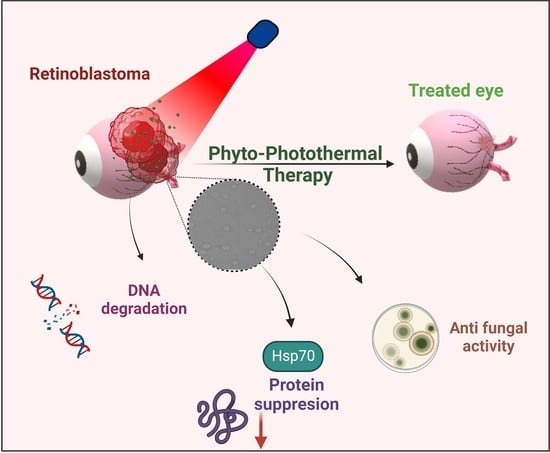
Bioactive Polymeric Nanoparticles of Moringa oleifera Induced Phyto-Photothermal Sensitization for the Enhanced Therapy of Retinoblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef] [PubMed]
- Parulekar, M.V. Retinoblastoma—Current treatment and future direction. Early Hum. Dev. 2010, 86, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Dimaras, H.; Kimani, K.; Dimba, E.A.O.; Gronsdahl, P.; White, A.; Chan, H.S.L.; Gallie, B.L. Retinoblastoma. Lancet 2012, 379, 1436–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimaras, H.; Corson, T.W.; Cobrinik, D.; White, A.; Zhao, J.; Munier, F.L.; Abramson, D.H.; Shields, C.L.; Chantada, G.L.; Njuguna, F.; et al. Retinoblastoma. Nat. Rev. Dis. Prim. 2015, 1, 15021. [Google Scholar] [CrossRef] [Green Version]
- White, L. Chemotherapy in retinoblastoma: Current status and future directions. Am. J. Pediatr. Hematol. Oncol. 1991, 13, 189–201. [Google Scholar] [CrossRef]
- Kaewkhaw, R.; Rojanaporn, D. Retinoblastoma: Etiology, Modeling, and Treatment. Cancers 2020, 12, 2304. [Google Scholar] [CrossRef]
- Jabbour, P.; Chalouhi, N.; Tjoumakaris, S.; Gonzalez, L.F.; Dumont, A.S.; Chitale, R.; Rosenwasser, R.; Bianciotto, C.G.; Shields, C. Pearls and pitfalls of intraarterial chemotherapy for retinoblastoma: A review. J. Neurosurg. Pediatr. 2012, 10, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Zanaty, M.; Barros, G.; Chalouhi, N.; Starke, R.M.; Manasseh, P.; Tjoumakaris, S.I.; Shields, C.L.; Hasan, D.; Bulsara, K.; Rosenwasser, R.H.; et al. Update on Intra-Arterial Chemotherapy for Retinoblastoma. Sci. World J. 2014, 2014, 869604. [Google Scholar] [CrossRef]
- Stacey, A.W.; De Francesco, S.; Borri, M.; Hadjistilianou, T. The Addition of Topotecan to Melphalan in the Treatment of Retinoblastoma with Intra-arterial Chemotherapy. Ophthalmol. Retin. 2021, 5, 824–830. [Google Scholar] [CrossRef]
- Kiratli, H.; Koç, I.; Öztürk, E.; Varan, A.; Akyüz, C. Comparison of intravitreal melphalan with and without topotecan in the management of vitreous disease in retinoblastoma. Jpn. J. Ophthalmol. 2020, 64, 351–358. [Google Scholar] [CrossRef]
- Delrish, E.; Jabbarvand, M.; Ghassemi, F.; Amoli, F.A.; Atyabi, F.; Lashay, A.; Soleimani, M.; Aghajanpour, L.; Dinarvand, R. Efficacy of topotecan nanoparticles for intravitreal chemotherapy of retinoblastoma. Exp. Eye Res. 2021, 204, 108423. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.M.; Federico, S.; Carcaboso, A.M.; Shen, Y.; Schaiquevich, P.; Zhang, J.; Egorin, M.; Stewart, C.; Dyer, M.A. Subconjunctival carboplatin and systemic topotecan treatment in preclinical models of retinoblastoma. Cancer 2011, 117, 421–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieślik, K.; Rogowska, A.; Hautz, W. Focal therapies for retinoblastoma. Klin. Ocz. Acta Ophthalmol. Pol. 2022, 124, 131–136. [Google Scholar] [CrossRef]
- Shields, C.L.; Honavar, S.G.; Meadows, A.T.; Shields, J.A.; Demirci, H.; Singh, A.; Friedman, D.L.; Naduvilath, T.J. Chemoreduction plus focal therapy for retinoblastoma: Factors predictive of need for treatment with external beam radiotherapy or enucleation11InternetAdvance publication at ajo.com April 8, 2002. Am. J. Ophthalmol. 2002, 133, 657–664. [Google Scholar] [CrossRef]
- Mudigunda, S.V.; Pemmaraju, D.B.; Paradkar, S.; Puppala, E.R.; Gawali, B.; Upadhyayula, S.M.; Vegi Gangamodi, N.; Rengan, A.K. Multifunctional Polymeric Nanoparticles for Chemo/Phototheranostics of Retinoblastoma. ACS Biomater. Sci. Eng. 2022, 8, 151–160. [Google Scholar] [CrossRef]
- Yang, X.-J.; Li, X.-L.; Chen, H.-Y.; Xu, J.-J. NIR-Activated Spatiotemporally Controllable Nanoagent for Achieving Synergistic Gene-Chemo-Photothermal Therapy in Tumor Ablation. ACS Appl. Bio Mater. 2019, 2, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, M.V.; Pleshko, N. Wavelength-dependent penetration depth of near infrared radiation into cartilage. Analyst 2015, 140, 2093–2100. [Google Scholar] [CrossRef] [Green Version]
- Rengan, A.K.; Bukhari, A.B.; Pradhan, A.; Malhotra, R.; Banerjee, R.; Srivastava, R.; De, A. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015, 15, 842–848. [Google Scholar] [CrossRef]
- Appidi, T.; PS, R.; Chinchulkar, S.A.; Pradhan, A.; Begum, H.; Shetty, V.; Srivastava, R.; Ganesan, P.; Rengan, A.K. A plasmon-enhanced fluorescent gold coated novel lipo-polymeric hybrid nanosystem: Synthesis, characterization and application for imaging and photothermal therapy of breast cancer. Nanoscale 2022, 14, 9112–9123. [Google Scholar] [CrossRef]
- Jogdand, A.; Alvi, S.B.; Rajalakshmi, P.S.; Rengan, A.K. NIR-dye based mucoadhesive nanosystem for photothermal therapy in breast cancer cells. J. Photochem. Photobiol. B Biol. 2020, 208, 111901. [Google Scholar] [CrossRef]
- Farhat, W.; Yeung, V.; Ross, A.; Kahale, F.; Boychev, N.; Kuang, L.; Chen, L.; Ciolino, J.B. Advances in biomaterials for the treatment of retinoblastoma. Biomater. Sci. 2022, 10, 5391–5429. [Google Scholar] [CrossRef] [PubMed]
- Bin, L.; Du, Y.; Zhang, Y.; Xiao, Q.; Chen, X.; Liu, Z.; Du, Z. Phase-changeable nanoparticles loaded with FeⅢ-tannic acid/paclitaxel for retinoblastoma treatment. J. Drug Deliv. Sci. Technol. 2022, 78, 103989. [Google Scholar] [CrossRef]
- Zheng, W.; Li, X.; Zou, H.; Xu, Y.; Li, P.; Zhou, X.; Wu, M. Dual-Target Multifunctional Superparamagnetic Cationic Nanoliposomes for Multimodal Imaging-Guided Synergistic Photothermal/Photodynamic Therapy of Retinoblastoma. Int. J. Nanomed. 2022, 17, 3217–3237. [Google Scholar] [CrossRef]
- Li, M.; Bian, X.; Chen, X.; Fan, N.; Zou, H.; Bao, Y.; Zhou, Y. Multifunctional liposome for photoacoustic/ultrasound imaging-guided chemo/photothermal retinoblastoma therapy. Drug Deliv. 2022, 29, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Pedrosa, P.; Lima, J.C.; Fernandes, A.R.; Baptista, P.V. Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of Gold Nanoparticles. Sci. Rep. 2017, 7, 10872. [Google Scholar] [CrossRef] [PubMed]
- Khademi, R.; Razminia, A. Selective nano-thermal therapy of human retinoblastoma in retinal laser surgery. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102102. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Chen, S.; Liu, J.; Wang, D.; Huang, Y. Liposome-based multifunctional nanoplatform as effective therapeutics for the treatment of retinoblastoma. Acta Pharm. Sin. B 2022, 12, 2731–2739. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology for Pediatric Retinoblastoma Therapy. Pharmaceuticals 2022, 15, 1087. [Google Scholar] [CrossRef]
- Evans, C.G.; Chang, L.; Gestwicki, J.E. Heat shock protein 70 (hsp70) as an emerging drug target. J. Med. Chem. 2010, 53, 4585–4602. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Chen, X.; Wang, X.; Liu, S.; Liu, J.; Xie, Z. Enhanced efficacy of photothermal therapy by combining a semiconducting polymer with an inhibitor of a heat shock protein. Mater. Chem. Front. 2019, 3, 127–136. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, W.; Du, L.; Xu, C.; Li, J. Synergy of hypoxia relief and heat shock protein inhibition for phototherapy enhancement. J. Nanobiotechnol. 2021, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ren, Q.; Hao, R.; Sun, Z. Innovative strategies to boost photothermal therapy at mild temperature mediated by functional nanomaterials. Mater. Des. 2022, 214, 110391. [Google Scholar] [CrossRef]
- Fraiser, L.H.; Kanekal, S.; Kehrer, J.P. Cyclophosphamide Toxicity. Drugs 1991, 42, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Latchman, J.; Guastella, A.; Tofthagen, C. 5-Fluorouracil toxicity and dihydropyrimidine dehydrogenase enzyme: Implications for practice. Clin. J. Oncol. Nurs. 2014, 18, 581–585. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Dhamija, E.; Meena, P.; Ramalingam, V.; Sahoo, R.; Rastogi, S.; Thulkar, S. Chemotherapy-induced pulmonary complications in cancer: Significance of clinicoradiological correlation. Indian J. Radiol. Imaging 2020, 30, 20–26. [Google Scholar] [CrossRef]
- Desai, A.; Qazi, G.; Ganju, R.; El-Tamer, M.; Singh, J.; Saxena, A.; Bedi, Y.; Taneja, S.; Bhat, H. Medicinal Plants and Cancer Chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Mehta, A. Anticancer potential of medicinal plants and their phytochemicals: A review. Braz. J. Bot. 2015, 38, 199–210. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Tekayev, M.; Bostancieri, N.; Saadat, K.A.S.M.; Turker, M.; Yuncu, M.; Ulusal, H.; Cicek, H.; Arman, K. Effects of Moringa oleifera Lam Extract (MOLE) in the heat shock protein 70 expression and germ cell apoptosis on experimentally induced cryptorchid testes of rats. Gene 2019, 688, 140–150. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Tiloke, C.; Phulukdaree, A.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera Gold Nanoparticles Modulate Oncogenes, Tumor Suppressor Genes, and Caspase-9 Splice Variants in A549 Cells. J. Cell. Biochem. 2016, 13, 2302–2314. [Google Scholar] [CrossRef] [PubMed]
- Barhoi, D.; Upadhaya, P.; Barbhuiya, S.N.; Giri, A.; Giri, S. Aqueous Extract of Moringa oleifera Exhibit Potential Anticancer Activity and can be Used as a Possible Cancer Therapeutic Agent: A Study Involving In Vitro and In Vivo Approach. J. Am. Coll. Nutr. 2021, 40, 70–85. [Google Scholar] [CrossRef]
- Khan, F.; Pandey, P.; Ahmad, V.; Upadhyay, T.K. Moringa oleifera methanolic leaves extract induces apoptosis and G0/G1 cell cycle arrest via downregulation of Hedgehog Signaling Pathway in human prostate PC-3 cancer cells. J. Food Biochem. 2020, 44, e13338. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, D.; Appidi, T.; Minhas, G.; Singh, S.P.; Khan, N.; Pal, M.; Srivastava, R.; Rengan, A.K. Chlorophyll rich biomolecular fraction of A. cadamba loaded into polymeric nanosystem coupled with Photothermal Therapy: A synergistic approach for cancer theranostics. Int. J. Biol. Macromol. 2018, 110, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of Free Radical Scavenging Activity of Plant Extracts Through DPPH Assay: An EPR and UV—Vis Study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Pagano, C.; Perioli, L.; Baiocchi, C.; Bartoccini, A.; Beccari, T.; Blasi, F.; Calarco, P.; Ceccarini, M.R.; Cossignani, L.; di Michele, A.; et al. Preparation and characterization of polymeric microparticles loaded with Moringa oleifera leaf extract for exuding wound treatment. Int. J. Pharm. 2020, 587, 119700. [Google Scholar] [CrossRef]
- Iliakis, G.; Rosidi, B.; Wang, M.; Wang, H. Plasmid-based assays for DNA end-joining in vitro. Methods Mol. Biol. 2006, 314, 123–131. [Google Scholar] [CrossRef]
- Kazeem, G.O.; Adedayo, F.E.; Thomas, A.O. Hyperthermal-induced stress effects on survival and expression of heat shock protein (HSP) genes in Nile tilapia, Oreochromis niloticus fingerlings fed aqueous extract from Moringa oleifera leaf. Livest. Res. Rural Dev. 2017, 29, 79. [Google Scholar]
- Alvi, S.B.; Appidi, T.; Deepak, B.P.; Rajalakshmi, P.S.; Minhas, G.; Singh, S.P.; Begum, A.; Bantal, V.; Srivastava, R.; Khan, N.; et al. The “nano to micro” transition of hydrophobic curcumin crystals leading to in situ adjuvant depots for Au-liposome nanoparticle mediated enhanced photothermal therapy. Biomater. Sci. 2019, 7, 3866–3875. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudigunda, S.V.; Pemmaraju, D.B.; Sankaranarayanan, S.A.; Rengan, A.K. Bioactive Polymeric Nanoparticles of Moringa oleifera Induced Phyto-Photothermal Sensitization for the Enhanced Therapy of Retinoblastoma. Pharmaceutics 2023, 15, 475. https://doi.org/10.3390/pharmaceutics15020475
Mudigunda SV, Pemmaraju DB, Sankaranarayanan SA, Rengan AK. Bioactive Polymeric Nanoparticles of Moringa oleifera Induced Phyto-Photothermal Sensitization for the Enhanced Therapy of Retinoblastoma. Pharmaceutics. 2023; 15(2):475. https://doi.org/10.3390/pharmaceutics15020475
Chicago/Turabian StyleMudigunda, Sushma Venkata, Deepak B. Pemmaraju, Sri Amruthaa Sankaranarayanan, and Aravind Kumar Rengan. 2023. "Bioactive Polymeric Nanoparticles of Moringa oleifera Induced Phyto-Photothermal Sensitization for the Enhanced Therapy of Retinoblastoma" Pharmaceutics 15, no. 2: 475. https://doi.org/10.3390/pharmaceutics15020475