 9, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.I.; Katas, H.; Jamil, A.; Mohd Amin, M.C.I.; Ng, S.-F.; Zulfakar, M.H.; Nadeem, S.M. Potential Treatment of Atopic Dermatitis: Tolerability and Safety of Cream Containing Nanoparticles Loaded with Hydrocortisone and Hydroxytyrosol in Human Subjects. Drug Deliv. Transl. Res. 2019, 9, 469–481. [Google Scholar] [CrossRef]
- Hussain, Z.; Katas, H.; Amin, M.C.I.M.; Kumolosasi, E.; Sahudin, S. Downregulation of Immunological Mediators in 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis-like Skin Lesions by Hydrocortisone-Loaded Chitosan Nanoparticles. Int. J. Nanomed. 2014, 9, 5143–5156. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Katas, H.; Amin, M.C.I.M.; Kumulosasi, E.; Sahudin, S. Antidermatitic Perspective of Hydrocortisone as Chitosan Nanocarriers: An Ex Vivo and in Vivo Assessment Using an NC/Nga Mouse Model. J. Pharm. Sci. 2013, 102, 1063–1075. [Google Scholar] [CrossRef]
- Hussain, Z.; Katas, H.; Mohd Amin, M.C.I.; Kumolosasi, E. Efficient Immuno-Modulation of TH1/TH2 Biomarkers in 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis: Nanocarrier-Mediated Transcutaneous Co-Delivery of Anti-Inflammatory and Antioxidant Drugs. PLoS ONE 2014, 9, e113143. [Google Scholar] [CrossRef]
- Zhuo, F.; Abourehab, M.A.S.; Hussain, Z. Hyaluronic Acid Decorated Tacrolimus-Loaded Nanoparticles: Efficient Approach to Maximize Dermal Targeting and Anti-Dermatitis Efficacy. Carbohydr. Polym. 2018, 197, 478–489. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical Formulations Containing Aptamer-Functionalized Nanocapsules Loaded with 5-Fluorouracil - An Innovative Concept for the Skin Cancer Therapy. Mater. Sci. Eng. C 2021, 119, 111591. [Google Scholar] [CrossRef]
- Sabitha, M.; Sanoj Rejinold, N.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Development and Evaluation of 5-Fluorouracil Loaded Chitin Nanogels for Treatment of Skin Cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef]
- Mumtaz, T.; Ahmed, N.; Hassan, N.; Badshah, M.; Khan, S.; Rehman, A. Voriconazole Nanoparticles-Based Film Forming Spray: An Efficient Approach for Potential Treatment of Topical Fungal Infections. J. Drug Deliv. Sci. Technol. 2022, 70, 102973. [Google Scholar] [CrossRef]
- Ho, H.N.; Le, T.G.; Dao, T.T.T.; Le, T.H.; Dinh, T.T.H.; Nguyen, D.H.; Tran, T.C.; Nguyen, C.N. Development of Itraconazole-Loaded Polymeric Nanoparticle Dermal Gel for Enhanced Antifungal Efficacy. J. Nanomater. 2020, 2020, 8894541. [Google Scholar] [CrossRef]
- Riezk, A.; van Bocxlaer, K.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of Amphotericin B-Loaded Chitosan Nanoparticles against Experimental Cutaneous Leishmaniasis. Molecules 2020, 25, 4002. [Google Scholar] [CrossRef]
- Abd-Elsalam, W.H.; Ibrahim, R.R. Span 80/TPGS Modified Lipid-Coated Chitosan Nanocomplexes of Acyclovir as a Topical Delivery System for Viral Skin Infections. Int. J. Pharm. 2021, 609, 121214. [Google Scholar] [CrossRef]
- Patarroyo, J.L.; Cifuentes, J.; Muñoz, L.N.; Cruz, J.C.; Reyes, L.H. Novel Antibacterial Hydrogels Based on Gelatin/Polyvinyl-Alcohol and Graphene Oxide/Silver Nanoconjugates: Formulation, Characterization, and Preliminary Biocompatibility Evaluation. Heliyon 2022, 8, e09145. [Google Scholar] [CrossRef]
- Hussain, Z.; Pandey, M.; Thu, H.E.; Kaur, T.; Jia, G.W.; Ying, P.C.; Xian, T.M.; Abourehab, M.A.S. Hyaluronic Acid Functionalization Improves Dermal Targeting of Polymeric Nanoparticles for Management of Burn Wounds: In Vitro, Ex Vivo and in Vivo Evaluations. Biomed. Pharmacother. 2022, 150, 112992. [Google Scholar] [CrossRef]
- Shafique, M.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Mahmood, A.; Abbasi, M.; Aziz, H.C.; et al. Bio-Functional Hydrogel Membranes Loaded with Chitosan Nanoparticles for Accelerated Wound Healing. Int. J. Biol. Macromol. 2021, 170, 207–221. [Google Scholar] [CrossRef]
- Ogunjimi, A.T.; Chahud, F.; Lopez, R.F.V. Isotretinoin-Delonix Polymeric Nanoparticles: Potentials for Skin Follicular Targeting in Acne Treatment. Int. J. Pharm. 2021, 610, 121217. [Google Scholar] [CrossRef]
- Kandekar, S.G.; Del Río-Sancho, S.; Lapteva, M.; Kalia, Y.N. Selective Delivery of Adapalene to the Human Hair Follicle under Finite Dose Conditions Using Polymeric Micelle Nanocarriers. Nanoscale 2018, 10, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and Anti-Inflammatory Activity of Chitosan-Alginate Nanoparticles: A Targeted Therapy for Cutaneous Pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Folle, C.; Marqués, A.M.; Díaz-Garrido, N.; Espina, M.; Sánchez-López, E.; Badia, J.; Baldoma, L.; Calpena, A.C.; García, M.L. Thymol-Loaded PLGA Nanoparticles: An Efficient Approach for Acne Treatment. J. Nanobiotechnol. 2021, 19, 1–21. [Google Scholar] [CrossRef]
- Kahraman, E.; Özhan, G.; Özsoy, Y.; Güngör, S. Polymeric Micellar Nanocarriers of Benzoyl Peroxide as Potential Follicular Targeting Approach for Acne Treatment. Colloids Surf. B Biointerfaces 2016, 146, 692–699. [Google Scholar] [CrossRef]
- World Health Organization Global Report on Psoriasis. Available online: https://apps.who.int/iris/handle/10665/204417 (accessed on 1 March 2022).
- Langley, R.G.B.; Krueger, G.G.; Griffiths, C.E.M. Psoriasis: Epidemiology, Clinical Features, and Quality of Life. Ann. Rheum. Dis. 2005, 64, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Tackling the Various Classes of Nano-Therapeutics Employed in Topical Therapy of Psoriasis. Drug Deliv. 2020, 27, 662–680. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, N.; Behl, T.; Sarkar, B.C.; Saha, H.R.; Garg, K.; Singh, S.K.; Arora, S.; Amran, M.S.; Abdellatif, A.A.H.; et al. Promising Strategies of Colloidal Drug Delivery-Based Approaches in Psoriasis Management. Pharmaceutics 2021, 13, 1978. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Gaies, E.; Jebabli, N. Methotrexate Side Effects: Review Article. J. Drug Metab. Toxicol. 2012, 3, 1–5. [Google Scholar] [CrossRef]
- Tedesco, D.; Haragsim, L. Cyclosporine: A Review. J. Transplant. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, L.K.; Gaar, L.R.; Yentzer, B.A.; O’Neill, J.L.; Feldman, S.R. Acitretin in Dermatology: A Review. J. Drugs Dermatol. 2011, 10, 772–782. [Google Scholar]
- Gesser, B.; Johansen, C.; Rasmussen, M.K.; Funding, A.T.; Otkjaer, K.; Kjellerup, R.B.; Kragballe, K.; Iversen, L. Dimethylfumarate Specifically Inhibits the Mitogen and Stress-Activated Kinases 1 and 2 (MSK1/2): Possible Role for Its Anti-Psoriatic Effect. J. Investig. Dermatol. 2007, 127, 2129–2137. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.C.U.; Listopad, J.J.; Rentzsch, C.U.; Igney, F.H.; Von Bonin, A.; Hennekes, H.H.; Asadullah, K.; Docke, W.D.F. Dimethylfumarate Induces Immunosuppression via Glutathione Depletion and Subsequent Induction of Heme Oxygenase 1. J. Investig. Dermatol. 2007, 127, 835–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balak, D.M. Fumaric Acid Esters in the Management of Psoriasis. Psoriasis 2015, 5, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, E. Psoriasis. Medicine 2017, 45, 368–378. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.X. A Clinical Review of Phototherapy for Psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.; Hsu, L.; Liao, W. Phototherapy in Psoriasis: A Review of Mechanisms of Action. J. Cutan. Med. Surg. 2013, 17, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Rønholt, K.; Iversen, L. Old and New Biological Therapies for Psoriasis. Int. J. Mol. Sci. 2017, 18, 2297. [Google Scholar] [CrossRef] [Green Version]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The Effect of Chitin and Chitosan on the Proliferation of Human Skin Fibroblasts and Keratinocytes in Vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic Dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1. [Google Scholar] [CrossRef]
- Eyerich, K.; Novak, N. Immunology of Atopic Eczema: Overcoming the Th1/Th2 Paradigm. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Dimitriades, V.R.; Wisner, E. Treating Pediatric Atopic Dermatitis: Current Perspectives. Pediatr. Health Med. Ther. 2015, 6, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenfield, L.F.; Boguniewicz, M.; Simpson, E.L.; Russell, J.J.; Block, J.K.; Feldman, S.R.; Clark, A.R.; Tofte, S.; Dunn, J.D.; Paller, A.S. Translating Atopic Dermatitis Management Guidelines Into Practice for Primary Care Providers. Pediatrics 2015, 136, 554–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, J.J.; Milner, J.D.; Stone, K.D. Atopic Dermatitis in Children: Clinical Features, Pathophysiology, and Treatment. Immunol. Allergy Clin. N. Am. 2015, 35, 161–183. [Google Scholar] [CrossRef] [Green Version]
- Parekh, K.; Mehta, T.A.; Dhas, N.; Kumar, P.; Popat, A. Emerging Nanomedicines for the Treatment of Atopic Dermatitis. AAPS PharmSciTech 2021, 22, 55. [Google Scholar] [CrossRef]
- Thomsen, S.F. Atopic Dermatitis: Natural History, Diagnosis, and Treatment. ISRN Allergy 2014, 2014, 354250. [Google Scholar] [CrossRef] [Green Version]
- Sathishkumar, D.; Moss, C. Topical Therapy in Atopic Dermatitis in Children. Indian J. Dermatol. 2016, 61, 656–661. [Google Scholar] [CrossRef]
- Łabędź, N.; Pawliczak, R. Efficacy and Safety of Topical Calcineurin Inhibitors for the Treatment of Atopic Dermatitis: Meta-Analysis of Randomized Clinical Trials. Adv. Dermatol. Allergol. 2019, 36, 752–759. [Google Scholar] [CrossRef]
- Wellington, K.; Jarvis, B. Spotlight on Topical Pimecrolimus in Atopic Dermatitis. Am. J. Clin. Dermatol. 2002, 3, 435–438. [Google Scholar] [CrossRef]
- Hajar, T.; Gontijo, J.R.V.; Hanifin, J.M. New and Developing Therapies for Atopic Dermatitis. An. Bras. Dermatol. 2018, 93, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Novak, N. Atopic Dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Dias-Ferreira, J.; Oliveira, J.; Sanchez-Lopez, E.; Lopez-Machado, A.; Espina, M.; Garcia, M.L.; Souto, S.B.; Martins-Gomes, C.; Silva, A.M. Trends in Atopic Dermatitis—from Standard Pharmacotherapy to Novel Drug Delivery Systems. Int. J. Mol. Sci. 2019, 20, 5659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, R.; Jabob, J.S.; Sahoo, S. Biomedical Applications of Green Biopolymer Guar Gum. J. Pharm. Biomed. Sci. 2013, 35, 1783–1787. [Google Scholar]
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Gigliotti, C.L.; Ciamporcero, E.S.; Daga, M.; Barrera, G. Drug Delivery Nanoparticles in Skin Cancers. Biomed Res. Int. 2014, 2014, 895986. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-Level Classification of Skin Cancer with Deep Neural Networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Su, Y.; Hu, J.; Huang, Z.; Huang, Y.; Peng, B.; Xie, N.; Liu, H. Paclitaxel-Loaded Star-Shaped Copolymer Nanoparticles for Enhanced Malignant Melanoma Chemotherapy against Multidrug Resistance. Drug Des. Dev. Ther. 2017, 11, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Omran, A.R. The Epidemiologic Transition: A Theory of the Epidemiology of Population Change. 1971. Milbank Q. 2005, 83, 731–757. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet Radiation and Skin Cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Bendesky, A.; Michel, A.; Sordo, M.; Calderón-Aranda, E.S.; Acosta-Saavedra, L.C.; Salazar, A.M.; Podoswa, N.; Ostrosky-Wegman, P. DNA Damage, Oxidative Mutagen Sensitivity, and Repair of Oxidative DNA Damage in Nonmelanoma Skin Cancer Patients. Environ. Mol. Mutagen. 2006, 47, 509–517. [Google Scholar] [CrossRef]
- Benjamin, C.L.; Ananthaswamy, H.N. P53 and the Pathogenesis of Skin Cancer. Toxicol. Appl. Pharmacol. 2007, 224, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seebode, C.; Lehmann, J.; Emmert, S. Photocarcinogenesis and Skin Cancer Prevention Strategies. Anticancer Res. 2016, 36, 1371–1378. [Google Scholar] [PubMed]
- Dréau, D.; Culberson, C.; Wyatt, S.; Holder, W.D.J. Human Papilloma Virus in Melanoma Biopsy Specimens and Its Relation to Melanoma Progression. Ann. Surg. 2000, 231, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Lugowska, I.; Koseła-Paterczyk, H.; Kozak, K.; Rutkowski, P. Trametinib: A MEK Inhibitor for Management of Metastatic Melanoma. OncoTargets Ther. 2015, 8, 2251–2259. [Google Scholar] [CrossRef] [Green Version]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.M.; Gherghel, D.; Popa, M. “In Vitro” Behaviour of Aptamer-Functionalized Polymeric Nanocapsules Loaded with 5-Fluorouracil for Targeted Therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef]
- Taveira, S.F. Topical Administration of Anticancer Drugs for Skin Cancer Treatment; IntechOpen: Rijeka, Croatia, 2011; Chapter 11. [Google Scholar]
- Remesh, A. Toxicities of Anticancer Drugs and Its Management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.L.; Wang, H.W.; Wang, H.S.; Xu, S.Z.; Liao, K.H.; Hillemanns, P. Topical 5-Aminolaevulinic Acid-Photodynamic Therapy for the Treatment of Urethral Condylomata Acuminata. Br. J. Dermatol. 2004, 151, 880–885. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Zhao, F.; Luan, H.; Tu, Q.; Huang, Z.; Wang, H.; Wang, H. In Vitro Evaluation of 5-Aminolevulinic Acid (ALA) Loaded PLGA Nanoparticles. Int. J. Nanomed. 2013, 8, 2669–2676. [Google Scholar] [CrossRef] [Green Version]
- de Campos Araújo, L.M.P.; Thomazine, J.A.; Lopez, R.F.V. Development of Microemulsions to Topically Deliver 5-Aminolevulinic Acid in Photodynamic Therapy. Eur. J. Pharm. Biopharm. 2010, 75, 48–55. [Google Scholar] [CrossRef]
- Ding, H.; Sumer, B.D.; Kessinger, C.W.; Dong, Y.; Huang, G.; Boothman, D.A.; Gao, J. Nanoscopic Micelle Delivery Improves the Photophysical Properties and Efficacy of Photodynamic Therapy of Protoporphyrin IX. J. Control. Release 2011, 151, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shi, L.; Tu, Q.; Wang, H.; Zhang, H.; Wang, P.; Zhang, L.; Huang, Z.; Zhao, F.; Luan, H.; et al. Treating Cutaneous Squamous Cell Carcinoma Using 5-Aminolevulinic Acid Polylactic-Co-Glycolic Acid Nanoparticle-Mediated Photodynamic Therapy in a Mouse Model. Int. J. Nanomed. 2015, 10, 347–355. [Google Scholar] [CrossRef] [Green Version]
- da Silva, C.L.; Del Ciampo, J.O.; Rossetti, F.C.; Bentley, M.V.L.B.; Pierre, M.B.R. Improved in Vitro and in Vivo Cutaneous Delivery of Protoporphyrin IX from PLGA-Based Nanoparticles. Photochem. Photobiol. 2013, 89, 1176–1184. [Google Scholar] [CrossRef]
- Petkovsek, Z.; Elersic, K.; Gubina, M.; Zgur-Bertok, D.; Starcic Erjavec, M. Virulence Potential of Escherichia Coli Isolates from Skin and Soft Tissue Infections. J. Clin. Microbiol. 2009, 47, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Rhody, C. Bacterial Infections of the Skin. Prim. Care 2000, 27, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Garber, G. An Overview of Fungal Infections. Drugs 2001, 61 (Suppl. 1), 1–12. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.C.; Reedijk, S.H.; Schallig, H.D.F.H. Cutaneous Leishmaniasis: Recent Developments in Diagnosis and Management. Am. J. Clin. Dermatol. 2015, 16, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Ramdass, P.; Mullick, S.; Farber, H.F. Viral Skin Diseases. Prim. Care 2015, 42, 517–567. [Google Scholar] [CrossRef]
- Ki, V.; Rotstein, C. Bacterial Skin and Soft Tissue Infections in Adults: A Review of Their Epidemiology, Pathogenesis, Diagnosis, Treatment and Site of Care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Cunha, B.A. Antibiotic Side Effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Kalita, S.; Devi, B.; Kandimalla, R.; Sharma, K.K.; Sharma, A.; Kalita, K.; Kataki, A.C.; Kotoky, J. Chloramphenicol Encapsulated in Poly-ε-Caprolactone-Pluronic Composite: Nanoparticles for Treatment of MRSA-Infected Burn Wounds. Int. J. Nanomed. 2015, 10, 2971–2984. [Google Scholar] [CrossRef] [Green Version]
- Briasoulis, A.; Agarwal, V.; Pierce, W.J. QT Prolongation and Torsade de Pointes Induced by Fluoroquinolones: Infrequent Side Effects from Commonly Used Medications. Cardiology 2011, 120, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Olliaro, P. Leishmaniasis Chemotherapy--Challenges and Opportunities. Clin. Microbiol. Infect. 2011, 17, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Vardy, D.; Barenholz, Y.; Naftoliev, N.; Klaus, S.; Gilead, L.; Frankenburg, S. Efficacious Topical Treatment for Human Cutaneous Leishmaniasis with Ethanolic Lipid Amphotericin B. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Désormeaux, A.; Gourde, P.; Juhász, J.; Bergeron, M.G. Efficacies of Topical Formulations of Foscarnet and Acyclovir and of 5-Percent Acyclovir Ointment (Zovirax) in a Murine Model of Cutaneous Herpes Simplex Virus Type 1 Infection. Antimicrob. Agents Chemother. 2000, 44, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Han, G. State-of-the-Art Wound Healing: Skin Substitutes for Chronic Wounds. Cutis 2014, 93, E13–E16. [Google Scholar]
- Chereddy, K.K.; Lopes, A.; Koussoroplis, S.; Payen, V.; Moia, C.; Zhu, H.; Sonveaux, P.; Carmeliet, P.; des Rieux, A.; Vandermeulen, G.; et al. Combined Effects of PLGA and Vascular Endothelial Growth Factor Promote the Healing of Non-Diabetic and Diabetic Wounds. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1975–1984. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA Based Drug Delivery Systems: Promising Carriers for Wound Healing Activity. Wound Repair Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef]
- Azevedo, F.; Pessoa, A.; Moreira, G.; Dos Santos, M.; Liberti, E.; Araujo, E.; Carvalho, C.; Saad, M.; Lima, M.H. Effect of Topical Insulin on Second-Degree Burns in Diabetic Rats. Biol. Res. Nurs. 2016, 18, 181–192. [Google Scholar] [CrossRef]
- Toy, L.W.; Macera, L. Evidence-Based Review of Silver Dressing Use on Chronic Wounds. J. Am. Acad. Nurse Pract. 2011, 23, 183–192. [Google Scholar] [CrossRef]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; Estes, C.; McLaren, A.; Spangehl, M.J. Chlorhexidine Antiseptic Irrigation Eradicates Staphylococcus Epidermidis From Biofilm: An In Vitro Study. Clin. Orthop. Relat. Res. 2018, 476, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hoey, C. Topical Antimicrobial Therapy for Treating Chronic Wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stashak, T.S.; Farstvedt, E.; Othic, A. Update on Wound Dressings: Indications and Best Use. Clin. Tech. Equine Pract. 2004, 3, 148–163. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel Membranes: A Review. Mater. Sci. Eng. C 2020, 114, 111023. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired Sodium Alginate Based Thermosensitive Hydrogel Membranes for Accelerated Wound Healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Nguyen, V.B.; Hsieh, M.F. Curcumin-Loaded Chitosan/Gelatin Composite Sponge for Wound Healing Application. Int. J. Polym. Sci. 2013, 2013, 106570. [Google Scholar] [CrossRef] [Green Version]
- Jahromi, M.A.M.; Al-Musawi, S.; Pirestani, M.; Ramandi, M.F.; Ahmadi, K.; Rajayi, H.; Hassan, Z.M.; Kamali, M.; Mirnejad, R. Curcumin-Loaded Chitosan Tripolyphosphate Nanoparticles as a Safe, Natural and Effective Antibiotic Inhibits the Infection of Staphylococcus Aureus and Pseudomonas Aeruginosa in Vivo. Iran. J. Biotechnol. 2014, 12, e1012. [Google Scholar] [CrossRef]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-Encapsulated Nanoparticles as Innovative Antimicrobial and Wound Healing Agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, M.S.; Alqahtani, A.; Kazi, M.; Ahmad, M.Z.; Alahmari, A.; Alsenaidy, M.A.; Syed, R. Wound-Healing Potential of Curcumin Loaded Lignin Nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 60, 102020. [Google Scholar] [CrossRef]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.S.; Malayandi, R. Curcumin Loaded Chitosan Nanoparticles Impregnated into Collagen-Alginate Scaffolds for Diabetic Wound Healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef]
- Ayer, J.; Burrows, N. Acne: More than Skin Deep. Postgrad. Med. J. 2006, 82, 500–506. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C. An Update on the Role of the Sebaceous Gland in the Pathogenesis of Acne. Dermatoendocrinology 2011, 3, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masterson, K.N. Acne Basics: Pathophysiology, Assessment, and Standard Treatment Options. J. Dermatol. Nurses. Assoc. 2018, 10, S2–S10. [Google Scholar] [CrossRef]
- Vyas, A.; Kumar Sonker, A.; Gidwani, B. Carrier-Based Drug Delivery System for Treatment of Acne. Sci. World J. 2014, 2014, 276260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motamedi, M.; Chehade, A.; Sanghera, R.; Grewal, P. A Clinician’s Guide to Topical Retinoids. J. Cutan. Med. Surg. 2022, 26, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Tanghetti, E. The Evolution of Benzoyl Peroxide Therapy. Cutis 2008, 82, 5–11. [Google Scholar] [PubMed]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [Green Version]
- Thiboutot, D.; Gollnick, H.; Bettoli, V.; Dréno, B.; Kang, S.; Leyden, J.J.; Shalita, A.R.; Lozada, V.T.; Berson, D.; Finlay, A.; et al. New Insights into the Management of Acne: An Update from the Global Alliance to Improve Outcomes in Acne Group. J. Am. Acad. Dermatol. 2009, 60, S1–S50. [Google Scholar] [CrossRef]
- Kraft, J.; Freiman, A. Management of Acne. Can. Med. Assoc. J. 2011, 183, E430–E435. [Google Scholar] [CrossRef] [Green Version]
- Gold, L.S.; Cruz, A.; Eichenfield, L.; Tan, J.; Jorizzo, J.; Kerrouche, N.; Dhuin, J.-C. Effective and Safe Combination Therapy for Severe Acne Vulgaris: A Randomized, Vehicle-Controlled, Double-Blind Study of Adapalene 0.1%-Benzoyl Peroxide 2.5% Fixed-Dose Combination Gel with Doxycycline Hyclate 100 Mg. Cutis 2010, 85, 94–104. [Google Scholar]
- Swallow, M.A.; Fan, R.; Cohen, J.M.; Bunick, C.G. Antibiotic Resistance Risk with Oral Tetracycline Treatment of Acne Vulgaris. Antibiotics 2022, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Worret, I.; Arp, W.; Zahradnik, H.P.; Andreas, J.O.; Binder, N. Acne Resolution Rates: Results of a Single-Blind, Randomized, Controlled, Parallel Phase III Trial with EE/CMA (Belara) and EE/LNG (Microgynon). Dermatology 2001, 203, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lainscak, M.; Pelliccia, F.; Rosano, G.; Vitale, C.; Schiariti, M.; Greco, C.; Speziale, G.; Gaudio, C. Safety Profile of Mineralocorticoid Receptor Antagonists: Spironolactone and Eplerenone. Int. J. Cardiol. 2015, 200, 25–29. [Google Scholar] [CrossRef]
- Wang, Y.; Lipner, S.R. Retrospective Analysis of Adverse Events with Spironolactone in Females Reported to the United States Food and Drug Administration. Int. J. Women’s Dermatol. 2020, 6, 272–276. [Google Scholar] [CrossRef]
- Webster, G.F.M. Isotretinoin: Mechanism of Action and Patient Selection. Semin. Cutan. Med. Surg. 2015, 34, S86-8. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Scharnitz, T.P.; Muscat, J.; Chen, A.; Gupta-Elera, G.; Kirby, J.S. Laboratory Monitoring During Isotretinoin Therapy for Acne: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2016, 152, 35–44. [Google Scholar] [CrossRef]
- Ertam, I.; Alper, S.; Unal, I. Is It Necessary to Have Routine Blood Tests in Patients Treated with Isotretinoin? J. Dermatol. Treat. 2006, 17, 214–216. [Google Scholar] [CrossRef]
- Główka, E.; Wosicka-Frąckowiak, H.; Hyla, K.; Stefanowska, J.; Jastrzębska, K.; Klapiszewski, Ł.; Jesionowski, T.; Cal, K. Polymeric Nanoparticles-Embedded Organogel for Roxithromycin Delivery to Hair Follicles. Eur. J. Pharm. Biopharm. 2014, 88, 75–84. [Google Scholar] [CrossRef]







| Type of Skin Disease | Type of Polymer(s) | Active Ingredient(s) | Drug Delivery System Design | PS, ZP | Study Design | Major Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Psoriasis | CS | TAC | TAC-CS-NPs | PS: 140.8 ± 50.0 nm | In vitro:
|
| [20] |
| Psoriasis | CS/ALG | CUR | CUR-CS/ALG PNPs combined with photo-irradiation | PS: 200–300 nm ZP: −20 to −30 mV | In vitro (normal and TNF-α-induced cultured HaCaT cells):
|
| [25] |
| Psoriasis | CS | CUR | CUR-CS-NPs incorporated in collagen-based patch | PS: 164 ± 52 nm ZP: +38 mV | In vitro:
|
| [32] |
| Psoriasis | CS | EGCG | EGCG-CS-NPs | PS: 80–225 nm ZP: 38.3 mV | In vitro:
|
| [33] |
| Psoriasis | CS | Gallic acid and rutin | Gallic acid and rutin-loaded CS-based Tween 80-coated PNPs | - | In vitro
|
| [34] |
| Psoriasis | CS | FUCO | FUCO-NLCs coated with CS | PS: 250–400 nm | In vitro:
|
| [35] |
| AD | CS | BMV | BMV-CS-NPs | PS: <250 ± 28 nm ZP: +58 ± 8 mV | In vitro:
|
| [36] |
| AD | CS | BMV | HA-BMV-CS-NPs | PS: <300 ± 28 nm ZP: +58 ± 8 mV | In vitro and ex vivo:
|
| [37] |
| AD | CS | HC and HT | HC-HT-CS-NPs AQ cream | PS: <250 nm | In vivo:
|
| [38] |
| AD | CS | HC | HC-CS-NPs | PS: 214 ± 12 nm ZP: +40 ± 4 mV | In vivo (AD-induced NC/Nga mouse model):
|
| [39] |
| ACD | CS | HC | HC-loaded CS-NPs (HC-CS-NPs) | PS: 382 ± 14 to 187 ± 12 nm ZP: +14 ± 2 to +45 ± 4 mV | Ex vivo (dermatome mouse skin):
|
| [40] |
| AD | CS | HC and HT | HC/HT co-loaded CS-NPs (HC-HT-CS-NPs) | PS: 244 ± 21 nm ZP: +38 ± 4 mV | In vivo (2,4-DNFB-induced AD NC/Nga mouse model:
|
| [41] |
| AD | CS | TAC | TAC-CS-HA NPs | PS: 117 ± 19 nm ZP: +63.8 ± 6.4 mV | In vitro:
|
| [42] |
| AD | GG | NA | GG-PNPs | PS: 80 nm | In vitro:
|
| [29] |
| Skin cancer | CS and poly(NVPAI) copolymer | 5-FU | 5-FU-CS-poly(NVPAI) copolymer nanocapsules incorporated in sodium ALG- and HA-based gel | - | Ex vivo:
|
| [43] |
| Skin cancer | Chitin | 5-FU | FCNGs | PS: 120–140 nmZP: +31.9 mV | In vitro:
|
| [44] |
| Fungal skin infection | CS | VRC | VRC-CS-NPs-FFS | PS: 238 nm | Ex vivo:
|
| [45] |
| Fungal skin infection | CS/XG | TB | TB-CS/XG NPs gel | PS: 221.3 nm ZP: +19.51 to +26.23 | Ex vivo:
|
| [30] |
| Fungal skin infection | EC | ITZ | ITZ-EC NPs gel | PS: 200 nm | Ex vivo:
|
| [46] |
| Parasitic skin infection | CS | AmB | AmB-CS-TPP or AmB-CS-Dex) PNPs | (AmB-CS-TPP) PS: 69 ± 8 nm ZP: 25.5 ± 1 mV (AmB-CS-Dex) PS: 174 ± 8 nm ZP: −11 ± 1 mV | In vitro:
|
| [47] |
| Viral skin infection | CS | ACR | Span 80/TPGS modified ACR-LCNCs | PS: 177.50 ± 1.41 nm ZP: −10.70 ± 0.85 mV | Ex vivo:
|
| [48] |
| Bacterial skin infection | Gelatin and PVA | GO/Ag | GO/Ag NPs loaded in gelatin/PVA-based hydrogel | - | In vitro:
|
| [49] |
| Skin wound | CS | CUR and REV | HA-CUR-REV-CS-NPs | PS: 138 ± 11 nm ZP: +35.4 ± 1.4 mV | In vitro:
|
| [28] |
| Burn wound | CS | CUR and QUE | HA-CUR-QUE-CS-NPs | PS: 177 ± 11 nm ZP: +37.0 ± 3.2 mV | In vitro:
|
| [50] |
| Infected skin wound | CS | Cefepime | Cefepime-CS-NPs embedded in HA-PVA-pullulan-based hydrogel membranes | PS: 172 nm ZP: +27.8 mV | In vitro:
|
| [51] |
| Acne | CS and HA | Clindamycin | Clindamycin-CS-NPs or Clindamycin-HA NPs | PS (CS): 362 ± 19 nm PS (HA): 417 ± 9 nm | In vitro:
|
| [27] |
| Acne | DLX | IST | IST-DLX NPs | PS: 230 nm ZP: negative | In vitro:
|
| [52] |
| Acne | TPGS | ADA | ADA-TPGS PNPs | PS: <20 nm | In vitro:
|
| [53] |
| Acne | CS/ALG | BP | BP-CS/ALG NPs | PS: 341.6 ± 11.1 nm | In vitro:
|
| [54] |
| Acne | PLGA | TH | TH-PLGA NPs | PS: 162–235 nm ZP: −22 to −31 mV | In vitro and ex vivo:
|
| [55] |
| Acne | Pluronic® F127 | BP | BP-Pluronic® F127 polymeric micelles | PS: 25.3 ± 0.3 nm ZP: −2.5 mV | In vitro:
|
| [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madawi, E.A.; Al Jayoush, A.R.; Rawas-Qalaji, M.; Thu, H.E.; Khan, S.; Sohail, M.; Mahmood, A.; Hussain, Z. Polymeric Nanoparticles as Tunable Nanocarriers for Targeted Delivery of Drugs to Skin Tissues for Treatment of Topical Skin Diseases. Pharmaceutics 2023, 15, 657. https://doi.org/10.3390/pharmaceutics15020657
Madawi EA, Al Jayoush AR, Rawas-Qalaji M, Thu HE, Khan S, Sohail M, Mahmood A, Hussain Z. Polymeric Nanoparticles as Tunable Nanocarriers for Targeted Delivery of Drugs to Skin Tissues for Treatment of Topical Skin Diseases. Pharmaceutics. 2023; 15(2):657. https://doi.org/10.3390/pharmaceutics15020657
Chicago/Turabian StyleMadawi, Eiman Abdalla, Alaa Raad Al Jayoush, Mutasem Rawas-Qalaji, Hnin Ei Thu, Shahzeb Khan, Mohammad Sohail, Asif Mahmood, and Zahid Hussain. 2023. "Polymeric Nanoparticles as Tunable Nanocarriers for Targeted Delivery of Drugs to Skin Tissues for Treatment of Topical Skin Diseases" Pharmaceutics 15, no. 2: 657. https://doi.org/10.3390/pharmaceutics15020657