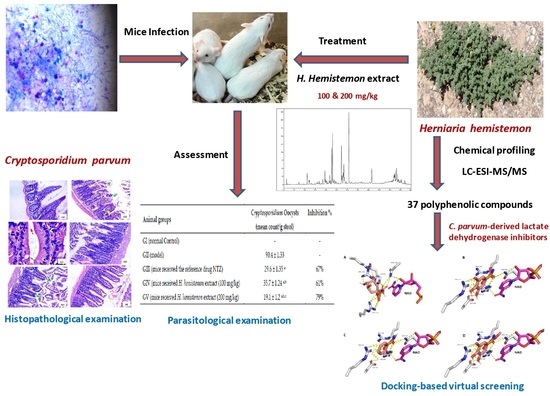
Polyphenolic Profile of Herniaria hemistemon Aerial Parts Extract and Assessment of Its Anti-Cryptosporidiosis in a Murine Model: In Silico Supported In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Extraction, and Fractionation
2.2. Phytochemical Analysis, Total Phenolic (TPC) and Total Flavonoid (TFC) Contents and Antioxidant Properties
2.3. In Vivo Anticryptosporidial Activity
2.3.1. Animals
2.3.2. Immunosuppression and Induction of Infection
- -
- GII: Immunosuppressed and infected (model);
- -
- -
- GIV: Immunosuppressed, infected, and received H. hemistemon extract at a dose of 100 mg/kg every day for 5 days;
- -
- GV: Immunosuppressed, infected, and received H. hemistemon extract at a dose of 200 mg/kg every day for 5 days.
2.3.3. Parasitological Examination
2.4. Histopathological Examination
2.5. Docking-Based Virtual Screening
2.6. Statistical Analysis
3. Results
3.1. Chemical Profiling
3.2. Total Polyphenols, Total Flavonoids and Antioxidant Properties
3.3. Parasitological Examination
3.4. Effects of H. hemistemon Aerial-Part Extract on the Small Intestine
3.5. Effects of H. hemistemon Aerial-Part Extract on the Liver
3.6. Docking-Based Virtual Screening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Abdelmaksoud, H.F.; El-Ashkar, A.M.; Elgohary, S.A.; El-Wakil, E.S. Potential Therapeutic and Prophylactic Effects of Asafoetida in Murine Cryptosporidiosis. J. Parasit. Dis. 2020, 44, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Guk, S.-M.; Seo, M.; Park, Y.-K.; Oh, M.-D.; Choe, K.-W.; Kim, J.-L.; Choi, M.-H.; Hong, S.-T.; Chai, J.-Y. Parasitic Infections in HIV-Infected Patients Who Visited Seoul National University Hospital during the Period 1995–2003. Korean J. Parasitol. 2005, 43, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Wakil, E.S.; Salem, A.E.; Al-Ghandour, A.M. Evaluation of Possible Prophylactic and Therapeutic Effect of Mefloquine on Experimental Cryptosporidiosis in Immunocompromised Mice. J. Parasit. Dis. 2021, 45, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Diptyanusa, A.; Sari, I.P. Treatment of Human Intestinal Cryptosporidiosis: A Review of Published Clinical Trials. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Krstin, S.; Sobeh, M.; Braun, M.S.; Wink, M. Anti-Parasitic Activities of Allium sativum and Allium cepa against Trypanosoma brucei and Leishmania tarentolae. Medicines 2018, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Krstin, S.; Sobeh, M.; Braun, M.S.; Wink, M. Tulbaghia violacea and Allium ursinum Extracts Exhibit Anti-Parasitic and Antimicrobial Activities. Molecules 2018, 23, 313. [Google Scholar] [CrossRef] [Green Version]
- Soufy, H.; Nadia, M.; Nasr, S.M.; Abd El-Aziz, T.H.; Khalil, F.A.; Ahmed, Y.F.; Abou Zeina, H.A. Effect of Egyptian Propolis on Cryptosporidiosis in Immunosuppressed Rats with Special Emphasis on Oocysts Shedding, Leukogram, Protein Profile and Ileum Histopathology. Asian Pac. J. Trop. Med. 2017, 10, 253–262. [Google Scholar] [CrossRef]
- El-Wakil, E.S.; El-Shazly, M.A.; El-Ashkar, A.M.; Aboushousha, T.; Ghareeb, M.A. Chemical Profiling of Verbena officinalis and Assessment of Its Anti-Cryptosporidial Activity in Experimentally Infected Immunocompromised Mice. Arab. J. Chem. 2022, 15, 103945. [Google Scholar] [CrossRef]
- Abd El-Hamed, W.F.; Yousef, N.S.; Mazrou, Y.S.; Elkholy, W.A.; El-Refaiy, A.I.; Elfeky, F.A.; Albadrani, M.; El-Tokhy, A.I.; Abdelaal, K. Anticryptosporidium Efficacy of Olea europaea and Ficus carica Leaves Extract in Immunocompromised Mice Associated with Biochemical Characters and Antioxidative System. Cells 2021, 10, 2419. [Google Scholar] [CrossRef]
- Ghazanfar, S.A. Handbook of Arabian Medicinal Plants; CRC Press: Boca Raton, FL, USA, 1994; ISBN 0-8493-0539-X. [Google Scholar]
- Tackholm, V.; Boulos, L. Students’ Flora of Egypt; Cairo University: Giza, Egypt, 1974. [Google Scholar]
- Sabitha, S.; Maher, K.; Mohamed, M. Medicinal Plants Diversity and Their Conservation Status in the United Arab Emirates (UAE). J. Med. Plants Res. 2012, 6, 1304–1322. [Google Scholar] [CrossRef]
- Chandra, S.; Rawat, D.S. Medicinal Plants of the Family Caryophyllaceae: A Review of Ethno-Medicinal Uses and Pharmacological Properties. Integr. Med. Res. 2015, 4, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Kozachok, S.; Kolodziejczyk-Czepas, J.; Marchyshyn, S.; Wojtanowski, K.K.; Zgórka, G.; Oleszek, W. Comparison of Phenolic Metabolites in Purified Extracts of Three Wild-Growing Herniaria L. Species and Their Antioxidant and Anti-Inflammatory Activities In Vitro. Molecules 2022, 27, 530. [Google Scholar] [CrossRef]
- Radwan, N.M.; Nazif, L.M.; Setta, A. The Lipid and Flavonoidal Constituents of Herniaria nemistemon J. Gay and Their Biological Activity. Egypt. J. Pharm. Sci. 2006, 47, 29–41. [Google Scholar]
- Elhagali, G.A.; Abozeed, A.E.; Youssif, Y.M. Investigation of Bioactive Constituents and Biological Activities of Different Fractions from Herniaria hemistemon J. Gay. Al-Azhar Bull. Sci. 2019, 30, 67–80. [Google Scholar] [CrossRef]
- Yousif, F.; Hifnawy, M.S.; Soliman, G.; Boulos, L.; Labib, T.; Mahmoud, S.; Ramzy, F.; Yousif, M.; Hassan, I.; Mahmoud, K.; et al. Large-Scale in Vitro. Screening of Egyptian Native and Cultivated Plants for Schistosomicidal Activity. Pharm. Biol. 2007, 45, 501–510. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Mohamed, T.; Saad, A.M.; Refahy, L.A.-G.; Sobeh, M.; Wink, M. HPLC-DAD-ESI-MS/MS Analysis of Fruits from Firmiana simplex (L.) and Evaluation of Their Antioxidant and Antigenotoxic Properties. J. Pharm. Pharmacol. 2018, 70, 133–142. [Google Scholar] [CrossRef]
- Tarazona, R.; Blewett, D.A.; Carmona, M.D. Cryptosporidium parvum Infestion in Experimentally Infected Mice: Infection Dynamics and Effect of Immunosuppression. Folia Parasitol. 1998, 45, 101–107. [Google Scholar]
- Li, X.; Brasseur, P.; Agnamey, P.; Leméteil, D.; Favennec, L.; Ballet, J.-J.; Rossignol, J.-F. Long-Lasting Anticryptosporidial Activity of Nitazoxanide in an Immunosuppressed Rat Model. Folia Parasitol. 2003, 50, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Barnes, J.M.; Paget, G.E. 2 Mechanisms of Toxic Action. Prog. Med. Chem. 1965, 4, 18–38. [Google Scholar]
- Henriksen, S.A.; Pohlenz, J.F.L. Staining of Cryptosporidia by a Modified Ziehl-Neelsen Technique. Acta Vet. Scand. 1981, 22, 594. [Google Scholar] [CrossRef]
- Benamrouz, S.; Guyot, K.; Gazzola, S.; Mouray, A.; Chassat, T.; Delaire, B.; Chabé, M.; Gosset, P.; Viscogliosi, E.; Dei-Cas, E.; et al. Cryptosporidium parvum Infection in SCID Mice Infected with Only One Oocyst: QPCR Assessment of Parasite Replication in Tissues and Development of Digestive Cancer. PLoS ONE 2012, 7, e51232. [Google Scholar] [CrossRef] [PubMed]
- Hosking, B.C.; Watson, T.G.; Leathwick, D.M. Multigeneric Resistance to Oxfendazole by Nematodes in Cattle. Vet. Rec. 1996, 138, 67. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. In Histopathology; Humana Press: New York, NY, USA, 2014; pp. 31–43. [Google Scholar]
- Cook, W.J.; Senkovich, O.; Hernandez, A.; Speed, H.; Chattopadhyay, D. Biochemical and Structural Characterization of Cryptosporidium Parvum Lactate Dehydrogenase. Int. J. Biol. Macromol. 2015, 74, 608–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Fu, A.; Zhang, L. Progress in Molecular Docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-C.; Chu, P.-Y.; Chen, C.-M.; Lin, J.-H. IdTarget: A Web Server for Identifying Protein Targets of Small Chemical Molecules with Robust Scoring Functions and a Divide-and-Conquer Docking Approach. Nucleic Acids Res. 2012, 40, W393–W399. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.; Morris, G.M.; Forli, S. Using AutoDock 4 and AutoDock Vina with AutoDockTools: A Tutorial; The Scripps Research Institute Molecular Graphics Laboratory: La Jolla, CA, USA, 2012; Volume 10550, p. 92037. [Google Scholar]
- Mead, J.R.; McNair, N. Antiparasitic Activity of Flavonoids and Isoflavones against Cryptosporidium parvum and Encephalitozoon Intestinalis. FEMS Microbiol. Lett. 2006, 259, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Scholz, E.; Heinrich, M.; Hunkler, D. Caffeoylquinic Acids and Some Biological Activities of Pluchea symphytifolia. Planta Med. 1994, 60, 360–364. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Valente, A.H.; Thamsborg, S.M.; Simonsen, H.T.; Boas, U.; Enemark, H.L.; López-Muñoz, R.; Williams, A.R. Antiparasitic Activity of Chicory (Cichorium intybus) and Its Natural Bioactive Compounds in Livestock: A Review. Parasites Vectors 2018, 11, 475. [Google Scholar] [CrossRef] [Green Version]
- Teichmann, K.; Kuliberda, M.; Schatzmayr, G.; Pacher, T.; Zitterl-Eglseer, K.; Joachim, A.; Hadacek, F. In Vitro Inhibitory Effects of Plant-Derived by-Products against Cryptosporidium parvum. Parasite 2016, 23, 41. [Google Scholar] [CrossRef] [Green Version]
- Aboelsoued, D.; Abo-Aziza, F.A.M.; Mahmoud, M.H.; Abdel Megeed, K.N.; Abu El Ezz, N.M.T.; Abu-Salem, F.M. Anticryptosporidial Effect of Pomegranate Peels Water Extract in Experimentally Infected Mice with Special Reference to Some Biochemical Parameters and Antioxidant Activity. J. Parasit. Dis. 2019, 43, 215–228. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Eastman, P.; Friedrichs, M.S.; Chodera, J.D.; Radmer, R.J.; Bruns, C.M.; Ku, J.P.; Beauchamp, K.A.; Lane, T.J.; Wang, L.-P.; Shukla, D.; et al. OpenMM 4: A Reusable, Extensible, Hardware Independent Library for High Performance Molecular Simulation. J. Chem. Theory Comput. 2013, 9, 461–469. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Chemical Biology; Hempel, J.E., Williams, C.H., Hong, C.C., Eds.; Springer: New York, NY, USA, 2015; pp. 243–250. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [Green Version]





| No. | Rt | M-H | MS/MS | Proposed Compounds |
|---|---|---|---|---|
| 1 | 1.80 | 191 | 111 | Quinic acid |
| 2 | 3.30 | 169 | 125 | Gallic acid |
| 3 | 4.86 | 315 | 153 | Protocatechuic acid glucoside |
| 4 | 7.20 | 353 | 191 | Chlorogenic acid |
| 5 | 7.59 | 341 | 135, 179 | Caffeoyl glucose |
| 6 | 8.05 | 285 | 153 | Protocatechuic acid pentoside |
| 7 | 8.67 | 339 | 177 | Esculetin glucoside |
| 8 | 9.01 | 137 | 108 | Hydroxybenzoic acid |
| 9 | 10.33 | 447 | 153, 315 | Protocatechuic acid caffeoyl pentoside |
| 10 | 10.31 | 183 | 125 | Methyl gallate |
| 11 | 10.51 | 257 | 121 | Esculetin sulfate |
| 12 | 11.05 | 353 | 191 | Neochlorogenic acid |
| 13 | 12.37 | 177 | 121, 133 | Esculetin |
| 14 | 12.67 | 329 | 167 | Vanillic acid glucoside |
| 15 | 13.75 | 329 | 167 | Caffeoyl vanillic acid |
| 16 | 14.59 | 431 | 153 | Protocatechuic acid coumaroyl pentoside |
| 17 | 15.34 | 337 | 163, 191 | Coumaroylquinic acid |
| 18 | 15.44 | 247 | 167 | Vanillic acid sulfate |
| 19 | 16.46 | 305 | 151, 287 | Gallocatechin |
| 20 | 18.50 | 337 | 191 | Coumaroylquinic acid |
| 21 | 18.62 | 303 | 137 | Hydroxybenzoic acid methyl gallate |
| 22 | 18.92 | 563 | 353, 383 | Schaftoside |
| 23 | 19.66 | 755 | 255, 301 | Quercetin rhamnosyl-rutinoside |
| 24 | 19.85 | 563 | 353, 383 | Vicenin 1 |
| 25 | 19.88 | 625 | 179, 317 | Myricetin rutinoside |
| 26 | 21.93 | 739 | 179, 285, 575 | Kaempferol dirhamnosyl-glucoside |
| 27 | 22.61 | 769 | 299, 315 | Isorhamnetin rhamnosyl-rutinoside |
| 28 | 23.15 | 771 | 299, 315, 477 | Isorhamnetin digalactosyl-pentoside |
| 29 | 23.67 | 609 | 271, 301 | Rutin |
| 30 | 24.18 | 463 | 271, 301 | Quercetin glucoside |
| 31 | 25.65 | 593 | 285 | Kaempferol rutinoside |
| 32 | 27.12 | 515 | 161, 179, 191 | Dicaffeoylquinic acid |
| 33 | 27.45 | 623 | 300, 315 | Isorhamnetin rutinoside |
| 34 | 28.66 | 395 | 300, 315 | Isorhamnetin sulfate |
| 35 | 29.98 | 593 | 179, 271, 315 | Isorhamnetin rhamnosyl-pentoside |
| 36 | 30.64 | 515 | 161, 179, 353 | Dicaffeoylquinic acid |
| 37 | 32.74 | 409 | 271, 299, 314 | Isorhamnetin derivative |
| Sample | TPC | TFC | DPPH | TAC |
|---|---|---|---|---|
| mg GAE/g Plant Extract | mg RE/g Plant Extract | IC50 (µg/mL) | mg AAE/g Extract | |
| H. hemistemon extract | 163.84 ± 3.91 | 61.54 ± 3.07 | 9.53 ± 0.67 | 438.67 ± 3.05 |
| Ascorbic acid | - | 3.39 ± 1.52 | - |
| Animal Groups | Cryptosporidium Oocysts (Mean Count/g Stool) | Inhibition % |
|---|---|---|
| GI (normal control) | - | - |
| GII (model) | 90.4 ± 1.33 | - |
| GIII (mice received the reference drug NTZ) | 29.6 ± 1.35 a | 67% |
| GIV (mice received H. hemistemon extract (100 mg/kg) | 35.7 ± 1.24 a,b | 61% |
| GV (mice received H. hemistemon extract (200 mg/kg) | 19.1 ± 1.2 a,b,c | 79% |
| Compound | Docking Score |
|---|---|
| Hydroxybenzoic acid | −7.4 |
| Quinic acid | −7.4 |
| Gallic acid | −7.3 |
| Methyl gallate | −7.1 |
| Esculetin | −4.6 |
| Dicaffeoylquinic acid | −4.4 |
| Isorhamnetin rhamnosyl-rutinoside | −4.1 |
| Hydroxybenzoic acid methyl gallate | −3.9 |
| Myricetin rutinoside | −3.8 |
| Kaempferol rutinoside | −3.6 |
| Kaempferol dirhamnosyl-glucoside | −3.4 |
| Isorhamnetin rutinoside | −3.3 |
| Rutin | −3.2 |
| Isorhamnetin sulfate | −3.2 |
| Caffeoyl glucose | −3.1 |
| Protocatechuic acid pentoside | −3.1 |
| Neochlorogenic acid | −3.1 |
| Vanillic acid glucoside | −3.1 |
| Quercetin glucoside | −3.1 |
| Caffeoyl vanillic acid | −3.0 |
| Chlorogenic acid | −2.8 |
| Vanillic acid sulfate | −2.8 |
| Gallocatechin | −2.7 |
| Coumaroylquinic acid | −2.6 |
| Vicenin 1 | −2.6 |
| Protocatechuic acid glucoside | −2.5 |
| Esculetin sulfate | −2.5 |
| Esculetin glucoside | −2.2 |
| Quercetin rhamnosyl-rutinoside | −2.1 |
| Schaftoside | −1.9 |
| Oxamic acid * | −6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghareeb, M.A.; Sobeh, M.; Aboushousha, T.; Esmat, M.; Mohammed, H.S.; El-Wakil, E.S. Polyphenolic Profile of Herniaria hemistemon Aerial Parts Extract and Assessment of Its Anti-Cryptosporidiosis in a Murine Model: In Silico Supported In Vivo Study. Pharmaceutics 2023, 15, 415. https://doi.org/10.3390/pharmaceutics15020415
Ghareeb MA, Sobeh M, Aboushousha T, Esmat M, Mohammed HS, El-Wakil ES. Polyphenolic Profile of Herniaria hemistemon Aerial Parts Extract and Assessment of Its Anti-Cryptosporidiosis in a Murine Model: In Silico Supported In Vivo Study. Pharmaceutics. 2023; 15(2):415. https://doi.org/10.3390/pharmaceutics15020415
Chicago/Turabian StyleGhareeb, Mosad A., Mansour Sobeh, Tarek Aboushousha, Marwa Esmat, Hala Sh. Mohammed, and Eman S. El-Wakil. 2023. "Polyphenolic Profile of Herniaria hemistemon Aerial Parts Extract and Assessment of Its Anti-Cryptosporidiosis in a Murine Model: In Silico Supported In Vivo Study" Pharmaceutics 15, no. 2: 415. https://doi.org/10.3390/pharmaceutics15020415