Topical Application of Siberian Pine Essential Oil Formulations Enhance Diabetic Wound Healing
Abstract
:1. Introduction
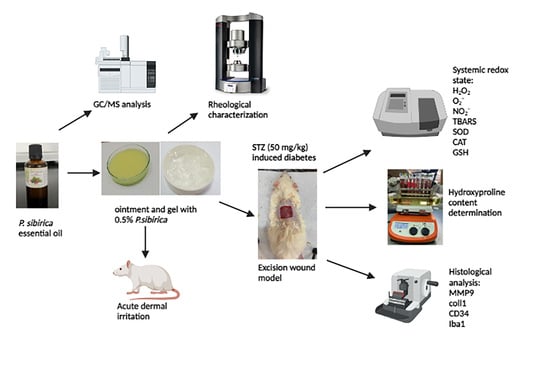
2. Materials and Methods
2.1. Pinus Sibrica Essential Oil Obtaining
2.2. Identification of Pinus Sibirica Essential Oil Chemical Composition
2.3. Composition and Preparation of Semi-Solid Samples
2.4. Rheological Characterization of Semi-Solid Formulations
2.5. In Vivo Animal Experiments
2.5.1. Ethics Statement
2.5.2. Acute Dermal Irritation Test
- Animals treated with 0.5% PSEO ointment (n = 3);
- Animals treated with 0.5% PSEO gel (n = 3);
- Animals treated with the ointment base (n = 3);
- Animals treated with the gel base (n = 3).
2.5.3. Wound-Healing Activity
Animals
Diabetes Mellitus Induction
Excision Wound Model
- Animals without treatment (control group (CTRL));
- Animas treated with a commercially available ointment 1% silver sulfadiazine (SSD group);
- Animals treated with an ointment base (OINT group);
- Animals treated with a gel base (GEL group);
- Animals treated with a 0.5% Pinus sibirica essential oil ointment (PSOINT group);
- Animals treated with a 0.5% Pinus sibirica essential oil gel (PSGEL group).
Wound Contraction
Hydroxyproline Content Determination
Systemic Redox State Determination
Histology Analysis of Wound Tissue
2.6. Statistic Analysis
3. Results
3.1. The Essential Oil Chemical Composition
3.2. Rheological Properties of Formulations
3.3. Acute Dermal Irritation Test
3.4. Wound-Healing Activity
3.4.1. Wound Contraction
3.4.2. Determination of Hydroxyproline Content
3.4.3. Systemic Redox State Markers
3.4.4. Histopathological Analysis
4. Discussion
5. Conclusions
- Conducted phytochemical analysis of PSEO revealed the presence of terpene compounds, which, in synergy, attributed to a significant wound-healing effect in animal models;
- Rheological measurements for the PSEO ointment and gel proved structure flexibility, indicating a good ability to spread on the skin;
- Novel plant formulations with P. sibirica essential oil are safe for application since there were no signs of dermal irritation;
- The PSEO ointment and gel formulations demonstrated a significant wound-healing effect in the diabetic rat model, supported by the increased contraction of wound area as well as hydroxyproline content, attenuation of oxidative stress, and histopathological analyzed markers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human wound and its burden: Updated 2020 compendium of estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Baldassarro, V.A.; Lorenzini, L.; Giuliani, A.; Cescatti, M.; Alastra, G.; Pannella, M.; Imbimbo, B.P.; Villetti, G.; Calza, L.; Giardino, L. Molecular mechanisms of skin wound healing in non-diabetic and diabetic mice in excision and pressure experimental wounds. Cell Tissue Res. 2022, 388, 595–613. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Caruso, G.; Privitera, A.; Ahmed, O.A.; Fahmy, U.A.; Md, S.; Mohamed, G.A.; Ibrahim, S.R.M.; Eid, B.G.; Abdel-Naim, A.B.; et al. Fluoxetine Ecofriendly Nanoemulsion Enhances Wound Healing in Diabetic Rats: In Vivo Efficacy Assessment. Pharmaceutics 2022, 14, 1133. [Google Scholar] [CrossRef] [PubMed]
- Quazi, A.; Patwekar, M.; Patwekar, F.; Mezni, A.; Ahmad, I.; Islam, F. Evaluation of Wound Healing Activity (Excision Wound Model) of Ointment Prepared from Infusion Extract of Polyherbal Tea Bag Formulation in Diabetes-Induced Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 1372199. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Acuña, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic foot ulcers: Current advances in antimicrobial therapies and emerging treatments. Antibiotics 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Andjić, M.; Božin, B.; Draginić, N.; Kočović, A.; Jeremić, J.N.; Tomović, M.; Bradić, J.V. Formulation and evaluation of helichrysum italicum essential oil-based topical formulations for wound healing in diabetic rats. Pharmaceuticals 2021, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Andjić, M.; Draginić, N.; Kočović, A.; Jeremić, J.; Vučićević, K.; Jeremić, N.; Bradić, J. Immortelle essential oil-based ointment improves wound healing in a diabetic rat model. Biomed. Pharmacother. 2022, 150, 112941. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Makarova, M.N. Anti-inflammatory effect of Pinus sibirica oil extract in animal models. J. Nat. Med. 2008, 62, 436–440. [Google Scholar] [CrossRef]
- Lantto, T.A.; Dorman, H.D.; Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R.; Raasmaja, A. Chemical composition, antioxidative activity and cell viability effects of a Siberian pine (Pinus sibirica Du Tour) extract. Food Chem. 2009, 112, 936–943. [Google Scholar] [CrossRef]
- Efremov, A.A.; Zykova, I.D.; Senashova, V.A.; Grodnitckaya, I.D.; Pashenova, N.V. Antimicrobial and Antiradical Activities of Individual Fractions of Pinus sibirica Du Tour and Abies sibirica Ledeb. Growing in Siberia. Russ. J. Bioorg. Chem. 2021, 47, 1439–1444. [Google Scholar] [CrossRef]
- Romanenko, E.P.; Domrachev, D.V.; Tkachev, A.V. Variations in Essential oils from South Siberian conifers of the Pinaceae family: New data towards identification and quality control. Chem. Biodivers. 2022, 19, e202100755. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, T.E.; Fedorov, S.V.; Babkin, V.A. Phenolic compounds of cedar (Siberian pine) wood Pinus sibirica Du Tour. Chemistry 2020, 3, 97–104. [Google Scholar] [CrossRef]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound healing activity of α-pinene and α-phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured: Carol Stream, IL, USA, 2005; Volume 456, pp. 544–545. ISBN 978-1-932633-21-4. [Google Scholar]
- Kouhihabibidehkordi, G.; Kheiri, S.; Karimi, I.; Taheri, F.; Bijad, E.; Bahadoram, M.; Alibabaie, Z.; Asgharian, S.; Zamani, H.; Rafieian-Kopaei, M. Effect of White Tea (Camellia sinensis) Extract on Skin Wound Healing Process in Rats. World J. Plast. Surg. 2021, 10, 85. [Google Scholar] [CrossRef]
- OECD. 404. Guidelines for the Testing of Chemicals. Acute Dermal Irritation/Corrosion; OECD Guidel Test; OECD: Paris, France, 2015; pp. 1–8. [Google Scholar]
- Boudjelal, A.; Smeriglio, A.; Ginestra, G.; Denaro, M.; Trombetta, D. Phytochemical profile, safety assessment and wound healing activity of Artemisia absinthium L. Plants 2020, 9, 1744. [Google Scholar] [CrossRef]
- Tazeze, H.; Mequanente, S.; Nigussie, D.; Legesse, B.; Makonnen, E.; Mengie, T. Investigation of Wound Healing and Anti-Inflammatory Activities of Leaf Gel of Aloe trigonantha LC Leach in Rats. J. Inflamm. Res. 2021, 14, 5567–5580. [Google Scholar] [CrossRef]
- Akinlade, O.M.; Owoyele, B.V.; Soladoye, A.O. Streptozotocin-induced type 1 and 2 diabetes in rodents: A model for studying diabetic cardiac autonomic neuropathy. Afr. Health Sci. 2021, 21, 719–727. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Zhao, L.; Liu, L.; Wang, X.; Fan, Z. Investigating the efficacy and safety of mineral smectite granules on wound healing. Exp. Ther. Med. 2021, 21, 160. [Google Scholar] [CrossRef]
- G/giorgis, S.G.; Ambikar, D.; Tsegaw, A.; Belayneh, Y.M. Wound Healing Activity of 80% Methanolic Crude Extract and Solvent Fractions of the Leaves of Justicia schimperiana (Hochst. ex Nees) T. Anderson (Acanthaceae) in Mice. J. Exp. Pharmacol. 2022, 14, 167–183. [Google Scholar] [CrossRef]
- Li, D.; Wu, N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 187, 109882. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The treatment of impaired wound healing in diabetes: Looking among old drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Prosekov, Y.A.; Dyshlyuk, L.S.; Milent’Eva, I.S.; Pavsky, V.A.; Ivanova, S.A.; Garmashov, S.Y. Study of the biofunctional properties of cedar pine oil with the use of in vitro testing cultures. Foods Raw Mater. 2018, 6, 136–143. [Google Scholar] [CrossRef]
- Rogachev, A.D.; Salakhutdinov, N.F. Chemical composition of Pinus sibirica (Pinaceae). Chem. Biodivers. 2015, 12, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Yu, J.S.; Lee, H.Y.; Kwon, D.J.; Han, W.; Heo, S.I.; Kim, S.Y. Evaluations on deodorization effect and anti-oral microbial activity of essential oil from Pinus koraiensis. Korean J. Plant Res. 2014, 27, 1–10. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Gomez, A.D.L.A.; Jimenez, C.M.; Lizarraga, E.F.; Ibatayev, Z.A.; Suleimen, Y.M.; Catalán, C.A. Chemical composition and antifungal activity of essential oils from medicinal plants of Kazakhstan. Nat. Prod. Res. 2017, 31, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Shatar, S.; Adams, R.P. Analyses of the leaf and resin essential oils of Pinus sibirica (Rupr.) Mayr from Mongolia. J. Essent. Oil Res. 1996, 8, 549–552. [Google Scholar] [CrossRef]
- Ghica, M.V.; Hîrjău, M.; Lupuleasa, D.; Dinu-Pîrvu, C.E. Flow and thixotropic parameters for rheological characterization of hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Verma, G.; Ingle, A.; Kumar, S.; Sarma, H.D.; Dutta, D.; Dutta, B.; Kunwar, A.; Ajish, K.; Bhainsa, K.C.; et al. Structural, rheological and therapeutic properties of pluronic F127 hydrogel and beeswax based lavender oil ointment formulations. J. Mol. Liq. 2022, 365, 120157. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Akkol, E.K. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar]
- Haas, M.R.; Nguyen, D.V.; Shook, B.A. Recovery of Altered Diabetic Myofibroblast Heterogeneity and Gene Expression Are Associated with CD301b+ Macrophages. Biomedicines 2021, 9, 1752. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan, O.I.; Kumar, J.A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]








| Ingredient | Ointment Base (%) | Ointment PSEO (%) |
|---|---|---|
| Cholesterol | 5.00 | 5 |
| Lanolin | 15.00 | 15 |
| Parafinum liquidum | 15.00 | 15 |
| Vaselinum album | 65.00 | 65 |
| PSEO * | / | 0.5 |
| Ingredient | Gel Base (%) | Gel PSEO * (%) |
|---|---|---|
| Carbomer 940 | 0.50 | 0.50 |
| Propylene glycol | 10.00 | 10.00 |
| TEA 10% | q.s | q.s |
| Sodium benzoate | 0.20 | 0.20 |
| PSEO * | / | 0.5 |
| Aqua purificata | ad 100.00 | ad 100.00 |
| Peak No | R.I. * | Compound | Rt (min) | % |
|---|---|---|---|---|
| 1 | 934 | α-Pinene | 7.481 | 40.19 |
| 2 | 938 | Camphene | 7.907 | 1.17 |
| 3 | 974 | β-Pinene | 8.804 | 19.64 |
| 4 | 983 | β-Myrcene | 9.146 | 1.13 |
| 5 | 1007 | δ-3-Carene | 9.902 | 16.14 |
| 6 | 1011 | α-Terpinen | 10.102 | 0.22 |
| 7 | 1019 | o-Cymene | 10.386 | 2.06 |
| 8 | 1020 | D-Limonene | 10.553 | 9.92 |
| 9 | 1022 | Eucalyptol | 10.66 | 0.12 |
| 10 | 1079 | Terpinolene | 12.825 | 0.25 |
| 11 | 1272 | Bornyl acetate | 21.099 | 0.13 |
| 12 | 1403 | Longifolene | 26.112 | 3.18 |
| 13 | 1424 | Caryophyllene | 26.668 | 1.97 |
| 14 | 1452 | Humulene | 28.028 | 2.59 |
| 15 | 1512 | δ–cadinene | 30.734 | 0.27 |
| 16 | 1578 | Caryophyllene oxide | 33.047 | 0.34 |
| Total % of identified compounds | 99.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolic, M.; Andjic, M.; Bradic, J.; Kocovic, A.; Tomovic, M.; Samanovic, A.M.; Jakovljevic, V.; Veselinovic, M.; Capo, I.; Krstonosic, V.; et al. Topical Application of Siberian Pine Essential Oil Formulations Enhance Diabetic Wound Healing. Pharmaceutics 2023, 15, 2437. https://doi.org/10.3390/pharmaceutics15102437
Nikolic M, Andjic M, Bradic J, Kocovic A, Tomovic M, Samanovic AM, Jakovljevic V, Veselinovic M, Capo I, Krstonosic V, et al. Topical Application of Siberian Pine Essential Oil Formulations Enhance Diabetic Wound Healing. Pharmaceutics. 2023; 15(10):2437. https://doi.org/10.3390/pharmaceutics15102437
Chicago/Turabian StyleNikolic, Milica, Marijana Andjic, Jovana Bradic, Aleksandar Kocovic, Marina Tomovic, Andjela Milojevic Samanovic, Vladimir Jakovljevic, Mirjana Veselinovic, Ivan Capo, Veljko Krstonosic, and et al. 2023. "Topical Application of Siberian Pine Essential Oil Formulations Enhance Diabetic Wound Healing" Pharmaceutics 15, no. 10: 2437. https://doi.org/10.3390/pharmaceutics15102437