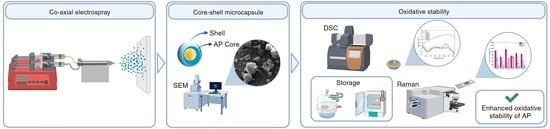
Carbohydrate Core–Shell Electrosprayed Microcapsules for Enhanced Oxidative Stability of Vitamin A Palmitate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Solutions for Co-Axial Electrospray
2.2. Co-Axial Electrospray Processing
2.3. Morphological Characterization of the Microcapsules by Scanning Electron Microscopy (SEM)
2.4. Evaluation of the Oxidative Stability of the Microcapsules
2.4.1. Evaluation of the Oxidative Stability by Differential Scanning Calorimetry (DSC)
2.4.2. Evaluation of the Oxidative Stability of AP by Raman Spectroscopy
2.4.3. Evaluation of the Interactions of AP with the Core Compounds of the Microcapsule by ATR-FTIR Spectroscopy
2.5. Encapsulation Efficiency
2.6. Statistical Analysis
3. Results and Discussion
3.1. Morphology of the Electrospray Core–Shell Microcapsules
3.2. Assessment of the Interactions of Vitamin A Palmitate with the Core Compounds of the Microcapsules by FTIR Spectroscopy
3.3. Oxidation Assessment of Non-Encapsulated and Encapsulated Vitamin A Palmitate by Raman Spectroscopy: Effect of Airflow and Temperature
3.4. Oxidation Assessment of Non-Encapsulated and Encapsulated Vitamin A Palmitate Using DSC
4. Further Discussion on the Enhanced Oxidative Stability of the Encapsulated Vitamin A Palmitate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combs, G.F., Jr.; McClung, J.P. Vitamin A. In The Vitamins, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 77–132. [Google Scholar]
- Salvador, A.; Chisvert, A. Vitamins. In Analysis of Cosmetic Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 364–379. [Google Scholar]
- Loveday, S.M.; Singh, H. Recent advances in technologies for vitamin A protection in foods. Trends Food Sci. Technol. 2008, 19, 657–668. [Google Scholar] [CrossRef]
- La Frano, M.R.; Cai, Y.; Burri, B.J.; Thilsted, S.H. Discovery and biological relevance of 3,4-didehydroretinol (vitamin A2) in small indigenous fish species and its potential as a dietary source for addressing vitamin A deficiency. Int. J. Food Sci. Nutr. 2018, 69, 253–261. [Google Scholar] [CrossRef]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Zayed, A.; Merghany, R.M.; Ezzat, S.M. Roles of antioxidants in the prevention and management of coronavirus disease 2019. In Coronavirus Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 85–104. [Google Scholar]
- WHO Iris. World Health Organization Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency. 2009, p. 55. Available online: http://apps.who.int//iris/handle/10665/44110 (accessed on 19 April 2023).
- Ottaway, P.B. Stability of vitamins during food processing and storage. In Chemical Deterioration and Physical Instability of Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2010; pp. 539–560. [Google Scholar]
- Pignitter, M.; Somoza, V. The Stability of Vitamins A and E in Edible Oils. In Sustainable Nutrition in a Changing World; Springer International Publishing: Cham, Switzerland, 2017; pp. 295–305. [Google Scholar]
- García-Moreno, P.J.; Mendes, A.C.; Jacobsen, C.; Chronakis, I.S. Biopolymers for the Nano-microencapsulation of Bioactive Ingredients by Electrohydrodynamic Processing. In Polymers for Food Applications; Springer International Publishing: Cham, Switzerland, 2018; pp. 447–479. [Google Scholar]
- Mendes, A.C.; Chronakis, I.S. Electrohydrodynamic encapsulation of probiotics: A review. Food Hydrocoll. 2021, 117, 106688. [Google Scholar] [CrossRef]
- Jacobsen, C.; Garcia-Moreno, P.J.; Mendes, A.C.; Mateiu, R.V.; Chronakis, I.S. Use of Electrospinning for Encapsulation of Sensitive Bioactive Compounds and Applications in Food. Annu. Rev. Food Sci. Technol. 2018, 9, 525–549. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Lemma, S.M.; Scampicchio, M.; Mahon, P.J.; Sbarski, I.; Wang, J.; Kingshott, P. Controlled Release of Retinyl Acetate from β-Cyclodextrin Functionalized Poly(vinyl alcohol) Electrospun Nanofibers. J. Agric. Food Chem. 2015, 63, 3481–3488. [Google Scholar] [CrossRef]
- Fahami, A.; Fathi, M. Development of cress seed mucilage/PVA nanofibers as a novel carrier for vitamin A delivery. Food Hydrocoll. 2018, 81, 31–38. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Perez-Masiá, R.; González-Barrio, R.; Periago, M.J.; López-Rubio, A. Potential of microencapsulation through emulsion-electrospraying to improve the bioaccesibility of β-carotene. Food Hydrocoll. 2017, 73, 1–12. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Micro- and nano-encapsulation of β-carotene in zein protein: Size-dependent release and absorption behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Niu, B.; Shao, P.; Feng, S.; Qiu, D.; Sun, P. Rheological aspects in fabricating pullulan-whey protein isolate emulsion suitable for electrospraying: Application in improving β-carotene stability. LWT 2020, 129, 109581. [Google Scholar] [CrossRef]
- Basar, A.O.; Prieto, C.; Durand, E.; Villeneuve, P.; Sasmazel, H.T.; Lagaron, J. Encapsulation of β-carotene by emulsion electrospraying using deep eutectic solvents. Molecules 2020, 25, 981. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; Ramos, P.E.; Cerqueira, M.F.; Teixeira, J.A.; Vicente, A.A.; Pastrana, L.M.; Pereira, R.N.; Cerqueira, M.A. Electrosprayed whey protein-based nanocapsules for β-carotene encapsulation. Food Chem. 2020, 314, 126157. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Y.; Fan, L.; Yan, W. Ethyl cellulose particles loaded with α-tocopherol for inhibiting thermal oxidation of soybean oil. Carbohydr. Polym. 2021, 252, 117169. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Li, J.; Fan, L.; Huang, S. Study on the antioxidative mechanism of tocopherol loaded ethyl cellulose particles in thermal-oxidized soybean oil. Carbohydr. Polym. 2022, 276, 118734. [Google Scholar] [CrossRef]
- Arayachukeat, S.; Wanichwecharungruang, S.P.; Tree-Udom, T. Retinyl acetate-loaded nanoparticles: Dermal penetration and release of the retinyl acetate. Int. J. Pharm. 2011, 404, 281–288. [Google Scholar] [CrossRef]
- Li, J.Z. The Use of Starch-Based Materials for Microencapsulation. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 195–210. [Google Scholar]
- Bajaj, R.; Singh, N.; Kaur, A. Properties of octenyl succinic anhydride (OSA) modified starches and their application in low fat mayonnaise. Int. J. Biol. Macromol. 2019, 131, 147–157. [Google Scholar] [CrossRef]
- Vidović, S.S.; Vladić, J.Z.; Vaštag, Ž.G.; Zeković, Z.P.; Popović, L.M. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
- Tesch, S.; Gerhards, C.; Schubert, H. Stabilization of emulsions by OSA starches. J. Food Eng. 2002, 54, 167–174. [Google Scholar] [CrossRef]
- Gangurde, A.B.; Amin, P.D. Microencapsulation by Spray Drying of Vitamin A Palmitate from Oil to Powder and Its Application in Topical Delivery System. J. Encapsulation Adsorpt. Sci. 2017, 7, 10–39. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Shahgol, M.; Estevinho, B.N.; Rocha, F. Microencapsulation of Vitamin A by spray-drying, using binary and ternary blends of gum arabic, starch and maltodextrin. Food Hydrocoll. 2020, 108, 106029. [Google Scholar] [CrossRef]
- Mujica-Álvarez, J.; Gil-Castell, O.; Barra, P.A.; Ribes-Greus, A.; Bustos, R.; Faccini, M.; Matiacevich, S. Encapsulation of Vitamins A and E as Spray-Dried Additives for the Feed Industry. Molecules 2020, 25, 1357. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-T.; Mendes, A.C.; Chronakis, I.S. Electrospinning and electrospraying technologies for food applications. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2019; Volume 88, pp. 167–234. [Google Scholar]
- García-Moreno, P.J.; Rahmani-Manglano, N.E.; Chronakis, I.S.; Guadix, E.M.; Yesiltas, B.; Sørensen, A.-D.M.; Jacobsen, C. Omega-3 nano-microencapsulates produced by electrohydrodynamic processing. In Omega-3 Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 345–370. [Google Scholar]
- Rahmani-Manglano, N.E.; Guadix, E.M.; Jacobsen, C.; García-Moreno, P.J. Comparative Study on the Oxidative Stability of Encapsulated Fish Oil by Monoaxial or Coaxial Electrospraying and Spray-Drying. Antioxidants 2023, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Encapsulation of β-carotene into food-grade nanofibers via coaxial electrospinning of hydrocolloids: Enhancement of oxidative stability and photoprotection. Food Hydrocoll. 2022, 133, 107949. [Google Scholar] [CrossRef]
- Gabrič, A.; Hodnik, Ž.; Pajk, S. Oxidation of Drugs during Drug Product Development: Problems and Solutions. Pharmaceutics 2022, 14, 325. [Google Scholar] [CrossRef] [PubMed]
- Micić, D.M.; Ostojić, S.B.; Simonović, M.B.; Krstić, G.; Pezo, L.L.; Simonović, B.R. Kinetics of blackberry and raspberry seed oils oxidation by DSC. Thermochim. Acta 2015, 601, 39–44. [Google Scholar] [CrossRef]
- Vilanova, N.; Solans, C. Vitamin A Palmitate–β-cyclodextrin inclusion complexes: Characterization, protection and emulsification properties. Food Chem. 2015, 175, 529–535. [Google Scholar] [CrossRef]
- Litwinienko, G.; Kasprzycka-Guttman, T. A DSC study on thermoxidation kinetics of mustard oil. Thermochim. Acta 1998, 319, 185–191. [Google Scholar] [CrossRef]
- Moomand, K.; Lim, L.T. Oxidative stability of encapsulated fish oil in electrospun zein fibres. Food Res. Int. 2014, 62, 523–532. [Google Scholar] [CrossRef]
- Moreno, J.S.; Dima, P.; Chronakis, I.S.; Mendes, A.C. Electrosprayed ethyl cellulose core-shell microcapsules for the encapsulation of probiotics. Pharmaceutics 2022, 14, 7. [Google Scholar] [CrossRef]
- Pezeshky, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Moghadam, M.; Babazadeh, A. Vitamin A palmitate-bearing nanoliposomes: Preparation and characterization. Food Biosci. 2016, 13, 49–55. [Google Scholar] [CrossRef]
- Huang, L.Y.; Yu, D.G.; Branford-White, C.; Zhu, L.M. Sustained release of ethyl cellulose micro-particulate drug delivery systems prepared using electrospraying. J. Mater. Sci. 2012, 47, 1372–1377. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R.K.; Jana, S. Characterization of Physicochemical and Thermal Properties of Biofield Treated Ethyl Cellulose and Methyl Cellulose. Int. J. Biomed. Mater. Res. 2015, 3, 83–91. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Spectroscopic manifestation of stretching vibrations of glycosidic linkage in polysaccharides. J. Mol. Struct. 2005, 752, 20–24. [Google Scholar] [CrossRef]
- Cherng, S.H.; Xia, Q.; Blankenship, L.R.; Freeman, J.P.; Wamer, W.G.; Howard, P.C.; Fu, P.P. Photodecomposition of retinyl palmitate in ethanol by UVA light-formation of photodecomposition products, reactive oxygen species, and lipid peroxides. Chem. Res. Toxicol. 2005, 18, 129–138. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Yin, J.J.; Cherng, S.-H.; Yan, J.; Mei, N.; Chen, T.; Boudreau, M.D.; Howard, P.C.; Wamer, W.G. Photodecomposition of Vitamin A and Photobiological Implications for the Skin. Photochem. Photobiol. 2007, 83, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yin, J.J.; Wamer, W.G.; Cherng, S.H.; Boudreau, M.D.; Howard, P.C.; Yu, H.; Fu, P.P. Photoirradiation of retinyl palmitate in ethanol with ultraviolet light–Formation of photodecomposition products, reactive oxygen species, and lipid peroxides. Int. J. Environ. Res. Public Health 2006, 3, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Failloux, N.; Bonnet, I.; Perrier, E.; Baron, M.H. Effects of light, oxygen and concentration on vitamin A1. J. Raman Spectrosc. 2004, 35, 140–147. [Google Scholar] [CrossRef]
- Galler, K.; Requardt, R.P.; Glaser, U.; Markwart, R.; Bocklitz, T.; Bauer, M.; Popp, J.; Neugebauer, U. Single cell analysis in native tissue: Quantification of the retinoid content of hepatic stellate cells. Sci. Rep. 2016, 6, 24155. [Google Scholar] [CrossRef]
- Larkin, P.J. IR and Raman Spectra–Structure Correlations. In Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 85–134. [Google Scholar]
- Wiley, J.H.; Atalla, R.H. Band assignments in the raman spectra of celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Karami, Z.; Pateiro, M.; Lorenzo, J.M. A Review on Health-Promoting, Biological, and Functional Aspects of Bioactive Peptides in Food Applications. Biomolecules 2021, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Manglano, N.E.; González-Sánchez, I.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Jacobsen, C.; Guadix, E.M. Development of Fish Oil-Loaded Microcapsules Containing Whey Protein Hydrolysate as Film-Forming Material for Fortification of Low-Fat Mayonnaise. Foods 2020, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Manglano, N.E.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Pérez-Gálvez, A.R.; Guadix-Escobar, E.M. The Role of Antioxidants and Encapsulation Processes in Omega-3 Stabilization. In Emulsion-Based Encapsulation of Antioxidants: Design and Performance; Springer: Cham, Switzerland, 2020; pp. 339–386. [Google Scholar]
- Malika, A.; Mohammed, A.; Boukhlifi, F. Kinetic and energy study of thermal degradation of biomass materials under oxidative atmosphere using TGA, DTA and DSC. J. Multidiscip. Eng. Sci. Technol. 2014, 1, 3159–3199. [Google Scholar]
- Xie, Y.L.; Zhou, H.M.; Liang, X.H.; He, B.S.; Han, X.X. Study on the morphology, particle size and thermal properties of Vitamin A microencapsulated by starch octenylsucciniate. Agric. Sci. China 2010, 9, 1058–1064. [Google Scholar] [CrossRef]
- Thomas, P.D.; Dill, K.A. Local and nonlocal interactions in globular proteins and mechanisms of alcohol denaturation. Protein Sci. 1993, 2, 2050–2065. [Google Scholar] [CrossRef]
- Liu, L.-L.; Li, X.-T.; Zhang, N.; Tang, C.-H. Novel soy β-conglycinin nanoparticles by ethanol-assisted disassembly and reassembly: Outstanding nanocarriers for hydrophobic nutraceuticals. Food Hydrocoll. 2019, 91, 246–255. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, X.; Kong, B.; Chen, Q.; Liu, Q. Ethanol induced changes in structural, morphological, and functional properties of whey proteins isolates: Influence of ethanol concentration. Food Hydrocoll. 2021, 111, 106379. [Google Scholar] [CrossRef]
- Feng, Y.; Yuan, D.; Cao, C.; Kong, B.; Sun, F.; Xia, X.; Liu, Q. Changes of in vitro digestion rate and antioxidant activity of digestion products of ethanol-modified whey protein isolates. Food Hydrocoll. 2022, 131, 107756. [Google Scholar] [CrossRef]
- Feng, Y.; Yuan, D.; Kong, B.; Sun, F.; Wang, M.; Wang, H.; Liu, Q. Structural changes and exposed amino acids of ethanol-modified whey proteins isolates promote its antioxidant potential. Curr. Res. Food Sci. 2022, 5, 1386–1394. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Haham, M.; Ish-Shalom, S.; Nodelman, M.; Duek, I.; Segal, E.; Kustanovich, M.; Livney, Y.D. Stability and bioavailability of vitamin D nanoencapsulated in casein micelles. Food Funct. 2012, 3, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Rosenberg, D.; Livney, Y.D. Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll. 2011, 25, 1270–1276. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Zhao, M.; Lin, L.; Ning, Z.; Sun, B. Soy peptide nanoparticles by ultrasound-induced self-assembly of large peptide aggregates and their role on emulsion stability. Food Hydrocoll. 2018, 74, 62–71. [Google Scholar] [CrossRef]
- Moure, A.; Domínguez, H.; Parajó, J.C. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006, 41, 447–456. [Google Scholar] [CrossRef]
- Chen, C.; Sun-Waterhouse, D.; Zhang, Y.; Zhao, M.; Sun, W. The chemistry behind the antioxidant actions of soy protein isolate hydrolysates in a liposomal system: Their performance in aqueous solutions and liposomes. Food Chem. 2020, 323, 126789. [Google Scholar] [CrossRef]
- Judde, A.; Villeneuve, P.; Rossignol-Castera, A.; Le Guillou, A. Antioxidant effect of soy lecithins on vegetable oil stability and their synergism with tocopherols. J. Am. Oil Chem. Soc. 2003, 80, 1209–1215. [Google Scholar] [CrossRef]
- Pan, Y.; Tikekar, R.V.; Nitin, N. Effect of antioxidant properties of lecithin emulsifier on oxidative stability of encapsulated bioactive compounds. Int. J. Pharm. 2013, 450, 129–137. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.-H.; Genot, C. Lipid Oxidation in Oil-in-Water Emulsions: Involvement of the Interfacial Layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Ohkuma, K.; Wakabayashi, S. Fibersol-2: A Soluble, Non-Digestible, Starch-Derived Dietary Fibre. In Advanced Dietary Fibre Technology; Barry, L.P., McCleary, V., Eds.; Blackwell Science: Hoboken, NJ, USA, 2000; pp. 509–523. [Google Scholar]
- Kishimoto, Y.; Yoshikawa, Y.; Miyazato, S.; Oga, H.; Yamada, T.; Tagami, H.; Hashizume, C.; Yamamoto, K. Effect of Resistant Maltodextrin on Digestion and Absorption of Lipids. J. Health Sci. 2009, 55, 838–844. [Google Scholar] [CrossRef]
- Chen, Q.; Zhong, F.; Wen, J.; McGillivray, D.; Quek, S.Y. Properties and Stability of Spray-Dried and Freeze-Dried Microcapsules Co-Encapsulated with Fish Oil, Phytosterol Esters, and Limonene. Dry. Technol. 2013, 31, 707–716. [Google Scholar] [CrossRef]
- Pai, D.A.; Vangala, V.R.; Ng, J.W.; Ng, W.K.; Tan, R.B.H. Resistant maltodextrin as a shell material for encapsulation of naringin: Production and physicochemical characterization. J. Food Eng. 2015, 161, 68–74. [Google Scholar] [CrossRef]
- Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Resistant maltodextrin’s effect on the physicochemical and structure properties of spray dried orange juice powders. Eur. Food Res. Technol. 2021, 247, 1125–1132. [Google Scholar] [CrossRef]










| Sample Designation | Shell Materials | Core Materials |
|---|---|---|
| H-EAP | Hi-Cap® 100, modified starch | Ethyl cellulose |
| Retinyl palmitate | ||
| Tween-20 | ||
| H-EAPL | Hi-Cap® 100 modified starch | Ethyl cellulose |
| Retinyl palmitate | ||
| Tween-20 Lecithin | ||
| H-EAPS | Hi-Cap® 100 modified starch | Ethyl cellulose |
| Retinyl palmitate | ||
| Tween-20 | ||
| Soy protein acid hydrolysate | ||
| H-EAPC | Hi-Cap® 100 modified starch | Ethyl cellulose |
| Retinyl palmitate | ||
| Tween-20 | ||
| Hy-case® SF | ||
| H-EAPF | Hi-Cap® 100 modified starch | Ethyl cellulose |
| Retinyl palmitate | ||
| Tween-20 Fibersol®–2 | ||
| HF-EAP | Ethyl cellulose | |
| Hi-Cap® 100 modified starch | Retinyl palmitate | |
| Fibersol®–2 | Tween-20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallahasghari, E.Z.; Højgaard Lynge, M.; Espholin Gudnason, E.; Munkerup, K.; Mendes, A.C.; Chronakis, I.S. Carbohydrate Core–Shell Electrosprayed Microcapsules for Enhanced Oxidative Stability of Vitamin A Palmitate. Pharmaceutics 2023, 15, 2633. https://doi.org/10.3390/pharmaceutics15112633
Fallahasghari EZ, Højgaard Lynge M, Espholin Gudnason E, Munkerup K, Mendes AC, Chronakis IS. Carbohydrate Core–Shell Electrosprayed Microcapsules for Enhanced Oxidative Stability of Vitamin A Palmitate. Pharmaceutics. 2023; 15(11):2633. https://doi.org/10.3390/pharmaceutics15112633
Chicago/Turabian StyleFallahasghari, Elnaz Z., Marie Højgaard Lynge, Emma Espholin Gudnason, Kristin Munkerup, Ana C. Mendes, and Ioannis S. Chronakis. 2023. "Carbohydrate Core–Shell Electrosprayed Microcapsules for Enhanced Oxidative Stability of Vitamin A Palmitate" Pharmaceutics 15, no. 11: 2633. https://doi.org/10.3390/pharmaceutics15112633