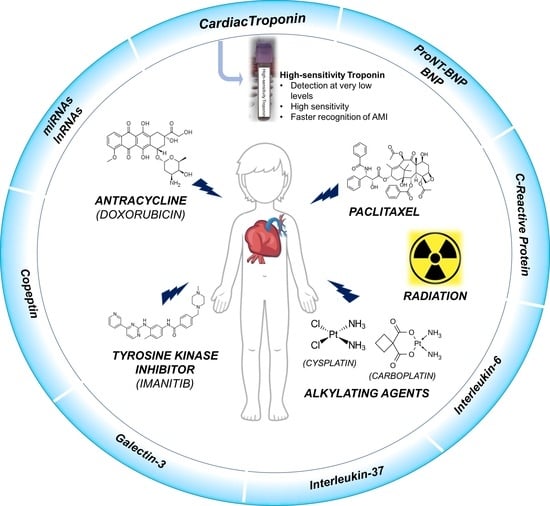
Circulating Biomarkers for Monitoring Chemotherapy-Induced Cardiotoxicity in Children
Abstract
:1. Introduction
2. Chemotherapy-Induced Cardiotoxicity in Children
2.1. Anthracyclines
Genetic Variants Associated with Anthracycline Sensitivity
2.2. Taxanes
2.3. Tyrosine Kinase Inhibitors
2.4. Alkylating Agents
2.5. Radiation Therapy
3. Cardiac Biomarkers
4. Role of hs-cTn in the Diagnosis and Monitoring of Chemotherapy-Induced Cardiotoxicity in Pediatric Population
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| cTn | cardiac troponin |
| hs-cTnI or hs-cTnT | high sensitive methods |
| NGS | next-generation sequencing |
| HSCT | hematopoietic stem cell transplantation |
| SMN | secondary malignant neoplasms |
| CNS | Central Nervous System |
| CTRCD | Cancer-therapy-related cardiac dysfunction |
| BNP | brain-type natriuretic peptide |
| TOP2 (TOP2A and TOP2B) | isoenzymes of topoisomerase II |
| ROS | reactive oxygen species |
| LVEF | left ventricular ejection fraction |
| SNP | single nucleotide polymorphism |
| ACT | anthracycline-associated cardiotoxicity |
| CBR3 | carbonyl reductase |
| 3′ UTR | 3′ Untranslated Region |
| ABCC1 | ATP binding cassette subfamily C member |
| ALL | Acute Lymphoblastic Leukemia |
| SULT2B1 | sulfotransferase family cytosolic member 2B1 |
| CAT | catalase |
| GSTP1 | glutathione S transferase |
| GPCR35 | G protein-coupled receptor 35 |
| HAS3 | Hyaluronan synthase-3 |
| CELF4 | Elav-like family member 4 |
| RARG | retinoic acid receptor-γ |
| hiPSC-CMs | pluripotent stem cell-derived cardiomyocytes |
| ERK | extracellular regulated kinase |
| CPNDS | The Canadian Pharmacogenomics Network for Drug Safety |
| TKIs | Tyrosine kinase inhibitors |
| CML | chronic myeloid leukemia |
| GIST | gastrointestinal stromal tumor |
| ER | endoplasmic reticulum |
| 5-FU | Fluorouracil |
| RT | radiation therapy |
| AMI | Acute Myocardial Infarction |
| CK | creatine kinase |
| CK-MB | CK isoenzyme MB |
| NT-proBNP | pro Brain Natriuretic Peptide |
| ELISA | enzyme linked immunoassay |
| RIA | radioimmunoassay |
| C-CB | Cardiac Bio-Markers |
| NPs | Natriuretic peptides |
| ANP | atrial natriuretic peptide |
| B-type | atrial natriuretic peptide or brain |
| CNP | C-type natriuretic peptide |
| NPRs | NP receptors |
| CRP | C-Reactive Protein |
| AVP | arginine vasopressin |
| IL-6 | Interleukin 6 |
| IL-37 | Interleukin 37 |
| Gal-3 | Galectin-3 |
| ESC | European Society of Cardiology |
| ESMO | European Society for Medical Oncology |
References
- Rahal, Z.; Abdulhai, F.; Kadara, H.; Saab, R. Genomics of Adult and Pediatric Solid Tumors. Am. J. Cancer Res. 2018, 8, 1356–1386. [Google Scholar] [PubMed]
- Mardis, E.R. The Impact of Next-Generation Sequencing on Cancer Genomics: From Discovery to Clinic. Cold Spring Harb. Perspect. Med. 2019, 9, a036269. [Google Scholar] [CrossRef] [PubMed]
- Cipri, S.; Abenavoli, L.; Boccuto, L.; Del Baldo, G.; Mastronuzzi, A. How Genetics and Genomics Advances Are Rewriting Pediatric Cancer Research and Clinical Care. Medicina 2022, 58, 1386. [Google Scholar] [CrossRef] [PubMed]
- Saletta, F.; Seng, M.S.; Lau, L.M.S. Advances in Paediatric Cancer Treatment. Transl. Pediatr. 2014, 3, 156–182. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.K.; Helenowski, I.; Hijiya, N. Secondary Malignancies in Pediatric Cancer Survivors: Perspectives and Review of the Literature. Int. J. Cancer 2014, 135, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and Management of Cancer Treatment-Related Cardiac Dysfunction and Heart Failure in Children—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9856743/#B5-children-10-00149 (accessed on 29 July 2023).
- Cheuk, D.K.L.; Sieswerda, E.; van Dalen, E.C.; Postma, A.; Kremer, L.C.M. Medical Interventions for Treating Anthracycline-Induced Symptomatic and Asymptomatic Cardiotoxicity during and after Treatment for Childhood Cancer. Cochrane Database Syst. Rev. 2016, 2016, CD008011. [Google Scholar] [CrossRef]
- Balis, F.M.; Womer, R.B.; Berg, S.; Winick, N.; Adamson, P.C.; Fox, E. Children’s Oncology Group Chemotherapy Standardization Task Force Dosing Anticancer Drugs in Infants: Current Approach and Recommendations from the Children’s Oncology Group’s Chemotherapy Standardization Task Force. Pediatr. Blood Cancer 2017, 64, e26636. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Armstrong, G.T.; Aune, G.; Chow, E.J.; Ehrhardt, M.J.; Ky, B.; Moslehi, J.; Mulrooney, D.A.; Nathan, P.C.; Ryan, T.D.; et al. Cardiovascular Disease in Survivors of Childhood Cancer: Insights Into Epidemiology, Pathophysiology, and Prevention. J. Clin. Oncol. 2018, 36, 2135–2144. [Google Scholar] [CrossRef]
- Kang, D.-W.; Wilson, R.L.; Christopher, C.N.; Normann, A.J.; Barnes, O.; Lesansee, J.D.; Choi, G.; Dieli-Conwright, C.M. Exercise Cardio-Oncology: Exercise as a Potential Therapeutic Modality in the Management of Anthracycline-Induced Cardiotoxicity. Front. Cardiovasc. Med. 2022, 8, 2194. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-Term Cardiovascular Toxicity in Children, Adolescents, and Young Adults Who Receive Cancer Therapy: Pathophysiology, Course, Monitoring, Management, Prevention, and Research Directions: A Scientific Statement from the American Heart Association. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef]
- Yester, J.W.; Kühn, B. Mechanisms of Cardiomyocyte Proliferation and Differentiation in Development and Regeneration. Curr. Cardiol. Rep. 2017, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Giacca, M.; Capogrossi, M.C.; Crescenzi, M.; Martelli, F. Knockdown of Cyclin-Dependent Kinase Inhibitors Induces Cardiomyocyte Re-Entry in the Cell Cycle. J. Biol. Chem. 2011, 286, 8644–8654. [Google Scholar] [CrossRef] [PubMed]
- Tocchetti, C.G.; Cadeddu, C.; Di Lisi, D.; Femminò, S.; Madonna, R.; Mele, D.; Monte, I.; Novo, G.; Penna, C.; Pepe, A.; et al. From Molecular Mechanisms to Clinical Management of Antineoplastic Drug-Induced Cardiovascular Toxicity: A Translational Overview. Antioxid. Redox Signal 2019, 30, 2110–2153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the Molecular Basis of Doxorubicin-Induced Cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Maillet, A.; Tan, K.; Chai, X.; Sadananda, S.N.; Mehta, A.; Ooi, J.; Hayden, M.R.; Pouladi, M.A.; Ghosh, S.; Shim, W.; et al. Modeling Doxorubicin-Induced Cardiotoxicity in Human Pluripotent Stem Cell Derived-Cardiomyocytes. Sci. Rep. 2016, 6, 25333. [Google Scholar] [CrossRef] [PubMed]
- Aminkeng, F.; Bhavsar, A.P.; Visscher, H.; Rassekh, S.R.; Li, Y.; Lee, J.W.; Brunham, L.R.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; et al. A Coding Variant in RARG Confers Susceptibility to Anthracycline-Induced Cardiotoxicity in Childhood Cancer. Nat. Genet. 2015, 47, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Varbiro, G.; Veres, B.; Gallyas, F.; Sumegi, B. Direct Effect of Taxol on Free Radical Formation and Mitochondrial Permeability Transition. Free Radic. Biol. Med. 2001, 31, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Zweier, J.L.; Levy, A.; Myers, C.E. Characterization of the Cycle of Iron-Mediated Electron Transfer from Adriamycin to Molecular Oxygen. J. Biol. Chem. 1985, 260, 6820–6826. [Google Scholar] [CrossRef]
- Singal, P.K.; Iliskovic, N.; Li, T.; Kumar, D. Adriamycin Cardiomyopathy: Pathophysiology and Prevention. FASEB J. 1997, 11, 931–936. [Google Scholar] [CrossRef]
- Blanco, J.G.; Sun, C.-L.; Landier, W.; Chen, L.; Esparza-Duran, D.; Leisenring, W.; Mays, A.; Friedman, D.L.; Ginsberg, J.P.; Hudson, M.M.; et al. Anthracycline-Related Cardiomyopathy After Childhood Cancer: Role of Polymorphisms in Carbonyl Reductase Genes—A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 1415–1421. [Google Scholar] [CrossRef]
- Kim, H.; Kang, H.J.; Park, K.D.; Koh, K.-N.; Im, H.J.; Seo, J.J.; Lee, J.W.; Chung, N.-G.; Cho, B.; Kim, H.K.; et al. Risk Factor Analysis for Secondary Malignancy in Dexrazoxane-Treated Pediatric Cancer Patients. Cancer Res. Treat. 2019, 51, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Eneh, C.; Lekkala, M.R. Dexrazoxane. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Langer, S.W. Dexrazoxane for the Treatment of Chemotherapy-Related Side Effects. Cancer Manag. Res. 2014, 6, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Semsei, A.F.; Erdelyi, D.J.; Ungvari, I.; Csagoly, E.; Hegyi, M.Z.; Kiszel, P.S.; Lautner-Csorba, O.; Szabolcs, J.; Masat, P.; Fekete, G.; et al. ABCC1 Polymorphisms in Anthracycline-Induced Cardiotoxicity in Childhood Acute Lymphoblastic Leukaemia. Cell Biol. Int. 2012, 36, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Visscher, H.; Ross, C.J.D.; Rassekh, S.R.; Barhdadi, A.; Dubé, M.-P.; Al-Saloos, H.; Sandor, G.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; et al. Pharmacogenomic Prediction of Anthracycline-Induced Cardiotoxicity in Children. J. Clin. Oncol. 2012, 30, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Visscher, H.; Ross, C.J.D.; Rassekh, S.R.; Sandor, G.S.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; van der Pal, H.J.; Rogers, P.C.; Rieder, M.J.; et al. Validation of Variants in SLC28A3 and UGT1A6 as Genetic Markers Predictive of Anthracycline-Induced Cardiotoxicity in Children. Pediatr. Blood Cancer 2013, 60, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; McLaughlin, D.; Robinson, E.; Harvey, A.P.; Hookham, M.B.; Shah, A.M.; McDermott, B.J.; Grieve, D.J. NOX2 NADPH Oxidase Promotes Pathologic Cardiac Remodeling Associated with Doxorubicin Chemotherapy. Cancer Res. 2010, 70, 9287–9297. [Google Scholar] [CrossRef] [PubMed]
- Rajić, V.; Aplenc, R.; Debeljak, M.; Prestor, V.V.; Karas-Kuzelicki, N.; Mlinaric-Rascan, I.; Jazbec, J. Influence of the Polymorphism in Candidate Genes on Late Cardiac Damage in Patients Treated Due to Acute Leukemia in Childhood. Leuk. Lymphoma 2009, 50, 1693–1698. [Google Scholar] [CrossRef]
- Windsor, R.E.; Strauss, S.J.; Kallis, C.; Wood, N.E.; Whelan, J.S. Germline Genetic Polymorphisms May Influence Chemotherapy Response and Disease Outcome in Osteosarcoma: A Pilot Study. Cancer 2012, 118, 1856–1867. [Google Scholar] [CrossRef]
- Min, K.-D.; Asakura, M.; Liao, Y.; Nakamaru, K.; Okazaki, H.; Takahashi, T.; Fujimoto, K.; Ito, S.; Takahashi, A.; Asanuma, H.; et al. Identification of Genes Related to Heart Failure Using Global Gene Expression Profiling of Human Failing Myocardium. Biochem. Biophys. Res. Commun. 2010, 393, 55–60. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Sun, C.-L.; Armenian, S.H.; Hakonarson, H.; Hageman, L.; Ding, Y.; Landier, W.; Blanco, J.G.; Chen, L.; et al. Hyaluronan Synthase 3 Variant and Anthracycline-Related Cardiomyopathy: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2014, 32, 647–653. [Google Scholar] [CrossRef]
- Wang, X.; Sun, C.-L.; Quiñones-Lombraña, A.; Singh, P.; Landier, W.; Hageman, L.; Mather, M.; Rotter, J.I.; Taylor, K.D.; Chen, Y.-D.I.; et al. CELF4 Variant and Anthracycline-Related Cardiomyopathy: A Children’s Oncology Group Genome-Wide Association Study. J. Clin. Oncol. 2016, 34, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.A.; Ordahl, C.P. A Single Cardiac Troponin T Gene Generates Embryonic and Adult Isoforms via Developmentally Regulated Alternate Splicing. J. Biol. Chem. 1985, 260, 11140–11148. [Google Scholar] [CrossRef] [PubMed]
- Magdy, T.; Jouni, M.; Kuo, H.-H.; Weddle, C.J.; Lyra-Leite, D.; Fonoudi, H.; Romero-Tejeda, M.; Gharib, M.; Javed, H.; Fajardo, G.; et al. Identification of Drug Transporter Genomic Variants and Inhibitors That Protect Against Doxorubicin-Induced Cardiotoxicity. Circulation 2022, 145, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Magdy, T.; Jiang, Z.; Jouni, M.; Fonoudi, H.; Lyra-Leite, D.; Jung, G.; Romero-Tejeda, M.; Kuo, H.-H.; Fetterman, K.A.; Gharib, M.; et al. RARG Variant Predictive of Doxorubicin-Induced Cardiotoxicity Identifies a Cardioprotective Therapy. Cell Stem Cell 2021, 28, 2076–2089.e7. [Google Scholar] [CrossRef] [PubMed]
- Loucks, C.M.; Yan, K.; Tanoshima, R.; Ross, C.J.D.; Rassekh, S.R.; Carleton, B.C. Pharmacogenetic Testing to Guide Therapeutic Decision-Making and Improve Outcomes for Children Undergoing Anthracycline-Based Chemotherapy. Basic Clin. Pharmacol. Toxicol. 2022, 130 (Suppl. S1), 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pinto, S.; Pita, G.; Patiño-García, A.; Alonso, J.; Pérez-Martínez, A.; Cartón, A.J.; Gutiérrez-Larraya, F.; Alonso, M.R.; Barnes, D.R.; Dennis, J.; et al. Exome Array Analysis Identifies GPR35 as a Novel Susceptibility Gene for Anthracycline-Induced Cardiotoxicity in Childhood Cancer. Pharmacogenetics Genom. 2017, 27, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Nižnanský, Ľ.; Osinová, D.; Kuruc, R.; Hengerics Szabó, A.; Szórádová, A.; Masár, M.; Nižnanská, Ž. Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity. Int. J. Mol. Sci. 2022, 23, 15619. [Google Scholar] [CrossRef]
- Gianni, L.; Viganò, L.; Locatelli, A.; Capri, G.; Giani, A.; Tarenzi, E.; Bonadonna, G. Human Pharmacokinetic Characterization and in Vitro Study of the Interaction between Doxorubicin and Paclitaxel in Patients with Breast Cancer. J. Clin. Oncol. 1997, 15, 1906–1915. [Google Scholar] [CrossRef]
- Saad, S.Y.; Najjar, T.A.O.; Alashari, M. Cardiotoxicity of Doxorubicin/Paclitaxel Combination in Rats: Effect of Sequence and Timing of Administration. J. Biochem. Mol. Toxicol. 2004, 18, 78–86. [Google Scholar] [CrossRef]
- Hurwitz, C.A.; Strauss, L.C.; Kepner, J.; Kretschmar, C.; Harris, M.B.; Friedman, H.; Kun, L.; Kadota, R. Paclitaxel for the Treatment of Progressive or Recurrent Childhood Brain Tumors: A Pediatric Oncology Phase II Study. J. Pediatr. Hematol. Oncol. 2001, 23, 277. [Google Scholar] [CrossRef]
- Adinani, H.; Campbell, L.; El-Mallawany, N.K.; Slone, J.; Mehta, P.; Bacha, J. Use of Paclitaxel to Successfully Treat Children, Adolescents, and Young Adults with Kaposi Sarcoma in Southwestern Tanzania. Children 2021, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Perez-Somarriba, M.; Moreno-Tejero, M.L.; Rozas, M.I.; Pelaez, I.; Madero, L.; Lassaletta, A. Gemcitabine, Paclitaxel, and Oxaliplatin (GEMPOX) in the Treatment of Relapsed/Refractory Intracranial Nongerminomatous Germ Cell Tumors. Pediatr. Blood Cancer 2020, 67, e28089. [Google Scholar] [CrossRef] [PubMed]
- Iwase, K.; Oyama, Y.; Tatsuishi, T.; Yamaguchi, J.; Nishimura, Y.; Kanada, A.; Kobayashi, M.; Maemura, Y.; Ishida, S.; Okano, Y. Cremophor EL Augments the Cytotoxicity of Hydrogen Peroxide in Lymphocytes Dissociated from Rat Thymus Glands. Toxicol. Lett. 2004, 154, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.B.; Miguel, B.S.; Villares, C.; Gallego, J.G.; Tuñón, M.J. Oxidative Stress Induced by Cremophor EL Is Not Accompanied by Changes in NF-kappaB Activation or iNOS Expression. Toxicology 2006, 222, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; McGuire, W.P.; Guarnieri, T.; Fisherman, J.S.; Christian, M.C.; Donehower, R.C. Cardiac Disturbances during the Administration of Taxol. J. Clin. Oncol. 1991, 9, 1704–1712. [Google Scholar] [CrossRef]
- Skolnik, J.M.; Adamson, P.C. Tyrosine Kinase Inhibitors in Pediatric Malignancies. Cancer Investig. 2007, 25, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Dong, S.; Zhang, A.; Lu, Y.; Li, Y.; Lv, S.; Zhang, J. Role of Oxidative Stress in Cardiotoxicity of Antineoplastic Drugs. Life Sci. 2019, 232, 116526. [Google Scholar] [CrossRef] [PubMed]
- Akam-Venkata, J.; Franco, V.I.; Lipshultz, S.E. Late Cardiotoxicity: Issues for Childhood Cancer Survivors. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 47. [Google Scholar] [CrossRef]
- Iqubal, A.; Iqubal, M.K.; Sharma, S.; Ansari, M.A.; Najmi, A.K.; Ali, S.M.; Ali, J.; Haque, S.E. Molecular Mechanism Involved in Cyclophosphamide-Induced Cardiotoxicity: Old Drug with a New Vision. Life Sci. 2019, 218, 112–131. [Google Scholar] [CrossRef]
- Fabin, N.; Bergami, M.; Cenko, E.; Bugiardini, R.; Manfrini, O. The Role of Vasospasm and Microcirculatory Dysfunction in Fluoropyrimidine-Induced Ischemic Heart Disease. J. Clin. Med. 2022, 11, 1244. [Google Scholar] [CrossRef]
- Bansal, N.; Blanco, J.G.; Sharma, U.; Pokharel, S.; Shisler, S.; Lipshultz, S.E. Cardiovascular Diseases in Survivors of Childhood Cancer. Cancer Metastasis Rev. 2020, 39, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.D.; Cehic, D.A.; Morgia, M.; Bergom, C.; Toohey, J.; Guerrero, P.A.; Ferencik, M.; Kikuchi, R.; Carver, J.R.; Zaha, V.G.; et al. Cardiovascular Manifestations from Therapeutic Radiation: A Multidisciplinary Expert Consensus Statement from the International Cardio-Oncology Society. JACC CardioOncol. 2021, 3, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Crocco, M.; d’Annunzio, G.; La Valle, A.; Piccolo, G.; Chiarenza, D.S.; Bigatti, C.; Molteni, M.; Milanaccio, C.; Garrè, M.L.; Di Iorgi, N.; et al. Endothelial Dysfunction in Childhood Cancer Survivors: A Narrative Review. Life 2021, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.F.; Cascino, G.J.; Murtagh, G.; Akhter, N. Circulating Biomarkers for Cardiotoxicity Risk Prediction. Curr. Treat. Options Oncol. 2021, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.F.; Coats, A.J.S.; Januzzi, J.L.; Maisel, A.S.; McDonald, K.; Mueller, T.; et al. Circulating Heart Failure Biomarkers beyond Natriuretic Peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632. [Google Scholar] [CrossRef]
- Karmen, A.; Wróblewski, F.; LaDue, J.S. Transaminase Activity in Human Blood. J. Clin. Investig. 1955, 34, 126–133. [Google Scholar] [CrossRef]
- Laurino, J.P.; Bender, E.W.; Kessimian, N.; Chang, J.; Pelletier, T.; Usategui, M. Comparative Sensitivities and Specificities of the Mass Measurements of CK-MB2, CK-MB, and Myoglobin for Diagnosing Acute Myocardial Infarction. Clin. Chem. 1996, 42, 1454–1459. [Google Scholar] [CrossRef]
- Panteghini, M.; Pagani, F.; Bonetti, G. The Sensitivity of Cardiac Markers: An Evidence-Based Approach. Clin. Chem. Lab. Med. (CCLM) 1999, 37, 1097–1106. [Google Scholar] [CrossRef]
- Garg, P.; Morris, P.; Fazlanie, A.L.; Vijayan, S.; Dancso, B.; Dastidar, A.G.; Plein, S.; Mueller, C.; Haaf, P. Cardiac Biomarkers of Acute Coronary Syndrome: From History to High-Sensitivity Cardiac Troponin. Intern. Emerg. Med. 2017, 12, 147–155. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, F.; Zhang, C.; Zheng, L.-R.; Yang, J. The Biomarkers for Acute Myocardial Infarction and Heart Failure. BioMed Res. Int. 2020, 2020, e2018035. [Google Scholar] [CrossRef] [PubMed]
- Katrukha, I.A. Human Cardiac Troponin Complex. Structure and Functions. Biochem. Mosc. 2013, 78, 1447–1465. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Rifai, N.; Sallan, S.E.; Lipsitz, S.R.; Dalton, V.; Sacks, D.B.; Ottlinger, M.E. Predictive Value of Cardiac Troponin T in Pediatric Patients at Risk for Myocardial Injury. Circulation 1997, 96, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Kremer, L.C.M.; Bastiaansen, B.A.J.; Offringa, M.; Lam, J.; van Straalen, J.P.; de Winter, R.J.; Voûte, P.A. Troponin T in the First 24 Hours after the Administration of Chemotherapy and the Detection of Myocardial Damage in Children. Eur. J. Cancer 2002, 38, 686–689. [Google Scholar] [CrossRef] [PubMed]
- de Antonio, M.; Lupon, J.; Galan, A.; Vila, J.; Urrutia, A.; Bayes-Genis, A. Combined Use of High-Sensitivity Cardiac Troponin T and N-Terminal pro-B Type Natriuretic Peptide Improves Measurements of Performance over Established Mortality Risk Factors in Chronic Heart Failure. Am. Heart J. 2012, 163, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Cediel, G.; Rueda, F.; García, C.; Oliveras, T.; Labata, C.; Serra, J.; Núñez, J.; Bodí, V.; Ferrer, M.; Lupón, J.; et al. Prognostic Value of New-Generation Troponins in ST-Segment–Elevation Myocardial Infarction in the Modern Era: The RUTI-STEMI Study. J. Am. Heart Assoc. 2017, 6, e007252. [Google Scholar] [CrossRef]
- Lazar, D.R.; Lazar, F.-L.; Homorodean, C.; Cainap, C.; Focsan, M.; Cainap, S.; Olinic, D.M. High-Sensitivity Troponin: A Review on Characteristics, Assessment, and Clinical Implications. Dis. Markers 2022, 2022, 9713326. [Google Scholar] [CrossRef]
- 2023 ESC Guidelines for the Management of Acute Coronary Syndromes|European Heart Journal|Oxford Academic. Available online: https://academic.oup.com/eurheartj/article/44/38/3720/7243210 (accessed on 21 November 2023).
- Biomarkers Reference Tables. Available online: https://ifcc.org/ifcc-education-division/emd-committees/committee-on-clinical-applications-of-cardiac-bio-markers-c-cb/biomarkers-reference-tables/ (accessed on 21 November 2023).
- Apple, F.S.; Collinson, P.O. IFCC Task Force on Clinical Applications of Cardiac Biomarkers Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays. Clin. Chem. 2012, 58, 54–61. [Google Scholar] [CrossRef]
- Collinson, P.; Aakre, K.M.; Saenger, A.; Body, R.; Hammarsten, O.; Jaffe, A.S.; Kavsak, P.; Omland, T.; Ordonez-Lianos, J.; Karon, B.; et al. Cardiac Troponin Measurement at the Point of Care: Educational Recommendations on Analytical and Clinical Aspects by the IFCC Committee on Clinical Applications of Cardiac Bio-Markers (IFCC C-CB). Clin. Chem. Lab. Med. (CCLM) 2023, 61, 989–998. [Google Scholar] [CrossRef]
- Alcidi, G.; Goffredo, G.; Correale, M.; Brunetti, N.D.; Iacoviello, M. Brain Natriuretic Peptide Biomarkers in Current Clinical and Therapeutic Scenarios of Heart Failure. J. Clin. Med. 2022, 11, 3192. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Marín, G.; Iraola, D.; Lorenzo, M.; De La Espriella, R.; Villar, S.; Santas, E.; Miñana, G.; Sanchis, J.; Carratalá, A.; Miró, Ò.; et al. An Update on Utilising Brain Natriuretic Peptide for Risk Stratification, Monitoring and Guiding Therapy in Heart Failure. Expert Rev. Mol. Diagn. 2023, 23, 521–533. [Google Scholar] [CrossRef] [PubMed]
- de Lemos, J.A.; Morrow, D.A.; Bentley, J.H.; Omland, T.; Sabatine, M.S.; McCabe, C.H.; Hall, C.; Cannon, C.P.; Braunwald, E. The Prognostic Value of B-Type Natriuretic Peptide in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2001, 345, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, D.G.J.; Papacosta, A.O.; Lennon, L.T.; Ramsay, S.E.; Whincup, P.H.; Wannamethee, S.G. Associations between Inflammation, Cardiovascular Biomarkers and Incident Frailty: The British Regional Heart Study. Age Ageing 2021, 50, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Villalpando, G.L.; Padilla-Gutiérrez, J.R.; Valdez-Haro, A.; Casillas-Muñoz, F.; Muñoz-Valle, J.F.; Castellanos-Nuñez, E.; Chávez-Herrera, J.C.; Valle, Y. Relationship Between C-Reactive Protein Serum Concentration and the 1846 C>T (Rs1205) Polymorphism in Patients with Acute Coronary Syndrome from Western Mexico. Genet. Test. Mol. Biomark. 2017, 21, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Afzali, D.; Erren, M.; Pavenstädt, H.-J.; Vollert, J.O.; Hertel, S.; Waltenberger, J.; Reinecke, H.; Lebiedz, P. Impact of Copeptin on Diagnosis, Risk Stratification, and Intermediate-Term Prognosis of Acute Coronary Syndromes. Clin. Res. Cardiol. 2013, 102, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Yalta, K.; Yetkin, E.; Yalta, T. Serum Copeptin in Cardiooncology Practice: Review of Pathophysiological and Clinical Implications. Balk. Med. J. 2023, 40, 82–92. [Google Scholar] [CrossRef]
- Folli, C.; Consonni, D.; Spessot, M.; Salvini, L.; Velati, M.; Ranzani, G.; Maiavacca, R.; Monzani, V. Diagnostic Role of Copeptin in Patients Presenting with Chest Pain in the Emergency Room. Eur. J. Intern. Med. 2013, 24, 189–193. [Google Scholar] [CrossRef]
- Jeong, J.H.; Seo, Y.H.; Ahn, J.Y.; Kim, K.H.; Seo, J.Y.; Chun, K.Y.; Lim, Y.S.; Park, P.W. Performance of Copeptin for Early Diagnosis of Acute Myocardial Infarction in an Emergency Department Setting. Ann. Lab. Med. 2020, 40, 7–14. [Google Scholar] [CrossRef]
- Abd El Baky Mahmoud, M.; Shaaban, M.A.A.; Ali Ramzy, A. Clinical Role of Serum Copeptin in Acute Coronary Syndrome. Egypt. Heart J. 2018, 70, 155–159. [Google Scholar] [CrossRef]
- Nickel, C.H.; Bingisser, R.; Morgenthaler, N.G. The Role of Copeptin as a Diagnostic and Prognostic Biomarker for Risk Stratification in the Emergency Department. BMC Med. 2012, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, J.; Koper, O.M.; Siedlecka-Czykier, E.; Matowicka-Karna, J.; Bychowski, J.; Kemona, H. The Utility of Inflammation and Platelet Biomarkers in Patients with Acute Coronary Syndromes. Saudi J. Biol. Sci. 2018, 25, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Liebetrau, C.; Hoffmann, J.; Dörr, O.; Gaede, L.; Blumenstein, J.; Biermann, H.; Pyttel, L.; Thiele, P.; Troidl, C.; Berkowitsch, A.; et al. Release Kinetics of Inflammatory Biomarkers in a Clinical Model of Acute Myocardial Infarction. Circ. Res. 2015, 116, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Held, C.; White, H.D.; Stewart, R.A.H.; Budaj, A.; Cannon, C.P.; Hochman, J.S.; Koenig, W.; Siegbahn, A.; Steg, P.G.; Soffer, J.; et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J. Am. Heart Assoc. 2017, 6, e005077. [Google Scholar] [CrossRef] [PubMed]
- Fanola, C.L.; Morrow, D.A.; Cannon, C.P.; Jarolim, P.; Lukas, M.A.; Bode, C.; Hochman, J.S.; Goodrich, E.L.; Braunwald, E.; O’Donoghue, M.L. Interleukin-6 and the Risk of Adverse Outcomes in Patients After an Acute Coronary Syndrome: Observations from the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib—Thrombolysis in Myocardial Infarction 52) Trial. J. Am. Heart Assoc. 2017, 6, e005637. [Google Scholar] [CrossRef] [PubMed]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 Is a Fundamental Inhibitor of Innate Immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Lucchesi, D.; Hainzl, S.; Leitner, M.; Maier, E.; Mangelberger, D.; Oostingh, G.J.; Pfaller, T.; Pixner, C.; Posselt, G.; et al. IL-37: A New Anti-Inflammatory Cytokine of the IL-1 Family. Eur. Cytokine Netw. 2011, 22, 127–147. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, C.; Tang, J.; Zhou, Y.; Bai, L.; Liu, Y.; Kijlstra, A.; Yang, P. Decreased Interleukin-37 Expression in Vogt-Koyanagi-Harada Disease and Upregulation Following Immunosuppressive Treatment. J. Interferon Cytokine Res. 2015, 35, 265–272. [Google Scholar] [CrossRef]
- Chai, M.; Ji, Q.; Zhang, H.; Zhou, Y.; Yang, Q.; Zhou, Y.; Guo, G.; Liu, W.; Han, W.; Yang, L.; et al. The Protective Effect of Interleukin-37 on Vascular Calcification and Atherosclerosis in Apolipoprotein E-Deficient Mice with Diabetes. J. Interferon Cytokine Res. 2015, 35, 530–539. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, R.; Wang, P.; Yang, H.; He, X.; Ji, Q.; Bai, W.; Chen, H.; Chen, J.; Peng, W.; et al. IL-37 Confers Protection against Mycobacterial Infection Involving Suppressing Inflammation and Modulating T Cell Activation. PLoS ONE 2017, 12, e0169922. [Google Scholar] [CrossRef]
- Yang, T.; Fang, F.; Chen, Y.; Ma, J.; Xiao, Z.; Zou, S.; Zheng, N.; Yan, D.; Liao, S.; Chen, S.; et al. Elevated Plasma Interleukin-37 Playing an Important Role in Acute Coronary Syndrome through Suppression of ROCK Activation. Oncotarget 2017, 8, 9686–9695. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, X.; Li, S.; Liu, Y.; Cui, Y.; Deng, X. Advances in Biomarkers for Detecting Early Cancer Treatment-Related Cardiac Dysfunction. Front. Cardiovasc. Med. 2021, 8, 753313. [Google Scholar] [CrossRef] [PubMed]
- French, B.; Wang, L.; Ky, B.; Brandimarto, J.; Basuray, A.; Fang, J.C.; Sweitzer, N.K.; Cappola, T.P. Prognostic Value of Galectin-3 for Adverse Outcomes in Chronic Heart Failure. J. Card. Fail. 2016, 22, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Zivlas, C.; Triposkiadis, F.; Psarras, S.; Giamouzis, G.; Skoularigis, I.; Chryssanthopoulos, S.; Kapelouzou, A.; Ramcharitar, S.; Barnes, E.; Papasteriadis, E.; et al. Cystatin C and Galectin-3 as Therapeutic Targets in Heart Failure. Ther. Adv. Cardiovasc. Dis. 2018, 12, 233–235. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs Are New and Sensitive Biomarkers of Myocardial Infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Vausort, M.; Wagner, D.R.; Devaux, Y. Long Noncoding RNAs in Patients with Acute Myocardial Infarction. Circ. Res. 2014, 115, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Devaux, Y. Transcriptome of Blood Cells as a Reservoir of Cardiovascular Biomarkers. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2017, 1864, 209–216. [Google Scholar] [CrossRef]
- Ikeda, S.; Pu, W.T. Expression and Function of microRNAs in Heart Disease. Curr. Drug Targets 2010, 11, 913–925. [Google Scholar] [CrossRef]
- Li, M.; Duan, L.; Li, Y.; Liu, B. Long Noncoding RNA/Circular Noncoding RNA-miRNA-mRNA Axes in Cardiovascular Diseases. Life Sci. 2019, 233, 116440. [Google Scholar] [CrossRef]
- Li Santi, A.; Gorrasi, A.; Alfieri, M.; Montuori, N.; Ragno, P. A Novel Oncogenic Role for Urokinase Receptor in Leukemia Cells: Molecular Sponge for Oncosuppressor microRNAs. Oncotarget 2018, 9, 27823–27834. [Google Scholar] [CrossRef]
- Alfieri, M.; Meo, L.; Ragno, P. Posttranscriptional Regulation of the Plasminogen Activation System by Non-Coding RNA in Cancer. Int. J. Mol. Sci. 2023, 24, 962. [Google Scholar] [CrossRef]
- Adachi, T.; Nakanishi, M.; Otsuka, Y.; Nishimura, K.; Hirokawa, G.; Goto, Y.; Nonogi, H.; Iwai, N. Plasma MicroRNA 499 as a Biomarker of Acute Myocardial Infarction. Clin. Chem. 2010, 56, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased MicroRNA-1 and MicroRNA-133a Levels in Serum of Patients with Cardiovascular Disease Indicate Myocardial Damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, L.-F.; Yang, X.-C.; Xu, L.; Li, W.-M.; Xia, K.; Zhang, D.-P.; Wu, R.-N.; Gan, T. Circulating Long Noncoding RNA LIPCAR Acts as a Novel Biomarker in Patients with ST-Segment Elevation Myocardial Infarction. Med. Sci. Monit. 2018, 24, 5064–5070. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating Long Noncoding RNA, LIPCAR, Predicts Survival in Patients with Heart Failure. Circ. Res. 2014, 114, 1569–1575. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.; Guo, S.; Yao, R.; Wu, L.; Xiao, L.; Wang, Z.; Liu, Y.; Zhang, Y. Circulating Long Noncoding RNA HOTAIR Is an Essential Mediator of Acute Myocardial Infarction. Cell. Physiol. Biochem. 2017, 44, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of Cardiac Disease in Cancer Patients throughout Oncological Treatment: ESMO Consensus Recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Leong, D.P.; Lenihan, D.J. Clinical Practice Guidelines in Cardio-Oncology. Heart Fail. Clin. 2022, 18, 489–501. [Google Scholar] [CrossRef]
- Katsurada, K.; Ichida, M.; Sakuragi, M.; Takehara, M.; Hozumi, Y.; Kario, K. High-Sensitivity Troponin T as a Marker to Predict Cardiotoxicity in Breast Cancer Patients with Adjuvant Trastuzumab Therapy. Springerplus 2014, 3, 620. [Google Scholar] [CrossRef]
- Cheung, Y.; Li, V.W.; Lai, C.T.; Shin, V.Y.; Keung, W.; Cheuk, D.K.; Kwong, A.; Li, R.A.; Chan, G.C. Circulating High-Sensitivity Troponin T and microRNAs as Markers of Myocardial Damage during Childhood Leukaemia Treatment. Pediatr. Res. 2021, 89, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Antón, B.; Madurga, R.; Zorita, B.; Wasniewski, S.; Moreno-Arciniegas, A.; López-Melgar, B.; Ramírez Merino, N.; Martín-Asenjo, R.; Barrio, P.; Amado Escañuela, M.G.; et al. Early Detection of Anthracycline- and Trastuzumab-induced Cardiotoxicity: Value and Optimal Timing of Serum Biomarkers and Echocardiographic Parameters. ESC Heart Fail. 2022, 9, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Prayogo, A.A.; Suryantoro, S.D.; Savitri, M.; Hendrata, W.M.; Wijaya, A.Y.; Pikir, B.S. High Sensitivity Troponin T as Complementary Modality for Determining Doxorubicin Regimen Cardiotoxicity in Non-Hodgkin Lymphoma Patients. Adv. Pharm. Bull. 2022, 12, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Bisoc, A.; Ciurescu, D.; Rădoi, M.; Tântu, M.M.; Rogozea, L.; Sweidan, A.J.; Bota, D.A. Elevations in High-Sensitive Cardiac Troponin T and N-Terminal Prohormone Brain Natriuretic Peptide Levels in the Serum Can Predict the Development of Anthracycline-Induced Cardiomyopathy. Am. J. Ther. 2020, 27, e142–e150. [Google Scholar] [CrossRef] [PubMed]
- Finke, D.; Romann, S.W.; Heckmann, M.B.; Hund, H.; Bougatf, N.; Kantharajah, A.; Katus, H.A.; Müller, O.J.; Frey, N.; Giannitsis, E.; et al. High-Sensitivity Cardiac Troponin T Determines All-Cause Mortality in Cancer Patients: A Single-Centre Cohort Study. ESC Heart Fail. 2021, 8, 3709–3719. [Google Scholar] [CrossRef] [PubMed]
- Petricciuolo, S.; Delle Donne, M.G.; Aimo, A.; Chella, A.; De Caterina, R. Pre-Treatment High-Sensitivity Troponin T for the Short-Term Prediction of Cardiac Outcomes in Patients on Immune Checkpoint Inhibitors. Eur. J. Clin. Investig. 2021, 51, e13400. [Google Scholar] [CrossRef] [PubMed]
- Demissei, B.G.; Hubbard, R.A.; Zhang, L.; Smith, A.M.; Sheline, K.; McDonald, C.; Narayan, V.; Domchek, S.M.; DeMichele, A.; Shah, P.; et al. Changes in Cardiovascular Biomarkers with Breast Cancer Therapy and Associations with Cardiac Dysfunction. J. Am. Heart Assoc. 2020, 9, e014708. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Zaninotto, M.; Passino, C.; Padoan, A.; Migliardi, M.; Plebani, M. Chapter Six—High-Sensitivity Methods for Cardiac Troponins: The Mission Is Not over Yet. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 103, pp. 215–252. [Google Scholar]
- Caselli, C.; Cangemi, G.; Masotti, S.; Ragusa, R.; Gennai, I.; Del Ry, S.; Prontera, C.; Clerico, A. Plasma Cardiac Troponin I Concentrations in Healthy Neonates, Children and Adolescents Measured with a High Sensitive Immunoassay Method: High Sensitive Troponin I in Pediatric Age. Clin. Chim. Acta 2016, 458, 68–71. [Google Scholar] [CrossRef]
- Bohn, M.K.; Higgins, V.; Kavsak, P.; Hoffman, B.; Adeli, K. High-Sensitivity Generation 5 Cardiac Troponin T Sex- and Age-Specific 99th Percentiles in the CALIPER Cohort of Healthy Children and Adolescents. Clin. Chem. 2019, 65, 589–591. [Google Scholar] [CrossRef]
- Jehlicka, P.; Rajdl, D.; Sladkova, E.; Sykorova, A.; Sykora, J. Dynamic Changes of High-Sensitivity Troponin T Concentration During Infancy: Clinical Implications. Physiol. Res. 2021, 70, 27–32. [Google Scholar] [CrossRef]
- Lam, L.; Ha, L.; Heron, C.; Chiu, W.; Kyle, C. Identification of Macrotroponin T: Findings from a Case Report and Non-Reproducible Troponin T Results. Clin. Chem. Lab. Med. 2021, 59, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.; Ryan, P.M.; Gupta, K.; Radovanovic, G.; Pugh, E.; Chan, A.K.C.; Hill, S. Cord-Blood High-Sensitivity Troponin-I Reference Interval and Association with Early Neonatal Outcomes. Am. J. Perinatol. 2022, 29, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Soldin, S.J.; Murthy, J.N.; Agarwalla, P.K.; Ojeifo, O.; Chea, J. Pediatric Reference Ranges for Creatine Kinase, CKMB, Troponin I, Iron, and Cortisol. Clin. Biochem. 1999, 32, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Engin, Y.; Ustün, Y.; Kurtay, G. Cardiac Troponin I Levels in Umbilical Cord Blood. Int. J. Gynaecol. Obs. 2002, 77, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Trevisanuto, D.; Pitton, M.; Altinier, S.; Zaninotto, M.; Plebani, M.; Zanardo, V. Cardiac Troponin I, Cardiac Troponin T and Creatine Kinase MB Concentrations in Umbilical Cord Blood of Healthy Term Neonates. Acta Paediatr. 2003, 92, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; George, K.P.; Tong, T.K.; Gaze, D.; Tian, Y.; Lin, H.; Shi, Q. The Influence of a Half-Marathon Race upon Cardiac Troponin T Release in Adolescent Runners. Curr. Med. Chem. 2011, 18, 3452–3456. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; George, K.P.; Tong, T.K.; Tian, Y.; Shi, Q. Effect of Repeated Endurance Runs on Cardiac Biomarkers and Function in Adolescents. Med. Sci. Sports Exerc. 2011, 43, 2081–2088. [Google Scholar] [CrossRef]
- Peretti, A.; Mauri, L.; Masarin, A.; Annoni, G.; Corato, A.; Maloberti, A.; Giannattasio, C.; Vignati, G. Cardiac Biomarkers Release in Preadolescent Athletes After an High Intensity Exercise. High. Blood Press. Cardiovasc. Prev. 2018, 25, 89–96. [Google Scholar] [CrossRef]
- Pompa, A.G.; Arora, G.; Harris, T.H. Effect of Treadmill Exercise Stress Testing on Troponin Levels in Children and Adolescents. Cardiol. Young 2023, 33, 380–382. [Google Scholar] [CrossRef]
- Cirer-Sastre, R.; Legaz-Arrese, A.; Corbi, F.; López-Laval, I.; Puente-Lanzarote, J.; Hernández-González, V.; Reverter-Masià, J. Effect of Training Load on Post-Exercise Cardiac Troponin T Elevations in Young Soccer Players. Int. J. Environ. Res. Public. Health 2019, 16, 4853. [Google Scholar] [CrossRef]
- Cirer-Sastre, R.; Legaz-Arrese, A.; Corbi, F.; López-Laval, I.; George, K.; Reverter-Masia, J. Influence of Maturational Status in the Exercise-Induced Release of Cardiac Troponin T in Healthy Young Swimmers. J. Sci. Med. Sport. 2021, 24, 116–121. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; Wang, D.; Brady, T.; Tang, O.; Ndumele, C.E.; Coresh, J.; Christenson, R.H.; Selvin, E. Myocardial Injury Thresholds for 4 High-Sensitivity Troponin Assays in a Population-Based Sample of US Children and Adolescents. Circulation 2023, 148, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Kelleman, M.; West, Z.; Peter, A.; Dove, M.; Butto, A.; Oster, M.E. Comparison of Multisystem Inflammatory Syndrome in Children-Related Myocarditis, Classic Viral Myocarditis, and COVID-19 Vaccine-Related Myocarditis in Children. J. Am. Heart Assoc. 2022, 11, e024393. [Google Scholar] [CrossRef] [PubMed]
- Dionne, A.; Kheir, J.N.; Sleeper, L.A.; Esch, J.J.; Breitbart, R.E. Value of Troponin Testing for Detection of Heart Disease in Previously Healthy Children. J. Am. Heart Assoc. 2020, 9, e012897. [Google Scholar] [CrossRef] [PubMed]
- Belhadjer, Z.; Méot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef]
- Alfieri, M.; Munno, C.; Savarese, M.; Pollice, A.; Antignani, A.; Leone, O. Upgrading to a High-Sensitivity Troponin Bedside Assay to Monitor Chemotherapy-Induced Cardiotoxicity in 15 Paediatric Patients. Ligand Assay 2022, 27, 233. [Google Scholar]
- Lv, X.; Pan, C.; Guo, H.; Chang, J.; Gao, X.; Wu, X.; Zhi, X.; Ren, C.; Chen, Q.; Jiang, H.; et al. Early Diagnostic Value of High-Sensitivity Cardiac Troponin T for Cancer Treatment-Related Cardiac Dysfunction: A Meta-Analysis. ESC Heart Fail. 2023, 10, 2170–2182. [Google Scholar] [CrossRef]


| SNP | Locus | Effects | Reference |
|---|---|---|---|
| rs2229774 | RARG | increased cardiomyocyte death | Aminkeng et al., 2015 [17] |
| rs1056892 | CBR3 | tripled risk of ACT | Blanco et al., 2012 [21] |
| rs3743527 | 3′ UTR of ABCC1 | ACT | Semsei et al., 2012 [25] |
| rs10426377 | SULT2B1 | ACT | Visscher et al., 2012 [26] Visscher et al., 2013 [27] |
| rs13058338 | RAC2 subunit of NADPH | ACT susceptibility | Zhao et al., 2010 [28] |
| rs10836235 | CAT | ACT resistance | Rajić et al., 2009 [29] |
| rs1695 | GSTP1 | susceptibility to ACT | Windsor et al., 2012 [30] |
| rs12468485 | GPCR35 | increased risk and severity of ACT | Min et al., 2010 [31] |
| rs2232228 | HAS3 | manipulate the risk of ACT | Wang et al., 2014 [32] |
| rs1786814 | CELF4 | increased cardiomyopathy | Wang et al., 2016 [33] |
| rs11140490 | SLC28A3 locus | cardio-protective effect | Magdy et al., 2022 [35] |
| rs2229774 | RARG | increased risk of ACT | Magdy et al., 2022 [35] |
| rs17863783 | UGT1A6 | increased risk of ACT | Loucks et al., 2022 [37] |
| rs7853758 | SLC28A3 | cardio-protective effect | Loucks et al., 2022 [37] |
| p.Thr253Met, c.758C>T variant | GPR35 | increased risk of ACT | Ruiz-Pinto et al., 2017 [38] |
| Characteristic | PATHFAST hs-cTnI | Quidel TriageTrue hs-cTnI | Siemens Atellica VTLi hs-cTnI |
|---|---|---|---|
| LoB | 1.23 ng/L | 0.4 ng/L (plasma) 0.5–0.8 ng/L (whole blood) | 0.55 ng/L |
| LoD | 2.33 ng/L | 0.7–1.6 ng/L (plasma) 1.5–1.9 ng/L (whole blood) | 1.2 ng/L (plasma) 1.6 ng/L (whole blood) |
| % CV at 99th Percentile | 6.1% | 5.0–5.9% at 21 ng/L (plasma) 5.9–6.5% at 22 ng/L (whole blood) | 6.5% (plasma) 6.1% at 22.9 ng/L (whole blood) |
| Concentration at 20% CV | 4 ng/L | 2.1–3.6 ng/L (plasma) 2.8 ng/L (whole blood) | 2.1 ng/L (plasma) 3.7 ng/L (whole blood) |
| Concentration at 10% CV | 15 ng/L | 4.4–8.4 ng/L (plasma) 5.8–6.2 ng/L (whole blood) | 6.7 ng/L (plasma) 8.9 ng/L (whole blood) |
| 99th Percentile | Overall: 27.9 ng/L F: 20.3 ng/L M: 29.7 ng/L | Overall: 20.5 ng/L F: 14.4 ng/L M: 25.7 ng/L | Overall: 22.9 ng/L F: 18.5 ng/L M: 27.1 ng/L |
| Reference population | Overall n = 734 F: 352 M: 382 | Overall n = 789 F: 391 M: 398 | Overall n = 694 F: 331 M: 363 |
| Specimen type | Heparin-Na, heparin-Li or EDTA whole blood or plasma | EDTA whole blood or plasma | Li Hep whole blood and plasma, capillary blood |
| POCT definition (according to Collinson 2023) | Desktop | Portable | Portable |
| Instrument size | 475 × 343 × 569 mm | 190 × 70 × 225 mm | 250 × 52 × 85 mm (analyzer) 290 × 60 × 100 mm (docking station) |
| Instrument weight | 28 kg | 0.7 kg | 780 g (analyzer) 460 g (docking station) |
| Single-test technology | No | Yes | Yes |
| Other markers available on the same analyzer | NT-proBNP, myoglobin, CK-MB, D-dimer, presepsin, CRP, PCT | BNP, NT-proBNP, myoglobin, CK-MB, D-dimer, drugs of abuse, PLGF | No |
| Included in ESC 2023 guidelines for ACS [74] | Yes | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meo, L.; Savarese, M.; Munno, C.; Mirabelli, P.; Ragno, P.; Leone, O.; Alfieri, M. Circulating Biomarkers for Monitoring Chemotherapy-Induced Cardiotoxicity in Children. Pharmaceutics 2023, 15, 2712. https://doi.org/10.3390/pharmaceutics15122712
Meo L, Savarese M, Munno C, Mirabelli P, Ragno P, Leone O, Alfieri M. Circulating Biomarkers for Monitoring Chemotherapy-Induced Cardiotoxicity in Children. Pharmaceutics. 2023; 15(12):2712. https://doi.org/10.3390/pharmaceutics15122712
Chicago/Turabian StyleMeo, Luigia, Maria Savarese, Carmen Munno, Peppino Mirabelli, Pia Ragno, Ornella Leone, and Mariaevelina Alfieri. 2023. "Circulating Biomarkers for Monitoring Chemotherapy-Induced Cardiotoxicity in Children" Pharmaceutics 15, no. 12: 2712. https://doi.org/10.3390/pharmaceutics15122712