Optimisation of the Manufacturing Process of Organic-Solvent-Free Omeprazole Enteric Pellets for the Paediatric Population: Full Factorial Design
Abstract
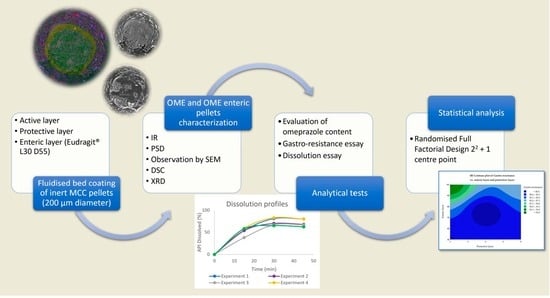
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Full Factorial Design (FFD)
2.2.2. Preparation of Omeprazole Enteric Pellets
2.2.3. Characterisation: API and Coated Pellets
Determination of Particle Size Distribution (PSD)
Determination of Flow Properties of OME Enteric Pellets
Determination of Coating Uniformity
Determination of API via Infrared Radiation
Differential Scanning Calorimetry (DSC)
Determination via X-ray Diffraction (XRD)
2.2.4. Evaluation of Omeprazole Content
2.2.5. Gastro-Resistance Trial
2.2.6. Dissolution Trial
3. Results and Discussion
3.1. Characterisation: Micronised OME and OME Enteric Pellets
3.1.1. Particle Size Distribution
3.1.2. Determination of Flow Properties of OME Enteric Pellets
3.1.3. Determination of Coating Uniformity
3.1.4. Infrared Radiation, Differential Scanning Calorimetry, and X-ray Diffraction
3.2. Experimental Responses of the FFD
3.2.1. Evaluation of Omeprazole Content
3.2.2. Gastro-Resistance Trial
3.2.3. Dissolution Trial
3.3. Statistical Analysis of the Full Factorial Design
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cañete Ramírez, C.; García Palomo, M.; García-Palop, B.; Cabañas Poy, M.J.; Cañete Ramírez, C. Formulación Magistral y Excipientes En Pediatría Correspondencia. Farmacéutico. Hosp. 2018, 213, 22–28. [Google Scholar]
- Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the Paediatric Population: A Review. Pharmaceutics 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Yafout, M.; Ousaid, A.; Lachguer, K.; Khayati, Y.; Ait Haj Said, A. Medication Use in Children: A Survey among Hospital Pediatricians in Morocco. Pharm. Clin. 2022, 57, 227–233. [Google Scholar] [CrossRef]
- Yang, S.; Trinh, N.T.H.; Chalumeau, M.; Kaguelidou, F.; Ruemmele, F.M.; Milic, D.; Lemaitre, M.; Cohen, J.F.; Taine, M. Pediatric Prescriptions of Proton Pump Inhibitors in France (2009–2019): A Time-Series Analysis of Trends and Practice Guidelines Impact. J. Pediatr. 2022, 245, 158–164.e4. [Google Scholar] [CrossRef] [PubMed]
- Tiengkate, P.; Lallemant, M.; Charoenkwan, P.; Angkurawaranon, C.; Kanjanarat, P.; Suwannaprom, P.; Borriharn, P. Gaps in Accessibility of Pediatric Formulations: A Cross-Sectional Observational Study of a Teaching Hospital in Northern Thailand. Children 2022, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Rang, H.; Dale, M. Farmacología, 10th ed.; Elsevier: Madrid, Spain, 2020. [Google Scholar]
- Esplugues, J.V.; Martí-Cabrera, M.; Flórez, J. Farmacología de La Secreción Gastrointestinal y de La Ulceración Mucosa Digestiva. In Farmacología Humana, 6th ed.; Elsevier: Madrid, Spain, 2014; pp. 708–722. [Google Scholar]
- Flórez, J.; Esplugues, J.V.; Martí-Cabrera, M. Farmacología Humana, 6th ed.; Elsevier: Madrid, Spain, 2014. [Google Scholar]
- Litalien, C.; Théorêt, Y.; Faure, C. Pharmacokinetics of Proton Pump Inhibitors in Children. Clin. Pharmacokinet. 2005, 44, 441–466. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kincl, M.; Turk, S.; Vrečer, F. Application of Experimental Design Methodology in Development and Optimization of Drug Release Method. Int. J. Pharm. 2005, 291, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kincl, M.; Vrečer, F.; Veber, M. Characterization of Factors Affecting the Release of Low-Solubility Drug from Prolonged Release Tablets. Anal. Chim. Acta 2004, 502, 107–113. [Google Scholar] [CrossRef]
- Bounouri, Y.; Berkani, M.; Zamouche, A.; Rycerz, L. Optimization and Modeling of Synthesis Parameters of Neodymium(III) Bromide by Dry Method Using Full Factorial Design Analysis. Arab. J. Chem. 2020, 13, 366–376. [Google Scholar] [CrossRef]
- Lazic, Z.R. Design of Experiments in Chemical Engineering: A Practical Guide; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Hanrahan, G.; Lu, K. Application of Factorial and Response Surface Methodology in Modern Experimental Design and Optimization. Crit. Rev. Anal. Chem. 2007, 36, 141–151. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Q8 (R2) Pharmaceutical Development—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q8-r2-pharmaceutical-development-scientific-guideline (accessed on 29 April 2023).
- Siepmann, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. Blends of Aqueous Polymer Dispersions Used for Pellet Coating: Importance of the Particle Size. J. Control. Release 2005, 105, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Varalakshmi, M.; Shaik, R. The Incluence of HPC-L and Eudragit L30 D-55 on Delayed Release Omeprazole Magnesium Multiple-Unit Pellet System. Asian J. Pharm. Clin. Res. 2018, 11, 178–184. [Google Scholar]
- Ronchi, F.; Sereno, A.; Paide, M.; Sacré, P.; Guillaume, G.; Stéphenne, V.; Goole, J.; Amighi, K. Development and Evaluation of an Omeprazole-Based Delayed-Release Liquid Oral Dosage Form. Int. J. Pharm. 2019, 567, 118416. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; The Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- European Pharmacopoeia 11.2 2.9.31. Particle Size Analysis by Laser Light Diffraction—European Pharmacopoeia 11.2. Available online: https://pheur.edqm.eu/app/11-2/content/11-2/20931E.htm?highlight=on&terms=particle&terms=particle-size%20distribution&terms=distribution&terms=particle%20size&terms=size%20distribution&terms=size&terms=particle-size (accessed on 7 May 2023).
- European Pharmacopoeia 2.9.38. Particle Size Distribution. Estimation by Analytical Sieving—European Pharmacopoeia 11.2. Available online: https://pheur.edqm.eu/app/11-2/content/11-2/20938E.htm?highlight=on&terms=particle&terms=size&terms=particle-size%20distribution&terms=particle-size&terms=particle%20size&terms=distribution (accessed on 17 May 2023).
- European Pharmacopoeia 11.2 2.9.16. Flowability. Available online: https://pheur.edqm.eu/app/11-2/content/default/20916E.htm (accessed on 28 October 2023).
- European Pharmacopoeia 11.2 2.9.36. Powder Flow. Available online: https://pheur.edqm.eu/app/11-2/content/default/20936E.htm (accessed on 28 October 2023).
- European Pharmacopoeia 2.9.6. Uniformity of Content of Single-Dose Prepparations—European Pharmacopoeia 11.2. Available online: https://pheur.edqm.eu/app/11-2/content/11-2/20906E.htm (accessed on 7 May 2023).
- USP-NF Omeprazole Delayed-Release Capsules. Available online: https://online.uspnf.com/uspnf/document/1_GUID-71E87DD7-0164-42C0-8027-A9211B069968_1_en-US?source=Search%20Results&highlight=omeprazole%20pellets (accessed on 7 May 2023).
- PubChem Triethyl Citrate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Triethyl-citrate (accessed on 30 October 2023).
- Evonik Industries. EUDRAGIT® L 30 D-55. Available online: https://healthcare.evonik.com/en/drugdelivery/oral-drug-delivery/oral-excipients/eudragit-portfolio/delayed-release?utm_source=website&utm_campaign=2023-7_reach_hc_productsearch_ddp_ods&utm_medium=link&utm_term=corporate (accessed on 30 October 2023).
- Rahman, M.A.; Ali, J. Development and in Vitro Evaluation of Enteric Coated Multiparticulate System for Resistant Tuberculosis. Indian J. Pharm. Sci. 2008, 70, 477. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Díaz, J.E.; Ticó Grau, J.R.; Pérez Lozano, P.; Miñarro Carmona, M.; García Montoya, E.; Suñé Negre, J.M. Contribución a La Aplicación En La Industria Farmacéutica Del Ensayo de Disolución Para Formas Farmacéuticas Sólidas. Cienc. Tecnol. Pharm. Rev. Esp. Medicam. Prod. Sanit. 2006, 16, 3–10. [Google Scholar]







| Statistical Order | Running Order | Block | Factor A | Factor B |
|---|---|---|---|---|
| 4 | 1 | 1 | + | + |
| 5 | 2 | 1 | 0 | 0 |
| 2 | 3 | 1 | + | - |
| 3 | 4 | 1 | - | + |
| 1 | 5 | 1 | - | - |
| Component | Functions | First Coating Layer | Second Coating Layer | Third Coating Layer |
|---|---|---|---|---|
| Omeprazole, micronised | API | 9.50% | --- | --- |
| Hypromellose (Grade 606) | Film-forming agent | 1.64% | 3.40 | --- |
| Hydroxypropyl cellulose | Film-forming agent and binder | 1.87% | --- | --- |
| Disodium phosphate · 12 H2O | Buffering agent | 0.50% | --- | --- |
| Lactose monohydrate | Filler, carrier, and dispersant agent | 2.50% | --- | --- |
| Sodium lauryl sulphate | Witting and dispersing agent | 0.15% | --- | --- |
| Eudragit® L-30 D-55 | Enteric polymer | --- | --- | 74.56% |
| Triethyl citrate | Plasticising and film-forming agent | --- | --- | 2.67% |
| Sodium hydroxide 1 N | pH regulator | --- | --- | 7.23% |
| Titanium dioxide | Adjuvant of the film-forming agent, opacifier, and pigment blocker | --- | --- | 0.77% |
| Talc | Opacifying agent | --- | --- | 5.03% |
| Experiment | Second Coating Layer: Average Pellet Weight Increase | Third Coating Layer: Average Pellet Weight Increase |
|---|---|---|
| 1 | 6% | 100% |
| 2 | 4% | 75% |
| 3 | 6% | 50% |
| 4 | 2% | 100% |
| 5 | 2% | 50% |
| Material | Polystyrene Latex |
|---|---|
| Refractive index | 1.59 |
| Control of particle distribution | |
| 50% |
| 2 Bar |
| Measurement cycles | |
| 6 s |
| 6000 |
| 6 s |
| 1 |
| Sieve Light (mm) | Sieve Tare (g) ± SD | Sieve Weight + Retained Sample (g) ± SD | Retained Fraction (g) ± SD | Retained Fraction (%) ± SD |
|---|---|---|---|---|
| 0.60 | 458.42 ± 0.03 | 458.42 ± 0.03 | 0.00 | 0.00 |
| 0.50 | 433.48 ± 0.05 | 440.66 ± 0.04 | 7.18 ± 0.04 | 70.47 ± 0.68 |
| 0.40 | 381.84 ± 0.05 | 384.73 ± 0.07 | 2.89 ± 0.07 | 28.38 ± 0.59 |
| 0.30 | 407.77 ± 0.11 | 407.85 ± 0.11 | 0.08 ± 0.01 | 0.79 ± 0.09 |
| Base | 378.41 ± 0.01 | 378.41 ± 0.0.1 | 0.00 | 0.00 |
| Angle of Repose (°) ± SD | Bulk Density (g/mL) ± SD | Tapped Density (g/mL) ± SD | Sliding Velocity ± SD | Hausner Ratio |
|---|---|---|---|---|
| 27.39 ± 0.84 | 0.81 ± 0.03 | 0.87 ± 0.01 | 6.05 ± 0.15 | 1.09 |
| Experiment | Theoretical Dose (mg) ± SD | Actual Dose (mg) ± SD | Dose Accuracy (% (w/w)) ± SD |
|---|---|---|---|
| Experiment 1 | 24.67 ± 0.18 | 24.72 ± 5.49 | 100.20 ± 1.13 |
| Experiment 2 | 28.64 ± 3.93 | 28.67 ± 8.23 | 100.10 ± 1.41 |
| Experiment 3 | 27.24 ± 5.32 | 27.26 ± 7.22 | 100.07 ± 1.43 |
| Experiment 4 | 25.19 ± 3.88 | 25.23 ± 15.40 | 100.16 ± 2.36 |
| Experiment 5 | 27.07 ± 10.25 | 27.06 ± 12.28 | 99.96 ± 0.40 |
| Experiment | Dose Accuracy after Gastro-Resistance Test (% (w/w)) ± SD | Amount of API Degraded after Gastro-Resistance Test (%) ± SD |
|---|---|---|
| 1 | 87.06 ± 1.06 | 12.94 ± 1.06 |
| 2 | 78.06 ± 1.88 | 21.93 ± 2.01 |
| 3 | 80.64 ± 1.34 | 19.36 ± 1.34 |
| 4 | 95.13 ± 1.29 | 4.87 ± 1.30 |
| 5 | 79.93 ± 1.59 | 20.07 ± 1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouaz-El-Hajoui, K.; García-Montoya, E.; López-Urbano, A.; Romero-Obon, M.; Chiclana-Rodríguez, B.; Fraschi-Nieto, A.; Nardi-Ricart, A.; Suñé-Pou, M.; Suñé-Negre, J.M.; Pérez-Lozano, P. Optimisation of the Manufacturing Process of Organic-Solvent-Free Omeprazole Enteric Pellets for the Paediatric Population: Full Factorial Design. Pharmaceutics 2023, 15, 2587. https://doi.org/10.3390/pharmaceutics15112587
Rouaz-El-Hajoui K, García-Montoya E, López-Urbano A, Romero-Obon M, Chiclana-Rodríguez B, Fraschi-Nieto A, Nardi-Ricart A, Suñé-Pou M, Suñé-Negre JM, Pérez-Lozano P. Optimisation of the Manufacturing Process of Organic-Solvent-Free Omeprazole Enteric Pellets for the Paediatric Population: Full Factorial Design. Pharmaceutics. 2023; 15(11):2587. https://doi.org/10.3390/pharmaceutics15112587
Chicago/Turabian StyleRouaz-El-Hajoui, Khadija, Encarnación García-Montoya, Andrea López-Urbano, Miquel Romero-Obon, Blanca Chiclana-Rodríguez, Alex Fraschi-Nieto, Anna Nardi-Ricart, Marc Suñé-Pou, Josep María Suñé-Negre, and Pilar Pérez-Lozano. 2023. "Optimisation of the Manufacturing Process of Organic-Solvent-Free Omeprazole Enteric Pellets for the Paediatric Population: Full Factorial Design" Pharmaceutics 15, no. 11: 2587. https://doi.org/10.3390/pharmaceutics15112587