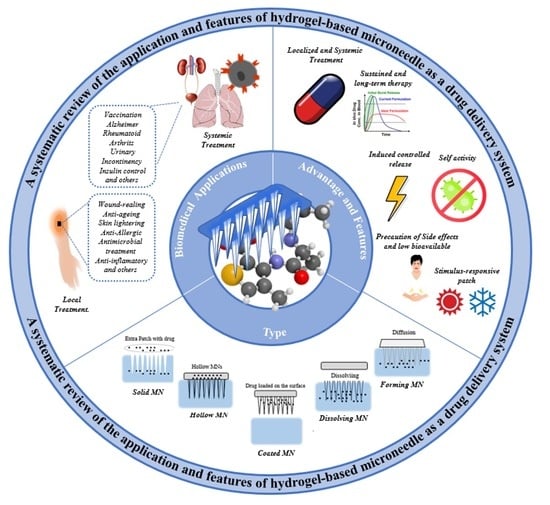
Hydrogel-Based Microneedle as a Drug Delivery System
Abstract
:1. Introduction
2. Hydrogel-Based Microneedle as a Drug Delivery System
3. New Approaches for Hydrogel-Based MN Patch
3.1. Stimulus Response
3.2. Induced Controlled Release
3.3. Sustained and Long-Term Therapy
3.4. Localized and Systemic Treatment
3.5. Self-Activity
3.6. Precaution of Side Effects and Low Bioavailable
4. Biomedical Applications of Hydrogel-Based Microneedles
4.1. General Characteristics for Area in Biomedical Application
4.2. General Characteristics for Hydrogel Type of HG–MNs
5. Drawbacks and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Z.; Xiang, Y.; Monroe, T.; Yu, S.; Dong, P.; Xian, S.; Webber, M.J. Microneedle Arrays with Glucose-Sensing Dynamic-Covalent Bonding for Insulin Delivery. Biomacromolecules 2022, 23, 4401–4411. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Morrow, D.I.; McCrudden, M.T.; Alkilani, A.Z.; Vicente-Pérez, E.M.; O’Mahony, C.; González-Vázquez, P.; McCarron, P.A.; Woolfson, A.D. Hydrogel-forming and dissolving microneedles for enhanced delivery of photosensitizers and precursors. Photochem. Photobiol. 2014, 90, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Tuan-Mahmood, T.M.; McElnay, J.C.; McCarthy, H.O.; Mooney, K.; Woolfson, A.D.; Donnelly, R.F. Potential of hydrogel-forming and dissolving MNs for use in paediatric populations. Int. J. Pharm. 2015, 489, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Malek-Khatabi, A.; Rad, Z.F.; Rad-Malekshahi, M.; Akbarijavar, H. Development of dissolvable microneedle patches by CNC machining and micromolding for drug delivery. Mater. Lett. 2023, 330, 133328. [Google Scholar] [CrossRef]
- Carreño, G.; Pereira, A.; Ávila-Salas, F.; Marican, A.; Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F. Development of “on-demand” thermo-responsive HG for anti-cancer drugs sustained release: Rational design, in silico prediction and in vitro validation in colon cancer models. Mater. Sci. Eng. 2021, 131, 112483. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-responsive HG for cancer treatment: The role of pH, light, ionic strength and magnetic field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Chen, H.; Fan, L.; Peng, N.; Yin, Y.; Mu, D.; Wang, J.; Xie, J. Galunisertib-Loaded Gelatin Methacryloyl Hydrogel Microneedle Patch for Cardiac Repair after Myocardial Infarction. ACS Appl. Mater. Interfaces 2022, 14, 40491–40500. [Google Scholar] [CrossRef]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-forming MNs: Current advancements and future trends. Macromol. Biosci. 2021, 21, 2000307. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, Z.; Baidya, A.; Kim, H.J.; Wang, C.; Jiang, X.; Khademhosseini, A. Biodegradable β-cyclodextrin conjugated gelatin methacryloyl microneedle for delivery of water-insoluble drug. Adv. Healthc. Mater. 2020, 9, 2000527. [Google Scholar] [CrossRef]
- Luo, X.; Sun, W.; Fang, J.; Lee, K.; Li, L.; Gu, Z.; Khademhosseini, A. Biodegradable gelatin methacryloyl MNs for transdermal drug delivery. Adv. Healthc. Mater. 2019, 8, 1801054. [Google Scholar] [CrossRef]
- Rong, X.; Mehwish, N.; Niu, X.; Zhu, N.; Lee, B.H. Human Albumin-Based HG for Their Potential Xeno-Free Microneedle Applications. Macromol. Biosci. 2023, 23, 2200463. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Fan, L.; Chen, H.; Zhang, Z.; Zhao, Y. Multifunctional Inverse Opal Microneedle Arrays for Drug Delivery and Monitoring. Small 2022, 18, 2201889. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lu, Z.; Shi, Y.; Du, Y.; Chen, X.; Kong, M. Systematic comparisons of dissolving and swelling hyaluronic acid MNs in transdermal drug delivery. Int. J. Biol. Macromol. 2021, 191, 783–791. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Huang, S.; Fu, T.; Xue, W.; Guo, R. Dextran methacrylate hydrogel MNs loaded with doxorubicin and trametinib for continuous transdermal administration of melanoma. Carbohydr. Polym. 2020, 246, 116650. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Poly (vinyl alcohol)/chitosan layer-by-layer MNs for cancer chemo-photothermal therapy. Int. J. Pharm. 2020, 576, 118907. [Google Scholar] [CrossRef]
- Sabri, A.H.; Cater, Z.; Gurnani, P.; Ogilvie, J.; Segal, J.; Scurr, D.J.; Marlow, M. Intradermal delivery of imiquimod using polymeric MNs for basal cell carcinoma. Int. J. Pharm. 2020, 589, 119808. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) MNs: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McCrudden, M.T.; McAvoy, K.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-mediated transdermal delivery of bevacizumab. Mol. Pharm. 2018, 15, 3545–3556. [Google Scholar] [CrossRef]
- Puri, A.; Nguyen, H.X.; Banga, A.K. Microneedle-mediated intradermal delivery of epigallocatechin-3-gallate. Int. J. Cosmet. Sci. 2016, 38, 512–523. [Google Scholar] [CrossRef]
- Darge, H.F.; Lee, C.Y.; La, L.Y.; Lin, S.Z.; Harn, H.J.; Chen, Y.S.; Tsai, H.C. Separable double-layered microneedle-based transdermal codelivery of DOX and LPS for synergistic immunochemotherapy of a subcutaneous glioma tumor. Chem. Eng. J. 2022, 433, 134062. [Google Scholar] [CrossRef]
- Huang, T.; Lai, H.; Jiang, J.; Xu, X.; Zeng, Z.; Ren, L.; Cui, S. pH-activatable oxidative stress amplifying dissolving MNs for combined chemo-photodynamic therapy of melanoma. Asian J. Pharm. Sci. 2022, 17, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Ge, Q.; Natabou, M.A.; Xu, W.; Liu, X.; Xu, B.; Chen, Y. Bolus delivery of palonosetron through skin by tip-loaded dissolving MNs with short-duration iontophoresis: A potential strategy to rapidly relieve emesis associated with chemotherapy. Int. J. Pharm. 2022, 628, 122294. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, X.; Huang, D.; Mao, L.; Qiu, Y.; Zhao, Y. Bioinspired adhesive microneedle patch with gemcitabine encapsulation for pancreatic cancer treatment. Chem. Eng. J. 2022, 431, 133362. [Google Scholar] [CrossRef]
- Hamdan, I.M.; Tekko, I.A.; Bell, S.E. Gold nanorods-loaded hydrogel-forming needles for local hyperthermia applications: Proof of concept. Eur. J. Pharm. Biopharm. 2022, 179, 105–117. [Google Scholar] [CrossRef]
- Alafnan, A.; Seetharam, A.A.; Hussain, T.; Gupta, M.S.; Rizvi, S.M.D.; Moin, A.; Balashanmugam, N. Development and Characterization of PEGDA Microneedles for Localized Drug Delivery of Gemcitabine to Treat Inflammatory Breast Cancer. Materials 2022, 15, 7693. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, J.; Zhao, Y.; Yan, X.; Zhang, L.; Choy, K.; Tang, T. Transdermal delivery of siRNA through microneedle array. Sci. Rep. 2016, 6, 21422. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, S.; Cheng, K. Microneedle Patch Delivery of PROTACs for Anti-Cancer Therapy. ACS Nano 2023, 17, 11855–11868. [Google Scholar] [CrossRef]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Gambotto, A. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. eBioMedicine 2020, 55, 102743. [Google Scholar] [CrossRef]
- Courtenay, A.J.; Rodgers, A.M.; McCrudden, M.T.; McCarthy, H.O.; Donnelly, R.F. Novel hydrogel-forming microneedle array for intradermal vaccination in mice using ovalbumin as a model protein antigen. Mol. Pharm. 2018, 16, 118–127. [Google Scholar] [CrossRef]
- Tekko, I.A.; Permana, A.D.; Vora, L.; Hatahet, T.; McCarthy, H.O.; Donnelly, R.F. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: Potential for enhanced treatment of psoriasis. Eur. J. Pharm. Sci. 2020, 152, 105469. [Google Scholar] [CrossRef]
- Liang, J.; Yu, Y.; Li, C.; Li, Q.; Chen, P.; Li, W.; Zhang, Z. Tofacitinib combined with melanocyte protector α-MSH to treat vitiligo through dextran-based hydrogel MNs. Carbohydr. Polym. 2023, 305, 120549. [Google Scholar] [CrossRef] [PubMed]
- Carcamo-Martinez, A.; Mallon, B.; Anjani, Q.B.; Dominguez-Robles, J.; Utomo, E.; Vora, L.K.; Donnelly, R.F. Enhancing intradermal delivery of tofacitinib citrate: Comparison between powder-loaded hollow microneedle arrays and dissolving microneedle arrays. Int. J. Pharm. 2021, 593, 120152. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Su, J.; An, M.; Yang, Y.; Zhang, Y.; Zuo, J.; Zhao, Y. Novel DEK-targeting aptamer delivered by a hydrogel microneedle attenuates collagen-induced arthritis. Mol. Pharm. 2020, 18, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Tekko, I.A.; Chen, G.; Domínguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.; Vora, L.L.; Donnelly, R.F. Development and characterization of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int. J. Pharm. 2020, 586, 119580. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hao, B.; Ju, D.; Liu, M.; Zhao, H.; Du, Z.; Xia, J. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under MNs on rats with collagen-induced arthritis. Acta Pharm. Sin. B 2015, 5, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yeo, D.C.; Wiraja, C.; Tey, H.L.; Mrksich, M.; Xu, C. Peptide delivery with poly (ethylene glycol) diacrylate MNs through swelling effect. Bioeng. Transl. Med. 2017, 2, 258–267. [Google Scholar] [CrossRef]
- Rojekar, S.; Vora, L.K.; Tekko, I.A.; Volpe-Zanutto, F.; McCarthy, H.O.; Vavia, P.R.; Donnelly, R.F. Etravirine-loaded dissolving microneedle arrays for long-acting delivery. Eur. J. Pharm. Biopharm. 2021, 165, 41–51. [Google Scholar] [CrossRef]
- Hu, W.; Peng, T.; Huang, Y.; Ren, T.; Chen, H.; Chen, Y.; Pan, X. Hyaluronidase-powered MNs for significantly enhanced transdermal delivery efficiency. J. Control. Release 2023, 353, 380–390. [Google Scholar] [CrossRef]
- Ryall, C.; Chen, S.; Duarah, S.; Wen, J. Chitosan-based microneedle arrays for dermal delivery of Centella asiatica. Int. J. Pharm. 2022, 627, 122221. [Google Scholar] [CrossRef]
- Younas, A.; Dong, Z.; Hou, Z.; Asad, M.; Li, M.; Zhang, N. A chitosan/fucoidan nanoparticle-loaded pullulan microneedle patch for differential drug release to promote wound healing. Carbohydr. Polym. 2023, 306, 120593. [Google Scholar] [CrossRef]
- Yao, Z.; Xue, T.; Xiong, H.; Cai, C.; Liu, X.; Wu, F.; Fan, C. Promotion of collagen deposition during skin healing through Smad3/mTOR pathway by parathyroid hormone-loaded microneedle. Mater. Sci. Eng. C 2021, 119, 111446. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Fan, L.; Zhao, Y. Multifunctional microneedle patches with aligned carbon nanotube sheet basement for promoting wound healing. Chem. Eng. J. 2021, 457, 141206. [Google Scholar] [CrossRef]
- Barnum, L.; Quint, J.; Derakhshandeh, H.; Samandari, M.; Aghabaglou, F.; Farzin, A.; Tamayol, A. 3D-printed hydrogel-filled microneedle arrays. Adv. Healthc. Mater. 2021, 10, 2001922. [Google Scholar] [CrossRef]
- Liu, Y.; Long, L.; Zhang, F.; Hu, X.; Zhang, J.; Hu, C.; Xu, J. Microneedle-mediated vascular endothelial growth factor delivery promotes angiogenesis and functional recovery after stroke. J. Control. Release 2021, 338, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.Y.; Lee, J.; Kim, B.J.; Joo, K.I.; Kim, K.H.; Lim, G.; Cha, H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 2019, 222, 119439. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, B.; Jiang, G.; Yu, W.; Zhang, Y.; Xu, B. Fabrication of composite MNs integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater. Sci. Eng. C 2018, 90, 180–188. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Huang, H.; Li, J.; Liu, H.; Guo, Z.; Lei, Y. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater. Sci. Eng. C 2020, 117, 111299. [Google Scholar] [CrossRef]
- Gholami, S.; Zarkesh, I.; Ghanian, M.H.; Hajizadeh-Saffar, E.; Hassan-Aghaei, F.; Mohebi, M.M.; Baharvand, H. Dynamically capped hierarchically porous MNs enable post-fabrication loading and self-regulated transdermal delivery of insulin. Chem. Eng. J. 2021, 421, 127823. [Google Scholar] [CrossRef]
- Chen, M.C.; Ling, M.H.; Kusuma, S.J. Poly-γ-glutamic acid MNs with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015, 24, 106–116. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Zheng, Y.; Ye, R.; Liu, B.; Huang, Y.; Jiang, L. Iontophoresis-driven porous microneedle array patch for active transdermal drug delivery. Acta Biomater. 2021, 121, 349–358. [Google Scholar] [CrossRef]
- Li, J.Y.; Feng, Y.H.; He, Y.T.; Hu, L.F.; Liang, L.; Zhao, Z.Q.; Guo, X.D. Thermosensitive hydrogel MNs for controlled transdermal drug delivery. Acta Biomater. 2022, 153, 308–319. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Li, J.; Lu, B.; Zhou, L.; Lam, L.; Giacca, A.; Wu, X.Y. Glucose-responsive composite microneedle patch for hypoglycemia-triggered delivery of native glucagon. Adv. Mater. 2019, 31, 1901051. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, H.; Wang, L.; Shen, D.; Ul Amin, B.; Feng, J.; Xiong, W. Microneedle Patch Prepared from a Hydrogel by a Mild Method for Insulin Delivery. ChemNanoMat 2021, 7, 1230–1240. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, Q.; Jiang, Z.; Liu, J.; Wan, J.; Jin, P.; Lv, Q. Multifunctional silk fibroin methacryloyl microneedle for diabetic wound healing. Small 2022, 18, 2203064. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Yi, Y.; Gong, Y.; Ji, H.; Gan, Y.; Wang, X. MXenes-integrated microneedle combined with asiaticoside to penetrate the cuticle for treatment of diabetic foot ulcer. J. Nanobiotechnol. 2022, 20, 259. [Google Scholar] [CrossRef]
- Chen, S.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Microneedle-array patch fabricated with enzyme-free polymeric components capable of on-demand insulin delivery. Adv. Funct. Mater. 2019, 29, 1807369. [Google Scholar] [CrossRef]
- Chen, X.; Yu, H.; Wang, L.; Wang, N.; Zhang, Q.; Zhou, W.; Uddin, M.A. Preparation of phenylboronic acid-based hydrogel microneedle patches for glucose-dependent insulin delivery. J. Appl. Polym. Sci. 2021, 138, 49772. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McAlister, E.; McCrudden, M.T.; Vora, L.; Steiner, L.; Levin, G.; Donnelly, R.F. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery. J. Control. Release 2020, 322, 177–186. [Google Scholar] [CrossRef]
- Kearney, M.C.; Caffarel-Salvador, E.; Fallows, S.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-mediated delivery of donepezil: Potential for improved treatment options in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2016, 103, 43–50. [Google Scholar] [CrossRef]
- Zhou, X.; Li, B.; Guo, M.; Peng, W.; Wang, D.; Guo, Q.; Zheng, B. Microneedle patch based on molecular motor as a spatio-temporal controllable dosing strategy of L-DOPA for Parkinson’s disease. Chem. Eng. J. 2022, 427, 131555. [Google Scholar] [CrossRef]
- Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Nowroozzadeh, M.H.; MTamaddon, A. In-situ nanomicelle forming MNs of poly NIPAAm-b-poly glutamic acid for trans-scleral delivery of dexamethasone. J. Ind. Eng. Chem. 2023, 119, 485–498. [Google Scholar] [CrossRef]
- Mahfufah, U.; Sultan, N.A.F.; Fitri, A.M.N.; Elim, D.; Mahfud, M.A.S.B.; Wafiah Permana, N.A.D. Application of multipolymers system in the development of hydrogel-forming microneedle integrated with polyethylene glycol reservoir for transdermal delivery of albendazole. Eur. Polym. J. 2023, 183, 111762. [Google Scholar] [CrossRef]
- Mir, M.; Permana, A.D.; Tekko, I.A.; McCarthy, H.O.; Ahmed, N.; Donnelly, R.F. Microneedle liquid injection system assisted delivery of infection responsive nanoparticles: A promising approach for enhanced site-specific delivery of carvacrol against polymicrobial biofilms-infected wounds. Int. J. Pharm. 2020, 587, 119643. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.R.; Khorram, M.; Barzegar, S.; Sarkari, B.; Asgari, Q.; Ahadian, S.; Zomorodian, K. Dissolvable carboxymethyl cellulose/polyvinylpyrrolidone microneedle arrays for transdermal delivery of Amphotericin B to treat cutaneous leishmaniasis. Int. J. Biol. Macromol. 2021, 182, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.H.B.; Anjani, Q.K.; Utomo, E.; Ripolin, A.; Donnelly, R.F. Development and characterization of a dry reservoir-hydrogel-forming MNs composite for minimally invasive delivery of cefazolin. Int. J. Pharm. 2022, 617, 121593. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Vora, L.K.; Domínguez-Robles, J.; Naser, Y.A.; Li, M.; Larrañeta, E.; Donnelly, R.F. Hydrogel-forming MNs for rapid and efficient skin deposition of controlled release tip-implants. Mater. Sci. Eng. C 2021, 127, 112226. [Google Scholar] [CrossRef]
- Ma, C.J.; He, Y.; Jin, X.; Zhang, Y.; Zhang, X.; Li, Y.; Lu, F. Light-regulated nitric oxide release from hydrogel-forming MNs integrated with graphene oxide for biofilm-infected-wound healing. Biomater. Adv. 2022, 134, 112555. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Permana, A.D.; Cárcamo-Martínez, Á.; Domínguez-Robles, J.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Versatility of hydrogel-forming microneedles in in vitro transdermal delivery of tuberculosis drugs. Eur. J. Pharm. Biopharm. 2021, 158, 294–312. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.; Fan, D. A dissolving microneedle patch for antibiotic/enzymolysis/photothermal triple therapy against bacteria and their biofilms. Chem. Eng. J. 2022, 437, 135475. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Yi, X.; Wang, C.; Yu, X.; Su, W.; Yuan, Z. Chitosan/zinc nitrate MNs for bacterial biofilm eradication. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 911–920. [Google Scholar] [CrossRef]
- Zan, P.; Than, A.; Duong, P.K.; Song, J.; Xu, C.; Chen, P. Antimicrobial microneedle patch for treating deep cutaneous fungal infection. Adv. Ther. 2019, 2, 1900064. [Google Scholar] [CrossRef]
- Yao, S.; Chi, J.; Wang, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Zn-MOF encapsulated antibacterial and degradable MNs array for promoting wound healing. Adv. Healthc. Mater. 2021, 10, 2100056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, P.; Yu, J.; Yang, J.; Zhao, J.; Wang, J.; Gu, Z. ROS-responsive microneedle patch for acne vulgaris treatment. Adv. Ther. 2018, 1, 1800035. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Hong, S.; Liu, J.; Cheng, J.; He, Y.; Hong, L. Collagen type I loaded methacrylamide hyaluronic acid hydrogel MNs alleviate stress urinary incontinence in mice: A novel treatment and prevention strategy. Colloids Surf. B Biointerfaces 2023, 222, 113085. [Google Scholar] [CrossRef] [PubMed]
- Elim, D.; Fitri, A.M.N.; Mahfud, M.A.S.B.; Afika, N.; Sultan, N.A.F.; Asri, R.M.; Permana, A.D. Hydrogel forming microneedle-mediated transdermal delivery of sildenafil citrate from polyethylene glycol reservoir: An ex vivo proof of concept study. Colloids Surf. B Biointerfaces 2023, 222, 113018. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, S.; Li, Y.; Tan, H.; Zhao, Y.; Sun, L. Antioxidant nanozyme MNs with stem cell loading for in situ endometrial repair. Chem. Eng. J. 2022, 449, 137786. [Google Scholar] [CrossRef]
- Indermun, S.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Van Vuuren, S.; Pillay, V. Ex vivo evaluation of a microneedle array device for transdermal application. Int. J. Pharm. 2015, 496, 351–359. [Google Scholar] [CrossRef]
- Castilla-Casadiego, D.A.; Carlton, H.; Gonzalez-Nino, D.; Miranda-Muñoz, K.A.; Daneshpour, R.; Huitink, D.; Almodovar, J. Design, characterization, and modeling of a chitosan microneedle patch for transdermal delivery of meloxicam as a pain management strategy for use in cattle. Mater. Sci. Eng. C 2021, 118, 111544. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhang, B.L.; Chu, H.Q.; Liang, L.; Chen, B.Z.; Zheng, H.; Guo, X.D. A high-dosage microneedle for programmable lidocaine delivery and enhanced local long-lasting analgesia. Biomater. Adv. 2022, 133, 112620. [Google Scholar] [CrossRef]
- Ronnander, P.; Simon, L.; Koch, A. Experimental and mathematical study of the transdermal delivery of sumatriptan succinate from polyvinylpyrrolidone-based MNs. Eur. J. Pharm. Biopharm. 2020, 146, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tao, Y.; Zhou, Y.; Gui, S. Development of sinomenine hydrochloride-loaded polyvinylalcohol/maltose microneedle for transdermal delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 1–7. [Google Scholar] [CrossRef]
- Yu, J.; Xia, Y.; Zhang, H.; Pu, X.; Gong, T.; Zhang, Z.; Deng, L. A semi-interpenetrating network-based microneedle for rapid local anesthesia. J. Drug Deliv. Sci. Technol. 2022, 78, 103984. [Google Scholar] [CrossRef]
- Indermun, S.; Choonara, Y.E.; Kumar, P.; du Toi, L.C.; Modi, G.; Luttge, R.; Pillay, V. In vitro and in vivo evaluation of a hydrogel-based microneedle device for transdermal electro-modulated analgesia. J. Pharm. Sci. 2017, 106, 1111–1116. [Google Scholar] [CrossRef]
- Nayak, A.; Das, D.B.; Vladisavljević, G.T. Microneedle-assisted permeation of lidocaine carboxymethylcellulose with gelatine co-polymer hydrogel. Pharm. Res. 2014, 31, 1170–1184. [Google Scholar] [CrossRef]
- Nayak, A.; Babla, H.; Han, M.T.; Das, D.B. Lidocaine carboxymethylcellulose with gelatine co-polymer hydrogel delivery by combined microneedle and ultrasound. Drug Deliv. 2016, 23, 658–669. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Pamornpathomkul, B.; Opanasopit, P. Fabrication, characterization and comparison of α-arbutin loaded dissolving and hydrogel forming MNs. Int. J. Pharm. 2020, 586, 119508. [Google Scholar] [CrossRef]
- Zhang, J.N.; Chen, B.Z.; Ashfaq, M.; Zhang, X.P.; Guo, X.D. Development of a BDDE-crosslinked hyaluronic acid based MNs patch as a dermal filler for anti-ageing treatment. J. Ind. Eng. Chem. 2018, 65, 363–369. [Google Scholar] [CrossRef]
- Pan, X.; Li, Y.; Pang, W.; Xue, Y.; Wang, Z.; Jiang, C.; Liu, L. Preparation, characterisation and comparison of glabridin-loaded hydrogel-forming MNs by chemical and physical cross-linking. Int. J. Pharm. 2022, 617, 121612. [Google Scholar] [CrossRef]
- Zhang, X.; Hasani-Sadrabadi, M.M.; Zarubova, J.; Dashtimighadam, E.; Haghniaz, R.; Khademhosseini, A.; Li, S. Immunomodulatory microneedle patch for periodontal tissue regeneration. Matter 2022, 5, 666–682. [Google Scholar] [CrossRef]
- Migdadi, E.M.; Courtenay, A.J.; Tekko, I.A.; McCrudden, M.T.; Kearney, M.C.; McAlister, E.; Donnelly, R.F. Hydrogel-forming MNs enhance transdermal delivery of metformin hydrochloride. J. Control. Release 2018, 285, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Brogden, N.K. Development of HG for microneedle-assisted transdermal delivery of naloxone for opioid-induced pruritus. J. Pharm. Sci. 2019, 108, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Fitri, A.M.N.; Elim, D.; Mahfud, M.A.S.B.; Sultan, N.A.F.; Saputra, M.D.; Afika, N.; Permana, A.D. Polymeric hydrogel forming microneedle-mediated transdermal delivery of sildenafil citrate from direct-compressed tablet reservoir for potential improvement of pulmonary hypertension therapy. Int. J. Pharm. 2023, 631, 122549. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Yu, H.; Wang, L.; Chen, X.; Feng, J.; Li, C.; Zhang, Q. Glucose-responsive hydrogel-based MNs containing phenylborate ester bonds and N-isopropylacrylamide moieties and their transdermal drug delivery properties. Eur. Polym. J. 2021, 148, 110348. [Google Scholar] [CrossRef]
- Jeong, J.O.; Lim, Y.M.; Lee, J.Y.; Park, J.S. Polyvinylpyrrolidone based graphene oxide HG by radiation crosslinking for conductive microneedle patches. Eur. Polym. J. 2023, 184, 111726. [Google Scholar] [CrossRef]
- Himawan, A.; Anjani, Q.K.; Detamornrat, U.; Vora, L.K.; Permana, A.D.; Ghanma, R.; Donnelly, R.F. Multifunctional Low Temperature-Cured PVA/PVP/Citric Acid-Based Hydrogel Forming Microarray Patches: Physicochemical Characteristics and Hydrophilic Drug Interaction. Eur. Polym. J. 2023, 186, 111836. [Google Scholar] [CrossRef]
- Zhou, Z.; Xing, M.; Zhang, S.; Yang, G.; Gao, Y. Process optimization of Ca2+ cross-linked alginate-based swellable MNs for enhanced transdermal permeability: More applicable to acidic drugs. Int. J. Pharm. 2022, 618, 121669. [Google Scholar] [CrossRef]
- Yang, S.J.; Jeong, J.O.; Lim, Y.M.; Park, J.S. Synthesis and characterization of PVP microneedle patch using metal bioelectrodes for novel drug delivery system. Mater. Des. 2021, 201, 109485. [Google Scholar] [CrossRef]
- Panda, A.; Sharma, P.K.; McCann, T.; Bloomekatz, J.; Repka, M.A.; Murthy, S.N. Fabrication and development of controlled release PLGA MNs for macromolecular delivery using FITC-Dextran as model molecule. J. Drug Deliv. Sci. Technol. 2022, 68, 102712. [Google Scholar] [CrossRef]
- Pillai, M.M.; Ajesh, S.; Tayalia, P. Two-photon polymerization based reusable master template to fabricate polymer MNs for drug delivery. MethodsX 2023, 10, 102025. [Google Scholar] [CrossRef]
- McAlister, E.; Dutton, B.; Vora, L.K.; Zhao, L.; Ripolin Zahari, A.D.S.Z.B.P.H.; Donnelly, R.F. Directly compressed tablets: A novel drug-containing reservoir combined with hydrogel-forming microneedle arrays for transdermal drug delivery. Adv. Healthc. Mater. 2021, 10, 2001256. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Lin, S.; Zhou, X.; Yang, F.; Lin, Z.; Li, S.; Zhu, J. Biodegradable double-network Gelatin methacryloyl (GelMA) -ACNM hydrogel MNs for transdermal drug delivery. Front. Bioeng. Biotechnol. 2023, 11, 1110604. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Larrañeta, E.; Donnelly, R.F.; McGoldrick, N.; Migalska, K.; McCrudden, M.T.; McCoy, C.P. Hydrogel-forming microneedle arrays made from light-responsive materials for on-demand transdermal drug delivery. Mol. Pharm. 2016, 13, 907–914. [Google Scholar] [CrossRef]
- Larraneta, E.; Lutton, R.E.; Brady, A.J.; Vicente-Pérez, E.M.; Woolfson, A.D.; Thakur, R.R.S.; Donnelly, R.F. Microwave-assisted preparation of hydrogel-forming microneedle arrays for transdermal drug delivery applications. Macromol. Mater. Eng. 2015, 300, 586–595. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCrudden, M.T.; Zaid Alkilani, A.; Larrañeta, E.; McAlister, E.; Courtenay, A.J.; Woolfson, A.D. Hydrogel-forming MNs prepared from “super swelling” polymers combined with lyophilised wafers for transdermal drug delivery. PLoS ONE 2014, 9, e111547. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, A.; Banga, A.K. Novel in situ forming hydrogel MNs for transdermal drug delivery. Drug Deliv. Transl. Res. 2017, 7, 16–26. [Google Scholar] [CrossRef]
- Pitakjakpipop, H.; Rajan, R.; Tantisantisom, K.; Opaprakasit, P.; Nguyen, D.D.; Ho, V.A.; Khanchaitit, P. Facile photolithographic fabrication of zwitterionic polymer microneedles with protein aggregation inhibition for transdermal drug delivery. Biomacromolecules 2021, 23, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, Y.; Mao, J.; Jiang, H.; Du, S.; Liu, P.; Zhu, J. Strong and Tough Supramolecular Microneedle Patches with Ultrafast Dissolution and Rapid-Onset Capabilities. Adv. Mater. 2022, 34, 2207832. [Google Scholar] [CrossRef]
- Chew, S.W.; Shah, A.H.; Zheng, M.; Chang, H.; Wiraja, C.; Steele, T.W.; Xu, C. A self-adhesive microneedle patch with drug loading capability through swelling effect. Bioeng. Transl. Med. 2020, 5, e10157. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, X.; Liu, X.; Sun, B.; Li, L.; Zhao, Y. Responsive Hydrogel Microcarrier-Integrated MNs for Versatile and Controllable Drug Delivery. Adv. Healthc. Mater. 2021, 10, 2002249. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, M.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Polyethylene glycol diacrylate (PEGDA)/PVP MNs with tailorable matrix constitutions for controllable transdermal drug delivery. Macromol. Mater. Eng. 2018, 303, 1800233. [Google Scholar] [CrossRef]
- Gaware, S.A.; Rokade, K.A.; Bala, P.; Kale, S.N. MNs of chitosan-porous carbon nanocomposites: Stimuli (pH and electric field)-initiated drug delivery and toxicological studies. J. Biomed. Mater. Res. Part A 2019, 107, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Pereira, B.; Lameirinhas, N.S.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Freire, C.S. Dissolvable Carboxymethylcellulose MNs for Noninvasive and Rapid Administration of Diclofenac Sodium. Macromol. Biosci. 2023, 23, 2200323. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Li, J.; Hong, Y.; Ruan, H.; Long, M.; Feng, N.; Zhang, Y. Advances and Prospects for Hydrogel-Forming Microneedles in Transdermal Drug Delivery. Biomedicines 2023, 11, 2119. [Google Scholar] [CrossRef]











| Hydrogel System | Bioactive Compound and Defined Specific Proposal (DSP) | Type | Response and Release Characteristics | Ref. |
|---|---|---|---|---|
| CANCER TREATMENT | ||||
| β-Cyclodextrin conjugated gelatin methacryloyl | Curcumin DSP: Melanoma | Dissolving MNs | The gelatin methacryloyl (GelMA)-β-CD/CUR MN exhibits relatively higher therapeutic efficacy through a more localized and deeper penetrated manner compared with a control nontransdermal patch for anticancer activities. In vivo, studies also verify the biocompatibility and degradability of the gelatin methacryloyl (GelMA)-β-CD MN arrays patch. | [9] |
| Gelatin methacryloyl (GelMA) | Doxorrubicin DSP: Melanoma | Dissolving MNs | The drug release rate can be adjusted by controlling the degree of polymer crosslinking. | [10] |
| Human serum albumin methacryloyl hydrogel | Doxorubicin DSP: Melanoma | Dissolving MNs | DOX-embedded human serum albumin methacryloyl displayed good anticancer efficacy with sustained activity up to 24 h. | [11] |
| Poly (ethylene glycol) diacrylate (PEGDA) hydrogel | Doxorubicin and capecitabine DSP: Mammary carcinome | Coated MNs | They used a new system to monitor the amounts of drugs release. | [12] |
| Hyaluronic acid dissolving MNs (HA DMNs) | Doxorubicin DSP: Skin cancer | Dissolving MNs | Two systems were used: swelling MNs and dissolving MNs. The relative bioavailability of Doxorubicin in swelling MNs towards Doxorubicin in dissolving MNs was 200% within 12 h. | [13] |
| Dextran methacrylate hydrogel | Doxorubicin and trametinib DSP: Melanoma | Dissolving MNs | The prepared hydrogel MNs can successfully penetrate the epidermal layer and achieve sustained drug release. Tested for 12 days. | [14] |
| Polyvinylpyrrolidone MNs coated with chitosan and poly (vinyl alcohol) hydrogel | Doxorubicin and AuMSS nanorods (Dox@MicroN) DSP: Cervical cancer | Coated MN | MNs structures can efficiently penetrate the tumor-mimicking agarose gel and release the Dox with a pH- and thermo-responsive profile. Furthermore, the system composed of Dox and AuMSS nanorods was able to simultaneously mediate the chemo- and photothermal therapies rendered a superior cytotoxic effect against the cervical cancer cells. | [15] |
| Polyvinylpyrollidone-co-vinyl acetate | Imiquimod DSP: Basal cell carcinoma (BBC) | Dissolving MNs | Permeation studies utilizing Franz diffusion cells demonstrated that the imiquimod-loaded polymeric MNs were capable of delivering similar quantities of imiquimod to the region of tumors, despite a six fold lower drug loading, relative to the current clinical dose of AldaraTM cream used in BCC treatment. | [16] |
| Poly (vinyl alcohol) | Doxorubicin DSP: Skin cancer | Forming MNs | The insertion of MNs resulted in significantly greater delivery of doxorubicin into and across human skin, as compared to passive diffusion. | [17] |
| Poly (vinyl alcohol) and poly (methyl vinyl ether co-maleic anhydride/acid (Gantrez® S-97), polyethylene glycol (PEG 10,000), and Na2CO3 | Bevacizumab DSP: Immunotherapy for cancer | Dissolving and forming MN | BEV was detected and measured in plasma across 7 days following one single application of MN arrays. BEV had lymphatic accumulation in vivo. This could prove to be a viable option for the treatment of lymphomas and secondary metastatic tumors. | [18] |
| Carbopol hydrogel | Epigallocatechin-3-gallate DSP: Skin cancer | Dissolving MNs | Microneedle-treated skin showed significant enhancement in the delivery of EGCG to viable epidermis and dermis from the hydrogel in comparison with the untreated skin. | [19] |
| Sodium alginate and sulfobetaine methacrylate using N, N′-methylenebisacrylamide and Ca2+ | Doxorrubicin and lipopolysaccharides DSP: Glioma tumor | Dissolving MNs | Adequate amounts of drugs were released from the MNs in the first 24 h, followed by a very gradual and sustained release for the next 7 d. | [20] |
| Protoporphyrin (PpIX) and dihydroartemisinin (DHA) with hyaluronic acid | Dihydroartemisinin DSP: Melanoma | Dissolving MN | The system was pH-sensitive and efficient to promote photodynamic therapy under light irradiation and amplified generation of reactive oxygen species. | [21] |
| Sodium hyaluronate (HA) | Palonosetron hydrochloride DSP: Skin cancer | Dissolving MN | The system showed rapid dissolution in the skin and there were no statistically significant differences in Tmax and AUC when compared to subcutaneous administration. | [22] |
| Gelatin methacryloyl (GelMA) | Gemcitabine DSP: Pancreatic cancer | Dissolving MNs | In vitro experiments demonstrated the outstanding adhesive ability on irregular surfaces in moist environments, and the drug-releasing kinetics were elucidated to be well controlled by adjusting the concentration of gelatin methacryloyl (GelMA). | [23] |
| Poly (methylvinylether/maelic acid) and crosslinked with glycerol hydrogel | 5-aminolevulinic acid (ALA) or meso-tetra (N-methyl-4-pyridyl) morphine tetra tosylate (TMP) DSP: Photodynamic therapy for cancer | Dissolving and forming MNs | TMP delivery was much more marked from the hydrogel-forming system, which offers another advantage over its dissolving counterpart in that the MN is removed intact, thus leaving no polymer behind in the skin. | [2] |
| Gantrez® S-97 hydrogel and polyethylene glycol (PEG) | Gold nanorods DSP: Basal cell carcinoma | Forming MNs | In the formulation, the heat generated rapidly by the combination allowed the temperature to reach 50 °C in a few seconds. Compared to conventional methods, delivering the component in a minimally invasive way and without leaving polymeric and particulate material on the skin are the main advantages. | [24] |
| Polyethylene glycol diacrylate (PEGDA) | Gencitabine DSP: Inflammatory breast cancer | Coated MNs | Thinking about a rapid release system, 100% of the drug was released in the first hour of administration with a system manufactured with high retention. | [25] |
| Silicon | Cholesterol-modified housekeeping gene (Gapdh) siRNA DSP: Skin cancer and other skin disorders | Solid MNs | The strategy reduced the expression of the gene of interest by up to 66% specifically in the skin without affecting other organs. | [26] |
| Methacrylated hyaluronic acid | Estrogen receptor alpha (ERα)-degrading PROTAC—ERD308 and Palbociclib DSP: ER-positive breast cancer | Dissolving MNs | The patch was effective in overcoming the problems involved with PROTAC systems, which had high rates of local drug retention (87%). Furthermore, the combination with pH-responsive micelles allowed the co-release of Palbociclib specific to the tumor acid environment for effective therapy against breast cancer. | [27] |
| VACCINATION | ||||
| Carboxymethyl cellulose | Protein MERS-S1f, MERS-S1fRS09, MERS-S1ffliC, SARS-CoV-2-S1, or SARS-CoV-2- S1fRS09 for antigen stimulator DSP: Coronavirus (SARS-CoV-2) | Dissolving MNs | The hydrogel-based vaccine MNs elicited strong and long-lasting antigen-specific antibody responses. | [28] |
| Gantrez® S-97 and poly (ethylene glycol) (PEG) | Protein antigen ovalbumin (OVA) DSP: Adjuvant for vaccination | Forming and dissolving MN | The system yielded enhanced immune responses, suggesting the possibility of lower dosing and equivalent outcomes as traditional needle and syringe methods. | [29] |
| AUTOIMMUNE DISORDERS | ||||
| Poly (vinyl alcohol)/poly vinyl pyrrolidone | Methotrexate DSP: Psoriasis | Dissolving MNs | The system acted as a drug depot and released the MTX in a sustained manner over 72 h, while minimizing MTX systemic exposure. Indeed, 24 h after application, a concentration of 322-fold higher than the amount of MTX retained in the skin after oral administration of MTX Na was reached. | [30] |
| Dextran methacrylate and cyclodextrin-adamantane | α-MSH and tofacitinib DSP: Vitiligo | Dissolving MNs | Under the treatment of α-MSH/tofacitinib MNs, massive deposition of melanin in epidermis and hair follicles significantly accelerated skin and hair pigmentation. | [31] |
| Gantrez® S-97, polyethylene glycol and poly (lactic acid) | Tofacitinib citrate DSP: Vitiligo, psoriasis, etc. | Hollow and dissolving MN | The dissolving MN arrays showed superiority in this regard, as around 835 μg/cm2 were found on the dermis after 24 h of study, providing proof of principle for intradermal delivery of tofacitinib citrate using MN arrays. | [32] |
| Hyaluronic acid | Aptamer DTA (DEK protein blocker) DSP: Rheumatoid arthritis | Dissolving MNs | The modification of the methoxy group, idT, and cholesterol enhanced the stability and anti-inflammatory efficacy of DTA. The system overcame the skin barrier without pain and quickly released aptamers into the skin. | [33] |
| Poly (vinyl alcohol)/poly (vinyl pyrrolidone) | Methotrexate DSP: Rheumatoid arthritis | Forming MNs | The integrated patch was able to bypass the skin barrier and deliver MTX in a sustained manner over 24 h. Importantly, the HFMNs were removed intact from the skin with only mild erythema, despite the cytotoxic nature of MTX. | [34] |
| Liposome hydrogel (LHP) | Triptolide (TP) DSP: Rheumatoid arthritis | Dissolving MNs | When the system was prepared, the hepatic first-pass metabolism and digestive toxicity were eliminated.TP-LHP provided stable, long-term release of triptolide, and had significant efficacy in the CIA model (long-term release 24 h). | [35] |
| Polyethylene glycol diacrylate (PEGDA) | Gap26 DSP: Keloid | Dissolving MN | Application of Gap 26-loaded MNs could inhibit collagen I expression efficiently, demonstrating the possibility of this peptide loading system in the treatment of keloid scar. | [36] |
| Poly (vinyl alcohol)/poly (vinyl pyrrolidone | Etravirine DSP: HIV | Dissolving MN | The microneedle formulations exhibited sustained delivery in rats in vivo, suggesting that, following further development, such patches may be clinically useful in that they could replace daily tablets, thus potentially enhancing adherence to therapy and health-related quality of life for HIV patients. | [37] |
| Poly vinyl pyrrolidone | Rhodamine B, hyaluronidase and millitin DSP: Rheumatoid arthritis | Dissolving MNs | Notably, the permeation-enhancement effect of hyaluronidase (HAase) could be affected by the dosage of HAase and the physicochemical properties of drugs, and therefor formulation optimization is usually required to achieve a satisfying result. Moreover, the interaction between Haase and MN polymers or APIs, like electrostatic force and hydrogen, may prolong the release of HAase and APIs from MNs, and further studies could be performed to illuminate how the formulation components affect the x’ release behavior and bioavailability of HAase MNs. | [38] |
| WOUND HEALING | ||||
| Chitosan | Centella asiática (medicinal herb) DSP: Wound healing | Dissolving MNs | The system was biocompatible with keratinocytes and fibroblasts and showed a sustained drug release over 48 h. | [39] |
| Chitosan/fucoidan nanoparticle-loaded pullulan microneedle | Moxifloxacin (MOX), thrombin (TH) and lidocaine (LH) DSP: Wound healing | Dissolving MNs | Rapid release of thrombin (TH) and lidocaine (LH) within 1 h, and sustained release of MOX for 24 h. The system heals mice skin wounds completely within 7 days and restores collagen deposition with accelerated cell proliferation, granulation, and reduced proinflammatory cytokines. | [40] |
| Polyvinyl alcohol | Parathyroid hormone (PTH) DSP: Wound healing | Forming MNs | demonstrated an intermittent systemic administration of PTH using our PTMN patches that accelerated skin wound healing, evaluated for 14 days. | [41] |
| Hyaluronic acid | Endothelial growth factor (VEGF) DSP: Wound healing | Dissolving MNs | The system could effectively deliver active substances and demonstrated induction of the orientation of fibroblasts; while VEGF release could facilitate tubular formation of endothelial cells. | [42] |
| Alginate/PEGDA hydrogel 3D-printed hydrogel-filled microneedle | Rhodamine B/bovine serum albumin (BSA) and VEGF DSP: Wound healing | Hollow MNs | The system was effective at containing and releasing bioactive VEGF and promoting gap closure in an HUVEC scratch assay. | [43] |
| Gelatin and methacrylic anhydride | Adeno-associated virus (AAV) expressing human VEGF (AAV-VEGF) DSP: Wound healing | Dissolving MNs | The implantation did not elicit an obvious inflammatory response and had good biocompatibility in the brain. In addition, the system increased VEGF expression and enhanced functional angiogenesis and neurogenesis. | [44] |
| Mussel adhesive protein (MAP)-based shell and a nonswellable silk fibroin (SF)-based core | Fluorescein isothiocyanate (FITC)-conjugated dextran DSP: Wound healing | Forming MN | The protein-based hydrogel system achieved in vitro sustained releases for at least 7 days via swelling-mediated diffusion and enzymatic degradation. | [45] |
| METABOLIC DISORDERS | ||||
| CaCO3 microparticles (INS-CaCO3 MPs) and poly vinyl pyrrolidone (PVP) | Insulin DSP: Diabetes | Dissolving MNs | This study suggests that the use of INS-CaCO3/poly vinyl pyrrolidone MNs achieved both high efficiency and constant release of insulin in comparison with the traditional subcutaneous injection approach. | [46] |
| Alginate with hydroxyapatite biolinker glucose responsive | Insulin DSP: Diabetes | Dissolving MNs | The microneedle patches regulated the blood glucose levels of diabetic mice in normoglycemic ranges for up to 40 h and alleviated the diabetic symptoms of the mice. | [47] |
| Chitosan | Insulin DSP: Diabetes | Coated MNs | In a type 1 diabetic mouse model, application of the smart MN system quickly causes normoglycemia within 1 h, which is prolonged for 5 h. | [48] |
| Poly-c-glutamic acid (c-PGA) and poly vinyl pyrrolidone-polyvinyl alcohol (PVP/PVA) | Insulin DSP: Diabetes | Dissolving MNs | The MNs patch after insertion dissolved into the skin within 4 min to deliver the entire drug load, without requiring the users to remove any sharps or waste. | [49] |
| Polyethylene glycol, 2-methoxyethanol, glycidyl methacrylate, Trimethylolpropane trimethacrylate, and triethylene glycol dimethacrylate (TEGDMA) | Insulin DSP: Diabetes | Dissolving MNs | The system exhibited excellent skin penetration ability and good biocompatibility without skin irritation and hypersensitivity. In vivo transdermal delivery of insulin nanovesicles in diabetic rats demonstrated that the system coupled with iontophoresis could effectively regulate blood glucose levels, maintain normoglycemia, and avoid the critical risk of hypoglycemia. | [50] |
| Gelatin grafted with carboxylic end-capped poly(N-isopropylacrylamide) (PNIPAm) | Insulin DSP: Diabetes | Solid MNs | The system had a temperature-responsive crosslinker for controlled release. Both in vivo and in vitro tests demonstrated the sufficient penetration ability and crosslinking speed of the system for maintaining blood glucose concentrations within the medicable levels for a long period of time. | [51] |
| Photocrosslinked methacrylated hyaluronic acid (MeHA) | Glucagon DSP: Diabetes treatment problems | Dissolving MN | The system successfully prevented hypoglycemia induced by overdosed insulin administration in rat models. The results from this work warrant further development of the transdermal glucagon delivery system as a solution to potentially life-threatening complications associated with intensive insulin therapy. | [52] |
| Phenylboronic acid grafted sodium hyaluronate and polyvinyl alcohol | Insulin DSP: Diabetes | Dissolving MNs | In the hypoglycemic experiment of diabetic rats, the microneedle patch effectively pierced the skin and maintained BGLs in the normal range for a long time. | [53] |
| Multifunctional silk fibroin methacryloyl | Prussian blue nanozymes (PBNs) and vascular endothelial growth factor (VEGF) DSP: Diabetic wound healing | Dissolving MNs | The system exhibits excellent biocompatibility, drug-sustained release, proangiogenesis, antioxidant, and antibacterial properties with sustained release after 9 days. | [54] |
| Ti2C3 MXenes-integrated poly-γ-glutamic acid (γ-PGA) hydrogel MNs | Asiaticoside DSP: Diabetic foot ulcer | Dissolving MNs | The system was shown to be a multifunctional subcutaneous drug-delivering system for accelerating diabetic wound healing for 14 days. | [55] |
| Poly (DMAA-co-PyPBA) and PEG4a−Diol | Insulin DSP: Diabetes | Dissolving MNs | The release of insulin from the system was accelerated in the presence of glucose. Moreover, short-term blood glucose control in a diabetic rat model following the application of the device to the skin confirms insulin activity and bioavailability. | [1] |
| NIPPAn and N,N′-Metilenobisacrilamida (MBA) crosslinked 4-(2-acrylamidoethylcarbamoyl)-3-fluorophenylboronic acid (AmECFPBA) | Insulin DSP: Diabetes | Forming MN | Aqueous stability of at least 2 months and sustained performance durability. | [56] |
| NIPAM, N-Vinylpyrrolidone (NVP), 3-(acrylamido) phenylboronic acid (AAPBA), MBA and photoinitiator were the base of hydrogel | Insulin DSP: Diabetes | Dissolving MNs | The release of insulin on the system surface was uncontrolled by MNs and rapidly finished after ~10 min. However, the release of insulin within MNs is dependent on glucose concentration. | [57] |
| CENTRAL NERVOUS SYSTEM DISORDERS | ||||
| Gantrez® S-97and PEG | Esketamine (ESK) DSP: Depression | Forming MNs | The authors aimed to achieve sustained therapeutic levels in plasma over 24 h using ESK-containing drug reservoirs in combination with hydrogel-forming MNs in this in vivo feasibility study. | [58] |
| Poly(vinylpyrrolidone) or Gantrez® S-97 | Donepezil DSP: Alzheimer’s | Forming MN | The authors aimed to achieve sustained therapeutic levels in plasma over 24 h, using the optimum patch formulation. | [59] |
| Gelatin methacryloyl (GelMA) | L-DOPA DSP: Parkinson’s | Dissolving MNs | The system released L-DOPA directly entering the blood, which reduces the side effects on the gastrointestinal tract and improves the utilization rate of the drug. | [60] |
| OCULAR DISORDERS | ||||
| NIPAAm-b-poly glutamic acid hydrogel | Dexamethasone DSP: Posterior ocular diseases | Forming MNs | The system was effective in delivering DEX, a hydrophobic drug, improving effective concentrations, and can be considered a promising system for drug delivery in this ocular region. | [61] |
| INFECTIONS DISORDERS | ||||
| PVA and polyvinyl pyrrolidone (PVP) | Albendazole (ABZ) DSP: Parasitic infections | Forming MNs | The overall results showed that this dosage form has a higher potential for transdermal bioavailability and can deliver ABZ more quickly. | [62] |
| Carbopol® 934 with poly (Ɛ-caprolactone) (PCL)-based nanoparticles (NPs) | Carvacrol (CAR) DSP: Biofilms-infected wounds | Dissolving MNs | Animal studies showed the successful in vivo delivery of CAR-PCL NPs to the skin, where bacteria can grow and form biofilms. | [63] |
| Carboxymethyl cellulose/polyvinylpyrrolidone | Amphotericin B (AMB) DSP: Cutaneous leishmaniasis | Dissolving MNs | After insertion to the skin, the system was rapidly dissolved to release the encapsulated drug, and the resulted micropores in the skin were quickly resealed within 30 min. Flow cytometry results showed a potent in vitro leishmanicidal activity of AMB-loaded MN patches against the Leishmania parasites (up to 86% of the parasites die). | [64] |
| Gantrez® S-97 and Carbopol® 974P | Cefazolin DSP: Bacterial infection | Forming MN | The system was capable of achieving up to 80 μg Cefazolin delivery into the epidermis within 2 h of application, with the effect lasting for more than 24 h. | [65] |
| Poly (lactic-co-glycolic acid) (PLGA) | Nile red and Amphotericin B DSP: fungal infections | Forming MN | An in vitro release study demonstrated that the release of amphotericin B inside the system would last for a week. An antifungal test revealed the effectiveness of the inserted tips against fungal growth. | [66] |
| PVA with S-nitrosoglutathione and graphene oxide (GO) | Nitric oxide (NO) DSP: biofilm-infected chronic wounds | Forming MN | The test results showed that an increase in temperature increased the release of NO from the patch and thus enhanced antibacterial performance. | [67] |
| Gantrez® S-97, PVA and polyvinyl pyrrolidone (PVP) | Rifampicin, Pyrazinamide, Ethambutol dihydrochloride and others DSP: Tuberculosis | Forming MN | The system was able to deliver optimal concentrations of the drugs used in the test. The results of this work demonstrated the versatility of hydrogel formulations to deliver a tuberculosis drug regimen using MNs. | [68] |
| PVA and polydopamine (PDA) NP | Levofloxacin and alfa-amilase DSP: Bacterial with biofilm infections | Dissolving MN | The system combined antibiotics and local moderate hyperthermia to kill bacteria and eliminate the biofilm in the wound, thereby shortening the period of inflammation to promote wound healing and tissue regeneration. | [69] |
| Chitosan-NIPAM hydrogel | Vascular endothelial growth factor (VEGF) DSP: Precaution of infections and promotion of wound healing | Dissolving MNs | The system exhibited a superior capability of acceleration in inflammatory inhibition, collagen deposition, angiogenesis, and granulation tissue formation. In addition, a controlled release can be achieved with thermoresponsive NIPAM. | [70] |
| Chitosan (CS) and zinc nitrate (CS–Zn [II] MNs) | Chitosan and Zn NP DSP: Bacterial biofilm | Dissolving MNs | The system not only can directly transport CS and Zn2+ into the bacterial biofilm but also offers a large specific surface area, to facilitate the diffusion within biofilm and effectively eradicate the bacterial biofilm. | [71] |
| Chitosan polyethylenimine antimicrobial polymer | Amphotericin B DSP: Fungal infections | Dissolving MN | The results of the combination system are attributable to the high bioavailability of therapeutics and synergistic actions of the antifungal polymer and drug. | [72] |
| Methacrylated hyaluronic acid (MeHA) | Zn-MOF DSP: Bacterial infections and wound healing | Dissolving MNs | The system significantly promotes angiogenesis, deposition of collagen, and reduced inflammation in the wounds. | [73] |
| PVA and phenylboronic acid | Clindamicyn DSP: Bacterial acne | Dissolving MNs | The drug-loaded MNs are able to penetrate the stratum corneum to improve the drug interaction against P. acnes. Meanwhile, the inflammation-mediated sustained drug release continuously keeps a sufficient drug concentration in the therapeutic levels around acne area (treatment of 6 days). | [74] |
| REPRODUCTIVE SYSTEM DISORDERS | ||||
| Methacrylamide hyaluronic acid hydrogel | Collagen type I DSP: Urinary incontinence | Dissolving MNs | The results of experiments showed that the system has good in vitro biocompatibility. In vivo experiments showed that the system could improve the urodynamic index of urinary stress incontinence in mice. | [75] |
| PVA and polyvinyl pyrrolidone (PVP) | Sildenafil citrate DSP: Erect disfunction | Forming MN | The ex vivo permeation study showed that up to 80% of sildenafil citrate was delivered transdermally from this combined dosage in the evaluation of 14 days. | [76] |
| Gelatin-methacryloyl (GelMA) | Antioxidant cerium oxide (CeO2) nanozyme MNs with stem cell loading DSP: Endometrial repair | Dissolving MNs | The combination in the system offered a flexible and elaborate platform to realize the combination therapy of endometrial injury. The system contributes to the in situ delivery of stem cells at the sites of injury in an efficient and noninvasive manner, possibly promoting the therapeutic effects of stem cells. | [77] |
| ANALGESIC, ANESTHETIC, AND/OR ANTI-INFLAMMATORY EFFECT | ||||
| Poly (methylvinylether/ maelic acid) (PMVE/MA) hydrogel | Caffeine and lidocaine DSP: Analgesic effect | Forming MNs | The system offered a simple and convenient route of drug administration while eliminating the pain associated with the use of hypodermic needles. | [3] |
| PVA and poly (ethyl- eneimine) with MBA crosslinking agent | Indomethacin DSP: Anti-inflammatory effect | Dissolving MNs | This investigation provided the efficacy of the device for attaining electromodulated drug release in the ex vivo porcine model. | [78] |
| Chitosan | Meloxican DSP: Anti-inflammatory effect | Dissolving MNs | The good bioavailability through the system demonstrates that chitosan/meloxicam MNs patches may be suitable to manage pain in cattle after routine procedures. | [79] |
| Gelatin-methacryloyl (GelMA) | Lidocaíne chlorideoride DSP: Anesthetic effect | Dissolving MNs | The system exhibited an analgesic effect for 6 h with good biological safety such that it did not cause irreversible wounds and irritation to the skin, nor did it bring potential side effects. | [80] |
| Polyvinyl pyrrolidone (PVP) | Sumatriptan succinate DSP: Analgesic effect | Dissolving MN | The calculated diffusion coefficients were one order of magnitude greater than the value estimated when the drug was directly applied to the skin surface. The dissolution rate constant was affected by the concentration of the polymer matrix. | [81] |
| PVA and maltose (MT) | Sinomenine hydrochloride DSP: Analgesic effect | Dissolving MNs | The in vitro study showed that the permeation rate was nearly stable within 48 h. Pharmacokinetic studies indicated that the system exhibited lower clearance, longer retention time, higher bioavailability, and stability versus SH-loaded hydrogel. | [82] |
| Polyvinyl pyrrolidone (PVP) | Lidocaíne hydrochloride DSP: Anesthetic effect | Dissolving MN | The system demonstrated sufficient biocompatibility without causing noticeable irritation to the skin. Also, the obtained MNs significantly shortened the onset time of lidocaine hydrochloride generating local anesthesia effects, comparable to creams. | [83] |
| PVA with poly (ethyleneimine) solution (PEI) and 1 vinylimidazole (VI) | Indomethacin DSP: Anti-inflammatory effect | Dissolving MNs | Both in vitro and in vivo studies revealed a good preliminary indication that the system has electroresponsive capabilities, ultimately facilitating the immediate release of indomethacin. | [84] |
| Sodium carboxymethyl cellulose (NaCMC) and gelatine A (GEL) | Lidocaine hydrochloride DSP: Anesthetic effect | Dissolving MNs | Long-term release of lidocaine and precaution of side effect in comparison to conventional methods. | [85] |
| Carboxymethylcellulose and gelatine | Lidocaine hydrochloride DSP: Anesthetic effect | Dissolving MNs | The diffusion experiments revealed a small increase in diffusional permeation when LFS was used in combination with an MN array pretreated skin. | [86] |
| SKIN LIGHTERING AND SKIN ANTIAGING AGENT | ||||
| Polyacrylic acid-co-maleic acid (PAMA) and p PVA | Alpha-arbutin DSP: Skin lightering | Dissolving and forming MNs | α-arbutin loaded in dissolving MNs had significantly enhanced α-arbutin permeation both in vitro and in vivo more than α-arbutin-loaded forming MNs. In a long-term stability test, α-arbutin remained stable at 25 °C for 3 months. | [87] |
| 1,4-butanediol diglycidyl ether (BDDE) crosslinked hyaluronic acid | Sulforhodamine B. DSP: Antiaging | Dissolving MNs | The system has prolonged effectiveness, with high swelling retention time or epidermal expansion time in mice up to 6 days or more. | [88] |
| PVA and carbomer | Glabridin DSP: Antiaging | Forming MN | The in vitro release studies showed that cumulative permeation amount within 24 h of the system with chemical crosslinker was significantly higher than that achieved by the system with physical crosslinker and that achieved by glabridin-loaded gel. | [89] |
| TISSUE REGENERATION | ||||
| Gelatin methacryloyl (GelMA) MNs with and without poly(lactic-coglycolic acid) nanoparticles and cytokine-loaded silica microparticles | Tetracycline and cytokines (IL-4 and TGF-b) DSP: Periodontal tissue regeneration | Dissolving MNs | In vivo delivery of the MN patch into periodontal tissues suppressed proinflammatory factors and promoted proregenerative signals and tissue healing. | [90] |
| Gelatin methacryloyl | Galunisertib DSP: Myocadiac infarction | Dissolving MNs | The drug-release properties can be controlled by adjusting the degree of crosslinking to ensure sustained release for at least 15 days after application. | [7] |
| MANAGEMENT OF PHARMACOKINETIC AND TOXICITY | ||||
| Poly (methylvinylether-co-maleic acid) crosslinked by esterification with poly (ethylene glycol) | Metformin hydrochloride DSP: Enhance bioavailability | Forming MNs | Metformin HCl plasma concentration was shown to be sustained over 24 h. This may indicate that the drug continues to be released from the MN patch while it is being cleared from the body of the rats. | [91] |
| Polyacrylic acid | Naloxone DSP: Management of side effects of opioids | Dissolving MNs | Naloxone permeation through intact skin was highest from pH 7.4 gels when naloxone is unionized. | [92] |
| PULMONARY DISORDER | ||||
| PVA and polyvinyl pyrrolidone (PVP) | Sildenafil citrate DSP: Pulmonary hypertension | Forming MN | Increasing SC bioavailability in treating pulmonary arterial hypertension is another benefit of this preparation. | [93] |
| NOT SPECIFIED | ||||
| Phenylborate ester bonds crosslinking NIPAAm/ poly(butyl acrylate) PBA | Rhodamine B drug DSP: Not specified | Dissolving MNs | The thermo and glucose-responsive system presented excellent biocompatibility for drug delivery at 37 °C. | [94] |
| Polyvinylpyrrolidone-based graphene oxide HG | L-Ascorbic acid DSP: Not specified | Dissolving MNs | New platform electricity responsive for drug delivery. The drug-release efficiency of the system with graphene oxide was increased by more than 2 times compared to the system without graphene oxide when 5 V was applied. | [95] |
| Poly (vinyl alcohol)/poly(vinylpyrrolidone)/citric acid composite crosslinked | Drug models theophylline (THEO), cyanocobalamin (CYAN), and fluorescein sodium (FLUO) DSP: Not specified | Forming MNs | The hydrogel shows good permeability with sufficient mechanical strength to aid skin insertion and hydrophilic drug retention capacity. | [96] |
| Alginate hydrogel | Ascorbic acid and tranexamic acid DSP: Not specified | Forming MN | The in vitro transdermal delivery efficiencies of drugs were significantly improved throughout 16 h. | [97] |
| PVP with gold and silver electrodes | Ascorbic acid DSP: Not specified | Dissolving MN | The system was extremely efficient in controlling drug release. It was influenced by temperature, irradiation, and electricity. | [98] |
| PLGA (poly (D, L-lactic co-glycolic acid), PVA, and polyvinyl pyrrolidone (PVP) | FITC Dextran DSP: Not specified | Dissolving MNs | The cumulative permeation of FITC-Dextran after 48 h was 2.34 ± 0.40 μg/cm2 and it showed a 31-fold higher permeation profile than its respective control. This study demonstrated that incorporating higher-molecular-weight molecules into PLGA MNs proved to be an effective strategy to sustain the release of macromolecules across the stratum corneum/epidermis for transdermal delivery. | [99] |
| PVA | Rhodamine B DSP: Not specified | Dissolving MN | In this study, the system showed dissolution after 17 min, good swelling properties, and a consistent release of the model drug RD for up to 12 days. | [100] |
| Gantrez® S-97 and PVA with crosslinker polyethylene glycol (PEG) and anhydrous sodium carbonate (Na2CO3) | Amoxicillin (AMX), atenolol (ATL), diltiazem (DLT), levodopa (LD), carbidopa (CD), levofloxacin (LVX), and primaquine (PQ) DSP: Not specified | Forming MN | The system was efficient for in vivo delivery of AMX, LD, and LVX at therapeutically relevant concentrations. | [101] |
| Gelatin methacrylate | Rhodamine B DSP: Not specified | Dissolving MNs | System with effectiveness for rapid release phase and sustained release phase. | [102] |
| 2-hydroxyethyl methacrylate (HEMA) and ethylene glycol dimethacrylate (EGDMA) | Ibuprofen DSP: Not specified | Forming MN | In vitro, this system was able to deliver up to three doses of 50 mg of ibuprofen upon application of an optical trigger over a prolonged period of time (up to 160 h). | [103] |
| Gantrez-PEG | Caffeine DSP: Not specified | Forming MN | The study demonstrated that the use of microwave radiation significantly reduces the time required for MN preparation. | [104] |
| Gantrez® S-97 and PEG | Ibuprofen and ovoalbumin DSP: Not specified | Forming MN | In in vitro delivery experiments across excised neonatal porcine skin, approximately 44 mg of the model high dose small molecule drug ibuprofen sodium was delivered in 24 h, equating to 37% of the loading in the lyophilized reservoir. | [105] |
| Poloxamer hydrogel (pluronic) | Methotrexate DSP: Not specified | Dissolving MN | The system embedded within the microporated skin site provided a steady and sustained delivery of methotrexate for 72 h. | [106] |
| Zwitterionic sulfobetaine (SPB) monomer blended with dextran-glycidyl methacrylate/acrylic acid | Rhodamine B DSP: Not specified | Coated MN | The hydrogel MNs with SPB side chains are suitable for biopharmaceutical transdermal drug delivery, particularly protein-based drugs with improved bioavailability. The MNs exhibited high drug loading capacity, efficient drug release, and protein preservation by suppressing aggregation. | [107] |
| Cyclodextrin (CD) | Ibuprofen DSP: Not specified | Dissolving MN | In this study, the system cannot only load hydrophilic drugs but also encapsulate hydrophobic drugs through the unique supramolecular structure of cyclodextrin. | [108] |
| Methacrylated hyaluronic acid (MeHA) | Doxorrubicin and others DSP: Not specified | Coated MNs | In this work, a self-adhesive MN platform with universal drug-loading capability was developed. | [109] |
| pNIPAM | Insulin DSP: Not specified | Hollow MNs | The system exhibited an evident volume shrinkage, excellent photothermal ability, and repeatable NIR-responsiveness. | [110] |
| Poly (ethylene glycol) diacrylate/polyvinylpyrrolidone (PEGDA/PVP) | Rhodamine B DSP: Not specified | Dissolving MNs | The system presented cumulative drug release kinetics in 172 h. | [111] |
| Chitosan-porous carbon nanocomposites | Cephalexin DSP: Not specified | Dissolving MNs | The system responded to acidic pH 4 and electric pulse 5 V for drug release. | [112] |
| Carboxymethylcellulose | Diclofenac sodium DSP: Not specified | Dissolving MN | The dissolution of the patches in saline buffer results in a maximum cumulative release of 98% of diclofenac after 40 min, and insertion in a skin simulant reveals that all MNs completely dissolve within 10 min. | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filho, D.; Guerrero, M.; Pariguana, M.; Marican, A.; Durán-Lara, E.F. Hydrogel-Based Microneedle as a Drug Delivery System. Pharmaceutics 2023, 15, 2444. https://doi.org/10.3390/pharmaceutics15102444
Filho D, Guerrero M, Pariguana M, Marican A, Durán-Lara EF. Hydrogel-Based Microneedle as a Drug Delivery System. Pharmaceutics. 2023; 15(10):2444. https://doi.org/10.3390/pharmaceutics15102444
Chicago/Turabian StyleFilho, David, Marcelo Guerrero, Manuel Pariguana, Adolfo Marican, and Esteban F. Durán-Lara. 2023. "Hydrogel-Based Microneedle as a Drug Delivery System" Pharmaceutics 15, no. 10: 2444. https://doi.org/10.3390/pharmaceutics15102444