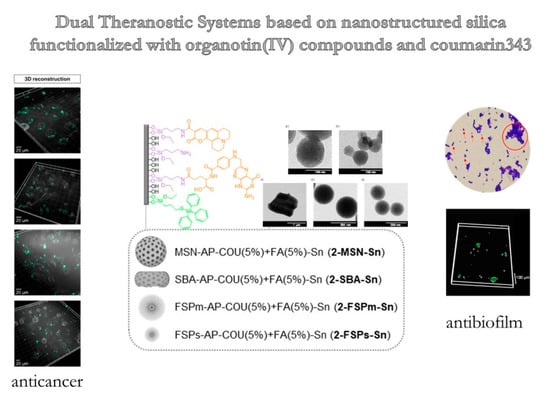
Dual Anticancer and Antibacterial Properties of Silica-Based Theranostic Nanomaterials Functionalized with Coumarin343, Folic Acid and a Cytotoxic Organotin(IV) Metallodrug
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Remarks on Characterization of the Materials
2.2. Synthesis of the Starting Silica Materials (MSN and SBA-15)
2.3. Functionalization of Silica Materials with Amino Ligand Synthesis of SiO2-AP
2.4. Incorporation of the Targeting and Imaging Agents
2.5. Synthesis and Incorporation of the Cytotoxic Agent
2.6. In Vitro Studies in Cancer Cells
2.6.1. Cell Culture
2.6.2. Cell Viability Assays
2.6.3. Cellular Uptake
2.7. In Vitro Studies in Bacteria
2.7.1. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
2.7.2. Attachment of Materials to Bacteria
2.7.3. Study of Oxidative Stress
2.7.4. S. aureus Biofilm–Nanoparticles Interaction
2.7.5. Inhibition of Adherence Stage during the Biofilm Formation
2.7.6. Effect on Biofilm Development
2.7.7. Optical and Fluorescence Microscopy
2.7.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Physicochemical Characterization of Functionalized NPs
3.1.1. Analysis of Size, Morphology, and Textural Properties
3.1.2. Quantification of the Degree of Functionalization by Thermogravimetry and Inductively Coupled Plasma Mass Spectroscopy
3.1.3. Characterization by Powder X-ray Diffraction Studies
3.2. In Vitro Studies of Antibacterial and Anticancer Activity
3.2.1. Cell Viability Studies in Cancer Cells
3.2.2. Minimum Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
3.2.3. Internalization of Materials in Bacteria
3.2.4. Study of Oxidative Stress
3.2.5. S. aureus Biofilm–Nanoparticles Interaction
3.2.6. Inhibition of Adherence Stage during the Biofilm Formation and during the Biofilm Development
3.3. Evaluation of In Vitro Imaging Capability in the Biological Environment
3.3.1. Cell Uptake Studies by Confocal Microscopy
3.3.2. Visualization of Inhibition of Biofilm Development by Optical and Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Berger, E.R.; Park, T.; Saridakis, A.; Golshan, M.; Greenup, R.A.; Ahuja, N. Immunotherapy Treatment for Triple Negative Breast Cancer. Pharmaceuticals 2021, 14, 763. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on Antimicrobial Resistance. Microbe Mag. 2015, 10, 354–355. [Google Scholar] [CrossRef]
- McKenna, M. Antibiotic Resistance: The Last Resort. Nature 2013, 499, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The Role of Bacterial Biofilms in Chronic Infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.S.; Otto, M. Molecular Basis of In Vivo Biofilm Formation by Bacterial Pathogens. Chem. Biol. 2012, 19, 1503–1513. [Google Scholar] [CrossRef]
- Whitney, J.D. Overview: Acute and Chronic Wounds. Nurs. Clin. N. Am. 2005, 40, 191–205. [Google Scholar] [CrossRef]
- Maslova, E.; Eisaiankhongi, L.; Sjöberg, F.; McCarthy, R.R. Burns and Biofilms: Priority Pathogens and in Vivo Models. NPJ Biofilms Microbiomes 2021, 7, 73. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Kahlmeter, G.; Jin, T. Biofilm Formation by Staphylococcus Aureus Isolates from Skin and Soft Tissue Infections. Curr. Microbiol. 2015, 70, 698–703. [Google Scholar] [CrossRef]
- Demir, C.; Demirci, M.; Yigin, A.; Tokman, H.B.; Cetik Yildiz, S. Presence of Biofilm and Adhesin Genes in Staphylococcus Aureus Strains Taken from Chronic Wound Infections and Their Genotypic and Phenotypic Antimicrobial Sensitivity Patterns. Photodiagnosis Photodyn. Ther. 2020, 29, 101584. [Google Scholar] [CrossRef]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global Burden of Cancer Attributable to Infections in 2018: A Worldwide Incidence Analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Kavanagh, K.A. Evaluation of Metal-Based Antimicrobial Compounds for the Treatment of Bacterial Pathogens. J. Med. Microbiol. 2021, 70, 001363. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper, An Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Galanski, M.; Jakupec, M.; Keppler, B. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef]
- Ott, I.; Gust, R. Non Platinum Metal Complexes as Anti-Cancer Drugs. Arch. Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin Resistance: A Cellular Self-Defense Mechanism Resulting from Multiple Epigenetic and Genetic Changes. Pharmacol. Rev. 2012, 64, 706. [Google Scholar] [CrossRef]
- Kaluderović, G.N.; Kommera, H.; Hey-Hawkins, E.; Paschke, R.; Gómez-Ruiz, S. Synthesis and Biological Applications of Ionic Triphenyltin(Iv) Chloride Carboxylate Complexes with Exceptionally High Cytotoxicity. Metallomics 2010, 2, 419–428. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. Anticancer Applications and Recent Investigations of Metallodrugs Based on Gallium, Tin and Titanium. Inorganics 2017, 5, 4. [Google Scholar] [CrossRef]
- Varela-Ramirez, A.; Costanzo, M.; Carrasco, Y.P.; Pannell, K.H.; Aguilera, R.J. Cytotoxic Effects of Two Organotin Compounds and Their Mode of Inflicting Cell Death on Four Mammalian Cancer Cells. Cell Biol. Toxicol. 2011, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Gulino, L.; Pellerito, L.; Fiore, T.; Pellerito, C.; Barbieri, G. Effects of Two Organotin(IV)(Sulfonatophenyl)Porphinates on MAPKs and on the Growth of A375 Human Melanoma Cells. Oncol. Rep. 2009, 21, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Rocamora-Reverte, L.; Carrasco-García, E.; Ceballos-Torres, J.; Prashar, S.; Kaluderović, G.N.; Ferragut, J.A.; Gómez-Ruiz, S. Study of the Anticancer Properties of Tin(IV) Carboxylate Complexes on a Panel of Human Tumor Cell Lines. ChemMedChem 2012, 7, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Abbas, Z.; Tuli, H.S.; Rani, A. Organotin Complexes with Promising Therapeutic Potential. Curr. Pharmacol. Rep. 2020, 6, 167–181. [Google Scholar] [CrossRef]
- Cooney, J.J.; Wuertz, S. Toxic Effects of Tin Compounds on Microorganisms. J. Ind. Microbiol. 1989, 4, 375–402. [Google Scholar] [CrossRef]
- White, J.S.; Tobin, J.M.; Cooney, J.J. Organotin Compounds and Their Interactions with Microoganisms. Can. J. Microbiol. 2011, 45, 541–554. [Google Scholar] [CrossRef]
- Babaer, D.; Amara, S.; Ivy, M.; Zhao, Y.; Lammers, P.E.; Titze, J.M.; Tiriveedhi, V.; Babaer, D.; Amara, S.; Ivy, M.; et al. High Salt Induces P-Glycoprotein Mediated Treatment Resistance in Breast Cancer Cells through Store Operated Calcium Influx. Oncotarget 2018, 9, 25193–25205. [Google Scholar] [CrossRef]
- Gueder, N.; Allan, G.; Telliez, M.S.; Hague, F.; Fernandez, J.M.; Sanchez-Fernandez, E.M.; Ortiz-Mellet, C.; Ahidouch, A.; Ouadid-Ahidouch, H. Sp2-Iminosugar α-Glucosidase Inhibitor 1-C-Octyl-2-Oxa-3-Oxocastanospermine Specifically Affected Breast Cancer Cell Migration through Stim1, Β1-Integrin, and FAK Signaling Pathways. J. Cell. Physiol. 2017, 232, 3631–3640. [Google Scholar] [CrossRef]
- Hammadi, M.; Chopin, V.; Matifat, F.; Dhennin-Duthille, I.; Chasseraud, M.; Sevestre, H.; Ouadid-Ahidouch, H. Human Ether À-Gogo K+ Channel 1 (HEag1) Regulates MDA-MB-231 Breast Cancer Cell Migration through Orai1-Dependent Calcium Entry. J. Cell. Physiol. 2012, 227, 3837–3846. [Google Scholar] [CrossRef]
- Necela, B.M.; Crozier, J.A.; Andorfer, C.A.; Lewis-Tuffin, L.; Kachergus, J.M.; Geiger, X.J.; Kalari, K.R.; Serie, D.J.; Sun, Z.; Aspita, A.M.; et al. Folate Receptor-α (FOLR1) Expression and Function in Triple Negative Tumors. PLoS ONE 2015, 10, e0122209. [Google Scholar] [CrossRef]
- Zeng, L.; Luo, L.; Pan, Y.; Luo, S.; Lu, G.; Wu, A. In Vivo Targeted Magnetic Resonance Imaging and Visualized Photodynamic Therapy in Deep-Tissue Cancers Using Folic Acid-Functionalized Superparamagnetic-Upconversion Nanocomposites. Nanoscale 2015, 7, 8946–8954. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, A.; Derrick, J.P. The Folic Acid Biosynthesis Pathway in Bacteria: Evaluation of Potential for Antibacterial Drug Discovery. BioEssays 2002, 24, 637–648. [Google Scholar] [CrossRef]
- Puligujja, P.; Balkundi, S.S.; Kendrick, L.M.; Baldridge, H.M.; Hilaire, J.R.; Bade, A.N.; Dash, P.K.; Zhang, G.; Poluektova, L.Y.; Gorantla, S.; et al. Pharmacodynamics of Long-Acting Folic Acid-Receptor Targeted Ritonavir-Boosted Atazanavir Nanoformulations. Biomaterials 2015, 41, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J. Physiology of Folate and Vitamin B 12 in Health and Disease. Nutr. Rev. 2004, 62 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured Materials Functionalized with Metal Complexes: In Search of Alternatives for Administering Anticancer Metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- US FDA. CFR—Code of Federal Regulations Title 21 CFR. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.1711&SearchTerm=silica (accessed on 4 November 2022).
- Gonçalves, M.C. Sol-Gel Silica Nanoparticles in Medicine: A Natural Choice. Design, Synthesis and Products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging In Vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Kim, J.; Suk Kim, H.; Lee, N.; Kim, T.; Kim, H.; Yu, T.; Chan Song, I.; Kyung Moon, W.; Hyeon, T.; Kim, H.S.; et al. Multifunctional Uniform Nanoparticles Composed of a Magnetite Nanocrystal Core and a Mesoporous Silica Shell for Magnetic Resonance and Fluorescence Imaging and for Drug Delivery. Angew. Chem. Int. Ed. 2008, 47, 8438–8441. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xue, M.; Xia, T.; Ji, Z.; Tarn, D.Y.; Zink, J.I.; Nel, A.E. Use of Size and a Copolymer Design Feature to Improve the Biodistribution and the Enhanced Permeability and Retention Effect of Doxorubicin-Loaded Mesoporous Silica Nanoparticles in a Murine Xenograft Tumor Model. ACS Nano 2011, 5, 4131–4144. [Google Scholar] [CrossRef] [PubMed]
- Souris, J.S.; Lee, C.H.; Cheng, S.H.; Chen, C.T.; Yang, C.S.; Ja-an, A.H.; Mou, C.Y.; Lo, L.W. Surface Charge-Mediated Rapid Hepatobiliary Excretion of Mesoporous Silica Nanoparticles. Biomaterials 2010, 31, 5564–5574. [Google Scholar] [CrossRef]
- Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Hosseini, M. Mesoporous Silica Nanoparticles: Synthesis, Pharmaceutical Applications, Biodistribution, and Biosafety Assessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.H.; Na, J.; Lee, C.H.; Sun, Z.; Wang, S.B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.Z.; et al. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Hooshmand, S.; Mollazadeh, S.; Akrami, N.; Ghanad, M.; El-Fiqi, A.; Baino, F.; Nazarnezhad, S.; Kargozar, S. Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy. Materials 2021, 14, 3337. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.H.; Jeong, H.; Hong, J.; Choi, W.S.; Lee, B.H.; Park, C.Y. An Evaluation of the in Vivo Safety of Nonporous Silica Nanoparticles: Ocular Topical Administration versus Oral Administration. Sci. Rep. 2017, 7, 8238. [Google Scholar] [CrossRef]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Chen, D.; Tang, F. The Absorption, Distribution, Excretion and Toxicity of Mesoporous Silica Nanoparticles in Mice Following Different Exposure Routes. Biomaterials 2013, 34, 2565–2575. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Bai, X.; Jiang, T.; Zhang, Q.; Wang, S. Mesoporous Silica Nanoparticles for Increasing the Oral Bioavailability and Permeation of Poorly Water Soluble Drugs. Mol. Pharm. 2012, 9, 505–513. [Google Scholar] [CrossRef]
- Bukara, K.; Schueller, L.; Rosier, J.; Martens, M.A.; Daems, T.; Verheyden, L.; Eelen, S.; Van Speybroeck, M.; Libanati, C.; Martens, J.A.; et al. Ordered Mesoporous Silica to Enhance the Bioavailability of Poorly Water-Soluble Drugs: Proof of Concept in Man. Eur. J. Pharm. Biopharm. 2016, 108, 220–225. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular Cancer Nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Ding, Y.; Tong, Z.; Jin, L.; Ye, B.; Zhou, J.; Sun, Z.; Yang, H.; Hong, L.; Huang, F.; Wang, W.; et al. An NIR Discrete Metallacycle Constructed from Perylene Bisimide and Tetraphenylethylene Fluorophores for Imaging-Guided Cancer Radio-Chemotherapy. Adv. Mater. 2022, 34, e2106388. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, S.; Yoo, D.; Shin, T.H.; Kim, H.; Gomes, M.D.; Kim, S.H.; Pines, A.; Cheon, J. Distance-Dependent Magnetic Resonance Tuning as a Versatile MRI Sensing Platform for Biological Targets. Nat. Mater. 2017, 16, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Tung, C.H.; Mahmood, U.; Bogdanov, A. In Vivo Imaging of Tumors with Protease-Activated near-Infrared Fluorescent Probes. Nat. Biotechnol. 1999, 17, 375–378. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Synthetic and Natural Coumarins as Cytotoxic Agents. Curr. Med. Chem. Anti Cancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kayser, O.; Woerdenbag, H.J.; van Uden, W.; Pras, N. Structure-Cytotoxicity Relationships of a Series of Natural and Semi-Synthetic Simple Coumarins as Assessed in Two Human Tumour Cell Lines. Z. Fur Nat. C J. Biosci. 1997, 52, 240–244. [Google Scholar] [CrossRef]
- Draoui, N.; Feron, O.; Riant, O.; Sonveaux, P.; Schicke, O.; Fernandes, A.; Kilonda, A.; Vanherck, J.-C.; Marchand, A. 3-Carboxy Substituted Coumarin Derivatives with a Potential Utility for the Treatment of Cancer Diseases 2014. Available online: https://www.lens.org/lens/patent/033-040-060-285-591/frontpage (accessed on 4 November 2022).
- Johnson, J.M.; Cotzia, P.; Fratamico, R.; Mikkilineni, L.; Chen, J.; Colombo, D.; Mollaee, M.; Whitaker-Menezes, D.; Domingo-Vidal, M.; Lin, Z.; et al. MCT1 in Invasive Ductal Carcinoma: Monocarboxylate Metabolism and Aggressive Breast Cancer. Front. Cell Dev. Biol. 2017, 5, 27. [Google Scholar] [CrossRef]
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Parages, M.L.; O’Gara, F. Coumarin: A Novel Player in Microbial Quorum Sensing and Biofilm Formation Inhibition. Appl. Microbiol. Biotechnol. 2018, 102, 2063–2073. [Google Scholar] [CrossRef]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Ovejero Paredes, K.; Díaz-García, D.; García-Almodóvar, V.; Lozano Chamizo, L.; Marciello, M.; Díaz-Sánchez, M.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers 2020, 12, 187. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Hernandes, C.; Coppede, J.D.S.; Bertoni, B.W.; França, S.D.C.; Pereira, A.M.S. Flash Microbiocide: A Rapid and Economic Method for Determination of MBC and MFC. Am. J. Plant Sci. 2013, 4, 850–852. [Google Scholar] [CrossRef]
- Tolosa, J.; Serrano de las Heras, G.; Carrión, B.; Segura, T.; Páez, P.L.; de Lera-Garrido, F.J.; Rodríguez-López, J.; García-Martínez, J.C. Structure-Activity Relationships for Poly(Phenylene)Vinylene Derivatives as Antibacterial Agents. ChemistrySelect 2018, 3, 7327–7332. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Gisbert-Garzarán, M.; Mediero, A.; Carias-Cálix, R.A.; Jiménez-Jiménez, C.; Esteban, J.; Vallet-Regí, M. Arabic Gum plus Colistin Coated Moxifloxacin-Loaded Nanoparticles for the Treatment of Bone Infection Caused by Escherichia coli. Acta Biomater. 2022, 137, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Díaz-García, D.; Sommerova, L.; Martisova, A.; Skoupilova, H.; Prashar, S.; Vaculovic, T.; Kanicky, V.; del Hierro, I.; Hrstka, R.; Gómez-Ruiz, S. Mesoporous Silica Nanoparticles Functionalized with a Dialkoxide Diorganotin(IV) Compound: In Search of More Selective Systems against Cancer Cells. Microporous Mesoporous Mater. 2020, 300, 110154. [Google Scholar] [CrossRef]
- Ovejero-Paredes, K.; Díaz-García, D.; Mena-Palomo, I.; Marciello, M.; Lozano-Chamizo, L.; Morato, Y.L.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Synthesis of a Theranostic Platform Based on Fibrous Silica Nanoparticles for the Enhanced Treatment of Triple-Negative Breast Cancer Promoted by a Combination of Chemotherapeutic Agents. Biomater. Adv. 2022, 137, 212823. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanaka, M.; Inagaki, A.; Wanibuchi, H.; Izumi, Y.; Miura, K.; Nagayama, K.; Shiota, M.; Iwao, H. Establishment of a 5-Fluorouracil-Resistant Triple-Negative Breast Cancer Cell Line. Int. J. Oncol. 2013, 43, 1985–1991. [Google Scholar] [CrossRef] [Green Version]
- Díaz-García, D.; Ardiles, P.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.; Gómez-Ruiz, S. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Matynia, B.; Młodzinska, E.; Hryniewicz, W. Antimicrobial Susceptibility Patterns of Staphylococcus Aureus in Poland Obtained by the National Quality Assurance Programme. Clin. Microbiol. Infect. 2005, 11, 379–385. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Lin, A.; Huang, N.; Long, L.; Gang, Y.; Liu, J. Folic Acid-Modified Mesoporous Silica Nanoparticles with PH-Responsiveness Loaded with Amp for an Enhanced Effect against Anti-Drug-Resistant Bacteria by Overcoming Efflux Pump Systems. Biomater. Sci. 2018, 6, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, K.; Bhise, K.; Sanchez, H.; Kebriaei, R.; Luong, D.; Sau, S.; Abdelhady, H.; Rybak, M.J.; Andes, D.; Iyer, A.K. Folate Functionalized Lipid Nanoparticles for Targeted Therapy of Methicillin-Resistant Staphylococcus Aureus. Pharmaceutics 2021, 13, 1791. [Google Scholar] [CrossRef]
- Albesa, I.; Becerra, M.C.; Battán, P.C.; Páez, P.L. Oxidative Stress Involved in the Antibacterial Action of Different Antibiotics. Biochem. Biophys. Res. Commun. 2004, 317, 605–609. [Google Scholar] [CrossRef]
- Becerra, M.C.; Albesa, I. Oxidative Stress Induced by Ciprofloxacin in Staphylococcus Aureus. Biochem. Biophys. Res. Commun. 2002, 297, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Foster, T.J. Immune Evasion by Staphylococci. Nat. Rev. Microbiol. 2005, 3, 948–958. [Google Scholar] [CrossRef]
- Mourenza, Á.; Gil, J.A.; Mateos, L.M.; Letek, M. Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance. Antioxidants 2020, 9, 361. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Bustos, P.S.; Deza-Ponzio, R.; Páez, P.L.; Cabrera, J.L.; Virgolini, M.B.; Ortega, M.G. Flavonoids as protective agents against oxidative stress induced by gentamicin in systemic circulation. Potent protective activity and microbial synergism of luteolin. Food Chem. Toxicol. 2018, 118, 294–302. [Google Scholar] [CrossRef]
- Scolari, I.R.; Páez, P.L.; Musri, M.M.; Petiti, J.P.; Torres, A.; Granero, G.E. Rifampicin loaded in alginate/chitosan nanoparticles as a promising pulmonary carrier against Staphylococcus aureus. Drug Deliv. Transl. Res. 2020, 10, 1403–1417. [Google Scholar] [CrossRef] [PubMed]









| Material | BET Surface (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| MSN | 1042 | 1.04 | 3.81 |
| 1-MSN-Sn | 691 | 0.41 | 2.06 |
| 2-MSN-Sn | 702 | 0.78 | 2.45 |
| SBA-15 | 650 | 0.80 | 4.74 |
| 2-SBA-Sn | 394 | 0.51 | 4.08 |
| FSPm | 317 | 0.49 | 5.80 |
| 2-FSPm-Sn | 132 | 0.23 | - |
| FSPs | 315 | 0.69 | 8.91 |
| 2-FSPs-Sn | 168 | 0.45 | - |
| Material | %AP | %COU + FA | Experimental Sn wt% |
|---|---|---|---|
| 1-MSN-Sn | 5.0 | 10.1 | 7.2 |
| 2-MSN-Sn | 5.0 | 2.4 | 2.0 |
| 2-SBA-Sn | 8.5 | 3.2 | 0.9 |
| 2-FSPm-Sn | 2.0 | 3.5 | 0.5 |
| 2-FSPs-Sn | 5.0 | 3.7 | 0.7 |
| Material | hkl | 2θ (°) | dhkl (nm) | a0 (nm) |
|---|---|---|---|---|
| MSN | 100 | 2.45 | 3.60 | 4.16 |
| 110 | 4.12 | 2.14 | 2.48 | |
| 200 | 4.67 | 1.89 | 2.18 | |
| MSN-AP | 100 | 2.45 | 3.60 | 4.16 |
| 1-MSN | 100 | 2.56 | 3.46 | 4.00 |
| 1-MSN-Sn | 100 | 2.61 | 3.39 | 3.91 |
| 2-MSN | 100 | 2.53 | 3.49 | 4.03 |
| 2-MSN-Sn | 100 | 2.53 | 3.49 | 4.03 |
| SBA-15 | 100 | 1.18 | 7.50 | 8.66 |
| 110 | 1.91 | 4.63 | 5.35 | |
| 200 | 2.17 | 4.08 | 4.70 | |
| SBA-AP | 100 | 1.21 | 7.34 | 8.47 |
| 2-SBA | 100 | 1.18 | 7.50 | 8.66 |
| 2-SBA-Sn | 100 | 1.20 | 7.34 | 8.47 |
| Material | IC50 µM vs. [Sn] | |
|---|---|---|
| HEK-293T | MDA-MB-231 | |
| 1-MSN-Sn | 2.11 ± 0.05 | 7.73 ± 0.24 |
| 2-MSN-Sn | 1.63 ± 0.52 | 0.69 ± 0.31 |
| 2-SBA-Sn | 5.31 ± 1.94 | 0.81 ± 0.05 |
| 2-FSPm-Sn | 0.18 ± 1.06 | 0.44 ± 0.03 |
| 2-FSPs-Sn | 0.91 ± 0.50 | 0.83 ± 0.42 |
| Material | ATCC29213 | |
|---|---|---|
| MIC | MBC | |
| 1-MSN-Sn | >2000 (>144.0) | >2000 (>144.0) |
| 2-MSN-Sn | 125 (2.5) | 2000 (40.0) |
| 2-SBA-Sn | >2000 (>17.0) | >2000 (>17.0) |
| 2-FSPm-Sn | >2000 (>10.0) | >2000 (>10.0) |
| 2-FSPs-Sn | >2000 (>14.0) | >2000 (>14.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugalde-Arbizu, M.; Aguilera-Correa, J.J.; García-Almodóvar, V.; Ovejero-Paredes, K.; Díaz-García, D.; Esteban, J.; Páez, P.L.; Prashar, S.; San Sebastian, E.; Filice, M.; et al. Dual Anticancer and Antibacterial Properties of Silica-Based Theranostic Nanomaterials Functionalized with Coumarin343, Folic Acid and a Cytotoxic Organotin(IV) Metallodrug. Pharmaceutics 2023, 15, 560. https://doi.org/10.3390/pharmaceutics15020560
Ugalde-Arbizu M, Aguilera-Correa JJ, García-Almodóvar V, Ovejero-Paredes K, Díaz-García D, Esteban J, Páez PL, Prashar S, San Sebastian E, Filice M, et al. Dual Anticancer and Antibacterial Properties of Silica-Based Theranostic Nanomaterials Functionalized with Coumarin343, Folic Acid and a Cytotoxic Organotin(IV) Metallodrug. Pharmaceutics. 2023; 15(2):560. https://doi.org/10.3390/pharmaceutics15020560
Chicago/Turabian StyleUgalde-Arbizu, Maider, John Jairo Aguilera-Correa, Victoria García-Almodóvar, Karina Ovejero-Paredes, Diana Díaz-García, Jaime Esteban, Paulina L. Páez, Sanjiv Prashar, Eider San Sebastian, Marco Filice, and et al. 2023. "Dual Anticancer and Antibacterial Properties of Silica-Based Theranostic Nanomaterials Functionalized with Coumarin343, Folic Acid and a Cytotoxic Organotin(IV) Metallodrug" Pharmaceutics 15, no. 2: 560. https://doi.org/10.3390/pharmaceutics15020560