Novel Collagen-Polyphenols-Loaded Silica Composites for Topical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mesoporous Silica Carriers Synthesis
2.3. Polyphenols-Loaded MSN Preparation
2.4. Radical Scavenger Activity Testing
2.5. Antimicrobial Activity Testing
2.6. Cytocompatibility Testing
2.7. Evaluation of Antiproliferative Activity
2.7.1. Cytotoxic Effect
2.7.2. Apoptotic Effect
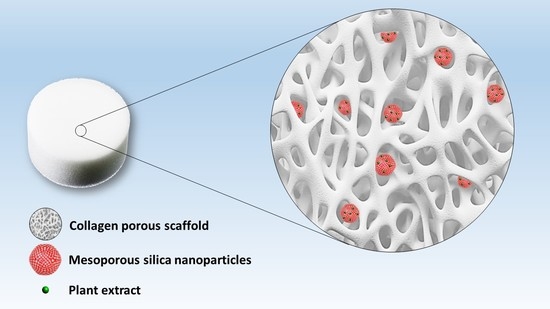
2.8. Preparation of Collagen Porous Scaffold Composites Preparation
2.9. Sterility Testing
2.10. Characterization Methods
3. Results
3.1. MSN Carriers Characterization
3.2. Polyphenols-Loaded MSN Composites Characterization
3.3. Collagen Porous Scaffold Composites Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn Injury: Challenges and Advances in Burn Wound Healing, Infection, Pain and Scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn Injury. Nat. Rev. Dis. Primers. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Burns. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 8 December 2022).
- Gaspar-Pintiliescu, A.; Stanciuc, A.M.; Craciunescu, O. Natural Composite Dressings Based on Collagen, Gelatin and Plant Bioactive Compounds for Wound Healing: A Review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent Advances of Collagen-Based Biomaterials: Multi-Hierarchical Structure, Modification and Biomedical Applications. Mater. Sci. Eng. C 2019, 99, 1509–1522. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Gaspar-Pintiliescu, A.; Stefan, L.M.; Anton, E.D.; Berger, D.; Matei, C.; Negreanu-Pirjol, T.; Moldovan, L. Physicochemical and Biological Properties of Gelatin Extracted from Marine Snail Rapana Venosa. Mar. Drugs 2019, 17, 589. [Google Scholar] [CrossRef] [Green Version]
- Lalzawmliana, V.; Anand, A.; Mukherjee, P.; Chaudhuri, S.; Kundu, B.; Nandi, S.K.; Thakur, N.L. Marine Organisms as a Source of Natural Matrix for Bone Tissue Engineering. Ceram. Int. 2019, 45, 1469–1481. [Google Scholar] [CrossRef]
- Castillo, R.R.; Vallet-Regí, M. Recent Advances toward the Use of Mesoporous Silica Nanoparticles for the Treatment of Bacterial Infections. Int. J. Nanomed. 2021, 16, 4409–4430. [Google Scholar] [CrossRef]
- Deaconu, M.; Nicu, I.; Tincu, R.; Brezoiu, A.M.; Mitran, R.A.; Vasile, E.; Matei, C.; Berger, D. Tailored Doxycycline Delivery from MCM-41-Type Silica Carriers. Chem. Pap. 2018, 72, 1869–1880. [Google Scholar] [CrossRef]
- Deaconu, M.; Brezoiu, A.M.; Mitran, R.A.; Nicu, I.; Manolescu, B.; Matei, C.; Berger, D. Exploiting the Zwitterionic Properties of Lomefloxacin to Tailor Its Delivery from Functionalized MCM-41 Silica. Microporous Mesoporous Mater. 2020, 305, 110323. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food and Drug Administration. Select Committee on GRAS Substances. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS&sort=Sortsubstance&order=ASC&startrow=1&type=basic&search=silica (accessed on 8 December 2022).
- Jurkić, L.M.; Cepanec, I.; Pavelić, S.K.; Pavelić, K. Biological and Therapeutic Effects of Ortho-Silicic Acid and Some Ortho-Silicic Acid-Releasing Compounds: New Perspectives for Therapy. Nutr. Metab. 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-Based Therapeutics for Antibiotic-Resistant Bacterial Infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.; Rodgers, A.M.; O’Brien, S.C.; Donnelly, R.F.; Gilmore, B.F. Gut Check Time: Antibiotic Delivery Strategies to Reduce Antimicrobial Resistance. Trends Biotechnol. 2020, 38, 447–462. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Qurtam, A.A.; Mechchate, H.; Es-Safi, I.; Al-Zharani, M.; Nasr, F.A.; Noman, O.M.; Aleissa, M.; Imtara, H.; Aleissa, A.M.; Bouhrim, M.; et al. Citrus Flavanone Narirutin, in Vitro and in Silico Mechanistic Antidiabetic Potential. Pharmaceutics 2021, 13, 1818. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Rowan Berries: A Potential Source for Green Synthesis of Extremely Monodisperse Gold and Silver Nanoparticles and Their Antimicrobial Property. Pharmaceutics 2022, 14, 82. [Google Scholar] [CrossRef]
- Soto, K.M.; Luzardo-Ocampo, I.; López-Romero, J.M.; Mendoza, S.; Loarca-Piña, G.; Rivera-Muñoz, E.M.; Manzano-Ramírez, A. Gold Nanoparticles Synthesized with Common Mullein (Verbascum Thapsus) and Castor Bean (Ricinus Communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells. Pharmaceutics 2022, 14, 2069. [Google Scholar] [CrossRef]
- Ilie, A.; Andronescu, E.; Ficai, D.; Voicu, G.; Ficai, M.; Maganu, M.; Ficai, A. New Approaches in Layer by Layer Synthesis of Collagen/Hydroxyapatite Composite Materials. Cent. Eur. J. Chem. 2011, 9, 283–289. [Google Scholar] [CrossRef]
- Desimone, M.F.; Hélary, C.; Rietveld, I.B.; Bataille, I.; Mosser, G.; Giraud-Guille, M.M.; Livage, J.; Coradin, T. Silica-Collagen Bionanocomposites as Three-Dimensional Scaffolds for Fibroblast Immobilization. Acta Biomater. 2010, 6, 3998–4004. [Google Scholar] [CrossRef]
- Mebert, A.M.; Alvarez, G.S.; Peroni, R.; Illoul, C.; Hélary, C.; Coradin, T.; Desimone, M.F. Collagen-Silica Nanocomposites as Dermal Dressings Preventing Infection in Vivo. Mater. Sci. Eng. C 2018, 93, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Hélary, C.; Coradin, T. Exploring the Cell–Protein–Mineral Interfaces: Interplay of Silica (Nano)Rods@collagen Biocomposites with Human Dermal Fibroblasts. Mater. Today Bio 2019, 1, 100004. [Google Scholar] [CrossRef]
- Quignard, S.; Hélary, C.; Boissière, M.; Fullana, J.M.; Lagrée, P.Y.; Coradin, T. Behaviour of Silica Nanoparticles in Dermis-like Cellularized Collagen Hydrogels. Biomater. Sci. 2014, 2, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Perumal, S.; Ramadass, S.K.; Gopinath, A.; Madhan, B.; Shanmugam, G.; Rajadas, J.; Mandal, A.B. Altering the Concentration of Silica Tunes the Functional Properties of Collagen-Silica Composite Scaffolds to Suit Various Clinical Requirements. J. Mech. Behav. Biomed. Mater. 2015, 52, 131–138. [Google Scholar] [CrossRef]
- Jing, S.; Jiang, D.; Wen, S.; Wang, J.; Yang, C. Preparation and Characterization of Collagen/Silica Composite Scaffolds for Peripheral Nerve Regeneration. J. Porous Mater. 2014, 21, 699–708. [Google Scholar] [CrossRef]
- Mahony, O.; Tsigkou, O.; Ionescu, C.; Minelli, C.; Ling, L.; Hanly, R.; Smith, M.E.; Stevens, M.M.; Jones, J.R. Silica-Gelatin Hybrids with Tailorable Degradation and Mechanical Properties for Tissue Regeneration. Adv. Funct. Mater. 2010, 20, 3835–3845. [Google Scholar] [CrossRef]
- Alvarez, G.S.; Hélary, C.; Mebert, A.M.; Wang, X.; Coradin, T.; Desimone, M.F. Antibiotic-Loaded Silica Nanoparticle-Collagen Composite Hydrogels with Prolonged Antimicrobial Activity for Wound Infection Prevention. J. Mater. Chem. B 2014, 2, 4660–4670. [Google Scholar] [CrossRef] [Green Version]
- Gaspar-Pintiliescu, A.; Seciu, A.M.; Miculescu, F.; Moldovan, L.; Ganea, E.; Craciunescu, O. Enhanced Extracellular Matrix Synthesis Using Collagen Dressings Loaded with Artemisia Absinthium Plant Extract. J. Bioact. Compat. Polym. 2018, 33, 516–528. [Google Scholar] [CrossRef]
- Gaspar, A.; Craciunescu, O.; Moldovan, L.; Ganea, E. New Composites Collagen-Polyphenols as Potential Dressing for Wound Care. Rom. J. Biochem. 2012, 49, 173–181. [Google Scholar]
- Perumal, R.K.; Gopinath, A.; Thangam, R.; Perumal, S.; Masilamani, D.; Ramadass, S.K.; Madhan, B. Collagen-Silica Bio-Composite Enriched with Cynodon Dactylon Extract for Tissue Repair and Regeneration. Mater. Sci. Eng. C 2018, 92, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pîrjol, T.; Muntean, D.; Danciu, C. Properties of Salvia Offcinalis l. And Thymus Serpyllum l. Extracts Free and Embedded into Mesopores of Silica and Titania Nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical Profile of Rosmarinus Officinalis and Salvia Officinalis Extracts and Correlation to Their Antioxidant and Anti-Proliferative Activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Prelipcean, A.M.; Iosageanu, A.; Gaspar-Pintiliescu, A.; Moldovan, L.; Craciunescu, O.; Negreanu-Pirjol, T.; Negreanu-Pirjol, B.; Mitran, R.A.; Marin, M.; D’Amora, U. Marine and Agro-Industrial By-Products Valorization Intended for Topical Formulations in Wound Healing Applications. Materials 2022, 15, 3507. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.M.; Bajenaru, L.; Berger, D.; Mitran, R.A.; Deaconu, M.; Lincu, D.; Guzun, A.S.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef]
- Badmus, J.A.; Ekpo, O.E.; Hussein, A.A.; Meyer, M.; Hiss, D.C. Antiproliferative and Apoptosis Induction Potential of the Methanolic Leaf Extract of Holarrhena Floribunda (G. Don). Evid. Based Complement. Altern. Med. 2015, 2015, 756482. [Google Scholar] [CrossRef] [Green Version]
- 2.6.1. Sterility. In European Pharmacopoeia 6.0; Council of Europe: Strasbourg, France, 2008.
- Prundeanu, M.; Brezoiu, A.M.; Deaconu, M.; Pircalabioru, G.G.; Lincu, D.; Matei, C.; Berger, D. Mesoporous Silica and Titania-Based Materials for Stability Enhancement of Polyphenols. Materials 2021, 14, 6457. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Matei, C.; Deaconu, M.; Stanciuc, A.M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols Extract from Grape Pomace. Characterization and Valorisation through Encapsulation into Mesoporous Silica-Type Matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Kaye, K.S.; Petty, L.A.; Shorr, A.F.; Zilberberg, M.D. Current Epidemiology, Etiology, and Burden of Acute Skin Infections in the United States. Clin. Infect. Dis. 2019, 68, S193–S199. [Google Scholar] [CrossRef] [Green Version]
- Bourgi, J.; Said, J.M.; Yaakoub, C.; Atallah, B.; al Akkary, N.; Sleiman, Z.; Ghanimé, G. Bacterial Infection Profile and Predictors among Patients Admitted to a Burn Care Center: A Retrospective Study. Burns 2020, 46, 1968–1976. [Google Scholar] [CrossRef]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Collagen Structural Hierarchy and Susceptibility to Degradation by Ultraviolet Radiation. Mater. Sci. Eng. C 2008, 28, 1420–1429. [Google Scholar] [CrossRef] [Green Version]
- Payne, K.J.; Veis, A. Fourier Transform IR Spectroscopy of Collagen and Gelatin Solutions: Deconvolution of the Amide I Band for Conformational Studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Bozec, L.; Odlyha, M. Thermal Denaturation Studies of Collagen by Microthermal Analysis and Atomic Force Microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Bonesi, M.; Brindisi, M.; Armentano, B.; Curcio, R.; Sicari, V.; Loizzo, M.R.; Cappello, M.S.; Bedini, G.; Peruzzi, L.; Tundis, R. Exploring the Anti-Proliferative, pro-Apoptotic, and Antioxidant Properties of Santolina Corsica Jord. & Fourr. (Asteraceae). Biomed. Pharmacother. 2018, 107, 967–978. [Google Scholar] [CrossRef]
- Garcia, C.S.C.; Menti, C.; Lambert, A.P.F.; Barcellos, T.; Moura, S.; Calloni, C.; Branco, C.S.; Salvador, M.; Roesch-Ely, M.; Henriques, J.A.P. Pharmacological Perspectives from Brazilian Salvia Officinalis (Lamiaceae): Antioxidant, and Antitumor in Mammalian Cells. Anm. Acad. Bras. Cienc. 2016, 88, 281–292. [Google Scholar] [CrossRef]
- el Hadri, A.; Ángeles Gómez Del Río, M.; Sanz, J.; Coloma, A.G.; Idaomar, M.; Ozonas, B.R.; Benedí González, J.; Isabel, M.; Reus, S. Cytotoxic Activity of α-Humulene and Transcaryo-Phyllene from Salvia Officinalis in Animal and Human Tumor Cells. Ann. R. Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]











| Sample | SBET m2/g | dDFT nm | V cm3/g | Vd < 10 nm cm3/g | SiO2:FG |
|---|---|---|---|---|---|
| MSN | 977 | 4.25 | 1.43 | 0.78 | - |
| MSN–NH2 | 512 | 3.18 | 0.42 | 0.32 | 1:0.22 |
| MSN–COOH | 388 | 3.66 | 0.47 | 0.29 | 1:0.05 |
| Sample | MIC (mg/mL) | MBC (mg/mL) | ||
|---|---|---|---|---|
| S. aureus | P. aeruginosa | S. aureus | P. aeruginosa | |
| S | 15.62 | 31.25 | 31.25 | 62.0 |
| S@MSN–NH2 | 7.81 | 15.62 | 15.62 | 31.25 |
| S@MSN–COOH | 3.90 | 15.62 | 7.81 | 31.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deaconu, M.; Prelipcean, A.-M.; Brezoiu, A.-M.; Mitran, R.-A.; Isopencu, G.; Matei, C.; Berger, D. Novel Collagen-Polyphenols-Loaded Silica Composites for Topical Application. Pharmaceutics 2023, 15, 312. https://doi.org/10.3390/pharmaceutics15020312
Deaconu M, Prelipcean A-M, Brezoiu A-M, Mitran R-A, Isopencu G, Matei C, Berger D. Novel Collagen-Polyphenols-Loaded Silica Composites for Topical Application. Pharmaceutics. 2023; 15(2):312. https://doi.org/10.3390/pharmaceutics15020312
Chicago/Turabian StyleDeaconu, Mihaela, Ana-Maria Prelipcean, Ana-Maria Brezoiu, Raul-Augustin Mitran, Gabriela Isopencu, Cristian Matei, and Daniela Berger. 2023. "Novel Collagen-Polyphenols-Loaded Silica Composites for Topical Application" Pharmaceutics 15, no. 2: 312. https://doi.org/10.3390/pharmaceutics15020312